Abstract
Survivability under carbon-starvation conditions was investigated in four species of purple phototrophic bacteria: Rhodopseudomonas palustris, Rhodobacter sphaeroides, Rhodospirillum rubrum, and Rubrivivax gelatinosus. All these test organisms survived longer in the light than in the dark. ATP levels in the cultures were maintained in the light, which indicated that survivability was supported by photosynthesis. Survivability and tolerance against hypertonic stress in the dark was higher in Rhodopseudomonas palustris, which is widely distributed in natural environments including soils, than in the three other species.
Keywords: starvation, survivability, purple phototrophic bacteria
Purple nonsulfur phototrophic bacteria grow by anoxygenic photosynthesis and utilize organic carbon as a carbon source for growth. They have been shown to markedly contribute to carbon, nitrogen, and sulfur cycles on the Earth (10). Previous studies examined the survivability of purple phototrophic bacteria under starvation conditions (2, 11). Breznak et al. (2) reported that the carbon-starved cells of Rhodospirillum rubrum strain Ha remained viable for longer in the light than in the dark. Oda et al. (11) demonstrated the positive effects of illumination on the survivability of Rhodopseudomonas palustris strain DCP3. The positive effects of light on starvation survival have also been reported in some strains of aerobic bacteriochlorophyll-containing bacteria (4, 7, 13, 14). Energy supply by photosynthesis appears to promote survivability; however, no direct correlation between the energy level of cells and viability has not yet been reported.
In the present study, we determined the viability and ATP levels of the test organisms under carbon-starvation conditions in the light and dark. Four species were used comparatively; Rhodopseudomonas palustris strain ATCC BAA-98 (=CGA009), Rhodobacter sphaeroides strain ATCC 17023T (=2.4.1T), Rhodospirillum rubrum strain S1T (=ATCC 11170T), and Rubrivivax gelatinosus strain IL144 (=NBRC 100245). The susceptibility of the starved cells of these species to osmotic stress was also investigated in order to characterize their survivability.
Rps. palustris ATCC BAA-98 and Rba. sphaeroides ATCC 17023T were obtained from the American Type Culture Collection and Rsp. rubrum S1T and Rvi. gelatinosus IL144 (=NBRC 100245) were kindly provided by K. Shimada (Tokyo Metropolitan University). These bacteria were anaerobically cultivated in a carbon-limited medium (pH 7.0) containing (per liter) 0.5 g sodium succinate as the sole source of carbon, 1 g (NH4)2SO4, 0.38 g KH2PO4, 0.39 g K2HPO4, 1 mL of a vitamin mixture (5), and 5 mL of a basal salt solution (5) at 30°C under illumination (tungsten lamp with a 750 nm longpass filter; 600 J s−1 m−2, quantitated by a pyranometer [LI-190SA, Meiwafosis, Tokyo, Japan]). The cultures were continuously and vigorously agitated using a magnetic stirrer. When increases in optical density at 660 nm ceased at the exponential growth phase, the culture was defined as being in carbon-starvation conditions. The starved cells in vials were incubated at 30°C with agitation in the light as described above or in the dark. Viability was measured by plate counting with 1.5% agar medium containing (per liter) 1 g sodium succinate, 1 g sodium acetate, 0.1 g yeast extract (Nihon Seiyaku, Tokyo, Japan), 0.1 g Na2S2O3·5H2O, 1 g (NH4)2SO4, 0.38 g KH2PO4, 0.39 g K2HPO4, 1 mL of a vitamin mixture (5), and 5 mL of a basal salt solution (5). To determine CFUs, serial dilutions of cultures were spread on 2 to 4 agar plates per one of the serial dilutions and cultivated aerobically at 30°C in the dark for approximately one week. The amount of ATP in the cultures was quantified using the BacTiter-Glo Microbial Cell Viability Assay Kit (Promega, Madison, WI, USA) and GloMax 20/20n Luminometer (Promega) according to the manufacturer’s protocol.
