Summary
Amphetamines modify the brain and alter behavior through mechanisms generally attributed to their ability to regulate extracellular dopamine concentrations. However, the actions of amphetamine are also linked to adaptations in glutamatergic signaling. We report here that when amphetamine enters dopamine neurons through the dopamine transporter, it stimulates endocytosis of an excitatory amino acid transporter, EAAT3, in dopamine neurons in vitro and in vivo. Consistent with this decrease in surface EAAT3, amphetamine potentiates excitatory synaptic responses in dopamine neurons. We also show that the process of internalization is dynamin- and Rho-mediated and requires a unique sequence in the cytosolic C-terminus of EAAT3. Introduction of a peptide based on this motif into dopamine neurons blocks the effects of amphetamine on EAAT3 internalization and its action on excitatory responses. These data indicate that the internalization of EAAT3 triggered by amphetamine increases glutamatergic signaling and thus contributes to the effects of amphetamine on neurotransmission.
Introduction
Psychostimulant drugs such as amphetamine (AMPH) and cocaine are thought to produce their acute behavioral effects and to activate neuronal pathways that contribute to addiction predominantly through their ability to increase extracellular dopamine (DA, Hyman et al., 2006). Although DA itself is clearly important for these actions, recent work underscores the importance of changes in glutamatergic signaling after AMPH treatment as well (Reissner and Kalivas, 2010; Wolf and Ferrario, 2010). For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. In vivo microdialysis studies indicate that the increases in extracellular glutamate are calcium independent (Del Arco et al., 1999; Del Arco et al., 1998; Xue et al., 1996) suggesting that synaptic release does not fully account for the observed increases in extracellular glutamate concentrations. A reduction in glutamate clearance through the changes in glutamate transport activity provides a potential mechanism to explain these calcium-independent increases in extracellular glutamate after AMPH treatment.
Sodium-dependent glutamate transporters are the primary means for clearing synaptic and extra-synaptically released glutamate in the central nervous system. There are five distinct isoforms of the excitatory amino acid transporters (EAATs) responsible for maintaining extracellular glutamate at concentrations that determine the temporal and spatial precision of glutamatergic neurotransmission and limit the excitotoxic actions of glutamate. In general, EAATs 1 and 2 are found predominantly in astrocytes, EAAT3 in neurons, EAAT4 in Purkinje cells, and EAAT5 expression is restricted to the retina (Danbolt, 2001). We hypothesized that modulation of a glutamate transporter, particularly the neuronal EAAT3 subtype present on the soma and processes of DA neurons, contributes to the increase in extracellular glutamate caused by AMPH.
We report here that AMPH influx through the DA transporter (DAT) induces endocytosis of EAAT3, the glutamate transporter subtype in DA neurons. We found that this internalization is dependent on dynamin and AMPH-mediated activation of a Rho-GTPase. Further, a unique sequence in the C-terminus of EAAT3 confers sensitivity to AMPH and the introduction of a peptide comprised of this domain can prevent internalization of EAAT3, most likely by binding competitively to a regulatory complex required for AMPH-mediated endocytosis. Trafficking of EAAT3 from the cell surface after AMPH treatment potentiates glutamatergic synaptic transmission by reducing glutamate clearance, demonstrating a new mechanism through which AMPH can regulate the actions of glutamate in the midbrain.
Results
AMPH decreases EAAT3 glutamate uptake in midbrain DA neurons
To examine the effects of AMPH on EAATs in midbrain neurons, we examined glutamate transport activity in primary midbrain cultures. AMPH (10μM) pretreatment for 30 minutes decreased total 3H-glutamate uptake in these cultures by 23±9%. There are several routes for glutamate entry into cells in these cultures including Na+-dependent EAAT-mediated and Na+-independent pathways. The Na+-independent component was assessed in sodium-free (choline-substituted) buffer and was not altered by AMPH pretreatment. EAAT1 was not detected in cultured mouse midbrain neurons when assessed by Western blot (Figure S1A). Moreover, the EAAT1 specific inhibitor 2-Amino-5,6,7,8-tetrahydro-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-4H-chromene-3-carbonitrile, UCPH-101 (Jensen et al., 2009), that selectively blocks EAAT1 uptake in HEK293 cells (Figure S1B), had no-effect on Na+-dependent glutamate uptake in the midbrain cultures indicating that EAAT1 does not contribute to glutamate transport in these cultures (Figure S1C). The contribution of EAAT2 to glutamate uptake was determined using the EAAT2-selective inhibitor di-hydrokainate (DHK, 100μM). DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH (Figure 1A). EAAT3 expression in these cultures was detected in DAT(+) and tyrosine hydroxylase(+) cells suggesting that DA neurons could be the site for AMPH-sensitive EAAT3 modulation (Figure 1B).