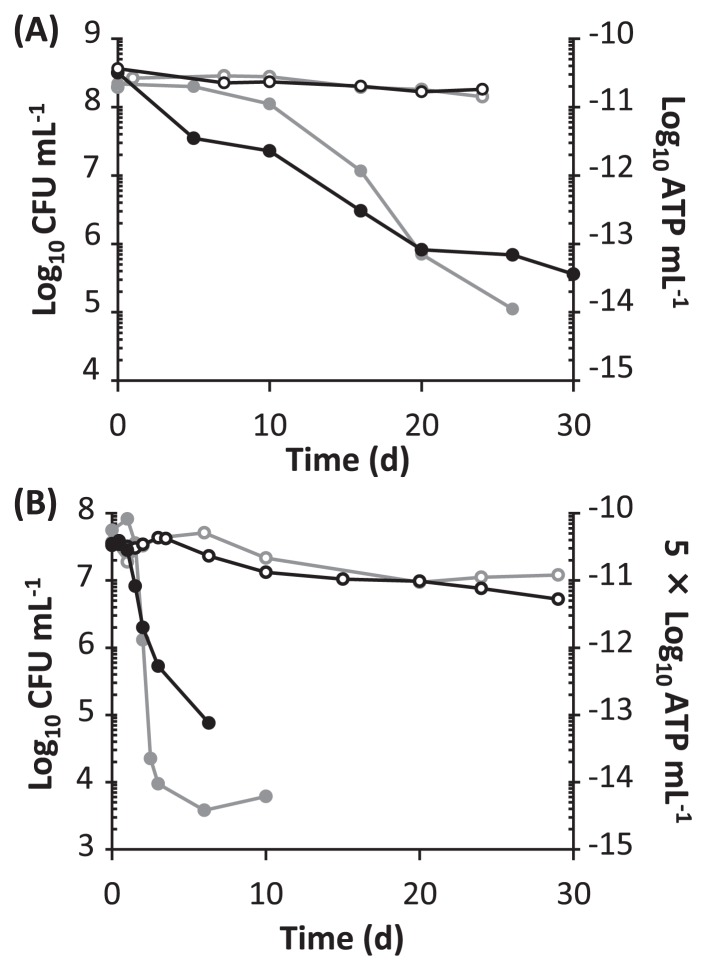
The four test strains of phototrophic bacteria were grown photoheterotrophically (Fig. S1). When sufficient organic carbon was available (5 g sodium succinate per liter), the cultures reached the stationary phase at which the optical densities at 660 nm were 0.5–1.6. This retardation of growth did not appear to be due to carbon starvation, but may have been attributed to a high cell density. When the initial concentration of sodium succinate as the carbon source was reduced to 0.5 g per liter, growth stopped at an optical density of 0.2–0.3 (Fig. S1); these cells were then defined as carbon-starved cells. Although the production of fumarate was observed in the culture of Rps. palustris ATCC BAA-98 during the exponential phase of growth, neither succinate nor fumarate were detected in the culture supernatant after growth stopped under carbon-limiting conditions (data not shown). Viability and ATP levels were determined in the starved cultures of Rps. palustris ATCC BAA-98 and Rsp. rubrum S1T after incubation in the light and dark (Fig. 1). In both strains, higher CFU values were observed in the light than in the dark. This higher CFU-value in the light was also confirmed for Rba. sphaeroides ATCC 17023T and Rvi. gelatinosus IL144 (Fig. S2). ATP levels were also maintained in the light (Fig. 1). The ATP produced through cyclic photophosphorylation may be consumed to keep viability by maintaining cytoplasmic homeostasis and/or synthesizing mending proteins. Our preliminary transcriptome analysis of Rps. palustris ATCC BAA-98 indicated that the genes related to transcription, translation, and protein decomposition were highly expressed under starvation conditions in the light (data not shown), which suggested that the decomposition and synthesis of cellular proteins may have been stimulated. A previous proteomic study for an aerobic phototrophic bacterium also demonstrated that light induced marked changes in the composition of proteins in cells under carbon-limiting conditions (18).
Fig. 1.
Changes in viability (black line) and ATP levels (gray line) during carbon-starvation conditions. The starved cells of Rps. palustris ATCC BAA-98 (A) and Rsp. rubrum S1T (B) were incubated in the light (open circle) and dark (filled circle). Time 0 was defined as the time when growth completely stopped. Viability was measured by plate counting and expressed as CFU mL−1.
During the period of incubation in the dark, the viable counts of the four strains of the phototrophic bacteria decreased at different rates from each other. After 10 d from the beginning of starvation, the CFUs of Rps. palustris ATCC BAA-98 decreased to 51% of the initial value, while the ATP level decreased more to 7%. When the starved cells that had been maintained in the dark for 10 d were illuminated for 1 min, ATP levels increased by more than 10-fold (data not shown). The decrease of up to 10% in the ATP concentrations in cells under growing conditions may not have been fatal to survival. The CFU and ATP levels of Rsp. rubrum S1T rapidly decreased in the dark and reached 0.007% and 0.3% of the initial values after 6 d of starvation, respectively (Fig. 1). This rapid decrease in ATP in Rsp. rubrum S1T may have been caused by the excessive consumption of ATP for cellular activities such as swimming motility, but not for maintenance of viability (8).
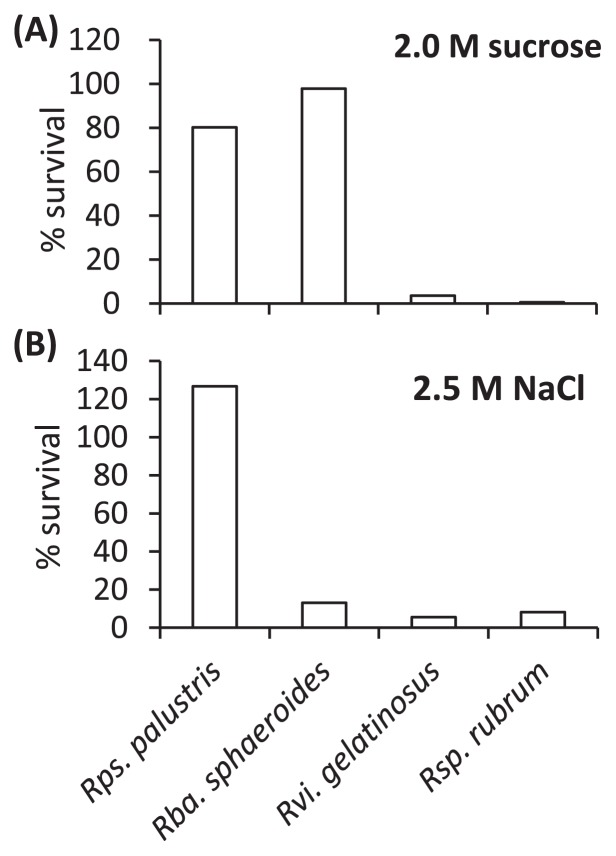
Susceptibility to osmotic stress was determined using the starved cells (Fig. 2). After exposure to 2.0 M sucrose solution, Rps. palustris ATCC BAA-98 and Rba. sphaeroides ATCC 17023T maintained high viabilities (Fig. 2a). In contrast, the sucrose stress markedly decreased the viabilities of Rsp. rubrum S1T and Rvi. gelatinosus IL144. Resistance to the sucrose stress was similar to survivability in the dark among the four species (Figs. 1, 2a and S2). Tolerance to osmotic stress may be achieved by membrane translocators and the composition of membrane fatty acids (1, 15–17). Although it is unlikely given the carbon-starvation conditions used in this study, it may be possible that an osmolyte such as trehalose accumulated in cells (15, 17).
Fig. 2.
Effects of osmotic stress on the viability of starved cells. Starved cells were suspended in a buffer solution containing (per liter) 0.38 g KH2PO4, 0.39 g K2HPO4, 1 mL of a vitamin mixture (5), and 5 mL of a basal salt solution (5), and either 2.0 M sucrose (A) or 2.5 M NaCl (B). CFUs were counted after incubation at 30°C for 10 min in the dark. The results are expressed as a percentage of the CFUs determined after incubation for 10 min without exposure to stress.
Osmotic stress with NaCl did not largely affect the viability of Rps. palustris ATCC BAA-98, whereas the other test organisms were susceptible to this stress (Fig. 2b). It is noteworthy that 15% of the genome in Rps. palustris ATCC BAA-98 is relevant to transport including sodium efflux systems (9). This may partially explain Rps. palustris being detected in a wide variety of environments such as paddy soil, freshwater marsh sediments, and aquatic sediments (3, 6, 12).
In summary, all four purple phototrophic bacteria used in this study exhibited long-term starvation-survival in the light. This result indicates that they effectively survive in natural environments in which light energy is available even if organic carbon sources are depleted. A physiological characterization of the non-growing cells of phototrophic bacteria under starved conditions will clarify how ATP is utilized to maintain viability.
Supplementary Information
Acknowledgements
We are grateful to R. Craig Everroad for critically reading this manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to SH (24117519) and Japan Society for the Promotion of Science (JSPS) to NK.
References
- 1.Alvarez-Ordonez A, Fernandez A, Lopez M, Arenas R, Bernardo A. Modifications in membrane fatty acid composition of Salmonella Typhimurium in response to growth conditions and their effect on heat resistance. Int J Food Microbiol. 2008;123:212–219. doi: 10.1016/j.ijfoodmicro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Breznak JA, Potrikus CJ, Pfennig N, Ensign JC. Viability and endogenous substrates used during starvation survival of Rhodospirillum rubrum. J Bacteriol. 1978;134:381–388. doi: 10.1128/jb.134.2.381-388.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Lin X, Yu Y, Zhu J. Elevated ground-level O3changes the diversity of anoxygenic purple phototrophic bacteria in paddy field. Microb Ecol. 2011;62:789–799. doi: 10.1007/s00248-011-9895-7. [DOI] [PubMed] [Google Scholar]
- 4.Fleischman DE, Evans WR, Miller IM. Bacteriochlorophyll-containing Rhizobium species. In: Blankenship RE, Madigan MT, Bauer CE, editors. Anoxygenic Photosynthetic Bacteria Advances in Photosynthesis. Vol. 2. Kluwer Academic Publishers; Dordrecht: 1995. pp. 123–136. [Google Scholar]
- 5.Hanada S, Hiraishi A, Shimada K, Matsuura K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int J Syst Bacteriol. 1995;45:676–681. doi: 10.1099/00207713-45-4-676. [DOI] [PubMed] [Google Scholar]
- 6.Harada N, Otsuka S, Nishiyama M, Matsumoto S. Characteristics of phototrophic purple bacteria isolated from a Japanese paddy soil. Soil Sci Plant Nutr. 2003;49:521–526. [Google Scholar]
- 7.Hiraishi A, Matsuzawa Y, Kanbe T, Wakao N. Acidisphaera rubrifaciens gen. nov., sp. nov., an aerobic bacteriochlorophyll-containing bacterium isolated from acidic environments. Int J Syst Evol Microbiol. 2000;50:1539–1546. doi: 10.1099/00207713-50-4-1539. [DOI] [PubMed] [Google Scholar]
- 8.Imhoff JF. Genus I, Rhospirillum Pfennig, and Trüper 1971, 17AL. In: Boone DR, Krieg NR, Staley JT, Garity GM, editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Vol. 2. Springer; New York: 2005. pp. 1–6. [Google Scholar]
- 9.Larimer FW, Chain P, Hauser L, et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat Biotechnol. 2004;22:55–61. doi: 10.1038/nbt923. [DOI] [PubMed] [Google Scholar]
- 10.Madigan MT, Jung DO. An overview of purple bacteria: systematics, physiology, and habitats. In: Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. The Purple Phototrophic Bacteria Advances in Photosynthesis and Respiration. Vol. 28. Springer; New York: 2009. pp. 1–15. [Google Scholar]
- 11.Oda Y, Slagman S-J, Meijer WG, Forney LJ, Gottschal JC. Influence of growth rate and starvation on fluorescent in situ hybridization of Rhodopseudomonas palustris. FEMS Microbiol Ecol. 2000;32:205–213. doi: 10.1111/j.1574-6941.2000.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 12.Oda Y, Wanders W, Huisman LA, Meijer WG, Gottschal JC, Forney LJ. Genotypic and phenotypic diversity within species of purple nonsulfur bacteria isolated from aquatic sediments. Appl Environ Microbiol. 2002;68:3467–3477. doi: 10.1128/AEM.68.7.3467-3477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiba T. Utilization of light energy by the strictly aerobic bacterium Erythrobacter sp. OCh 114. J Gen Appl Microbiol. 1984;30:239–244. [Google Scholar]
- 14.Suyama T, Shigematsu T, Suzuki T, Tokiwa Y, Kanagawa T, Nagashima KVP, Hanada S. Photosynthetic apparatus in Roseateles depolymerans 61A is transcriptionally induced by carbon limitation. Appl Environ Microbiol. 2002;68:1665–1673. doi: 10.1128/AEM.68.4.1665-1673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuzuki M, Moskvin OV, Kuribayashi M, Sato K, Retamal S, Abo M, Zeilstra-Ryalls J, Gomelsky M. Salt stress-induced changes in the transcriptome, compatible solutes, and membrane lipids in the facultatively phototrophic bacterium Rhodobacter sphaeroides. Appl Environ Microbiol. 2011;77:7551–7559. doi: 10.1128/AEM.05463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tymczyszyn EE, Gómez-Zavaglia A, Disalvo EA. Influence of the growth at high osmolality on the lipid composition, water permeability and osmotic pressure of Lactobacillus bulgaricus. Arch Biochem Biophys. 2005;443:66–73. doi: 10.1016/j.abb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Wood JM. Osmotic stress. In: Storz G, Hengge R, editors. Bacterial Stress Responses. 2nd ed. American Society for Microbiology Press; Washington, DC: 2011. pp. 133–156. [Google Scholar]
- 18.Zong R, Jiao N. Proteomic responses of Roseobacter litoralis OCh149 to starvation and light regimen. Microbes Environ. 2012;27:430–442. doi: 10.1264/jsme2.ME12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.