Figure 1. AMPH stimulates internalization of the glutamate transporter EAAT3.

A. Glutamate uptake in primary midbrain cultures after AMPH treatment EAAT2-mediated 3H-glutamate transport (DHK-sensitive, Na+-dependent uptake) was not significantly altered by AMPH pretreatment (112.2±15% of vehicle treated control). The EAAT3-mediated 3H-glutamate transport (DHK-insensitive, Na+-dependent uptake) in these cultures is mediated by EAAT3 and this component of the glutamate transport was significantly attenuated by AMPH treatment (38.4±16%). B. Characterization of DA neurons in culture Neurons in primary midbrain cultures that express tyrosine hydroxylase (TH, green) also express DAT and EAAT3 (red). All DAT(+) cells were also EAAT3(+). C.Glutamate uptake in HEK cells transiently transfected with EAATs1, 2 and 3 HEK cells transiently expressing DAT with either EAAT 1, 2 or 3 were treated with AMPH, 10μM, or vehicle for thirty minutes and then 3H-glutamate transport was measured. Only transport mediated by EAAT3 exhibited sensitivity to AMPH. (*p<0.05 by 2-way ANOVA). D. TIRF quantification of EAAT3 internalization EAAT2 or EAAT3 cells treated with vehicle or AMPH were monitored by TIRF. The vehicle treated traces indicate that both carriers are relatively stable over this time-course in these cultures. EAAT3 membrane localization decreased under AMPH conditions while the EAAT2 protein remained stable. (See also Figure S1.)
Effects of AMPH were also determined for HEK293 cells selectively co-expressing EAATs 1, 2 or 3 with DAT. Pretreatment of the cells with AMPH (10μM) for 30 minutes resulted in a decrease in the transport capacity of EAAT3, whereas EAATs 1 and 2 were unaffected (Figure 1C). The Vmax of EAAT3 was diminished by 64% but there was no significant change in the Km for glutamate (Km vehicle=57.9± 25μM versus AMPH treated = 33.9 ±33μM). These data suggest that trafficking of the carrier out of the cell membrane causes the AMPH-mediated decrease in EAAT3 activity.
Previous work has shown that amphetamines, unlike cocaine and other non-transported blockers, have the ability to stimulate internalization of DAT from the cell surface (Luscher and Malenka, 2011; Saunders et al., 2000). We addressed the possibility that AMPH also stimulated internalization of EAAT3 using total internal reflection fluorescence (TIRF) microscopy to monitor trafficking of a tagged eGFP-EAAT3 in HEK293 cells. We observed a loss of eGFP-EAAT3 fluorescence at the membrane after AMPH application, but no change in fluorescence in parallel experiments in eGFP-EAAT2-expressing cells (Figure 1D). These data suggest that internalization of cell surface EAAT3 may contribute to the rise in extracellular glutamate observed following AMPH administration.
AMPH induces trafficking of endogenous EAAT3 in midbrain DA neurons
The effects of AMPH on endogenous EAAT3 carriers were examined in ventral tegmental area (VTA) DA neurons in adult midbrain slices. VTA DA neurons could be readily identified by robust DAT immunolabeling and all DAT(+) cells were colabeled with EAAT3 (Figure 2A). Similar colocalization was observed in the SN (Figure S2A). Additional EAAT3(+)/TH(-) cells were also evident. Processes projecting away from the VTA to the cortex and nucleus accumbens were also DAT(+) and EAAT3(+) suggesting that the two carriers colocalize in neuronal soma and processes. To assess internalization of endogenous EAAT3, brain slices were exposed to vehicle or AMPH for 30 minutes at 37° in ACSF. Surface proteins were biotinylated with a cell-impermeable biotin reagent, isolated with NeutrAvidin beads, separated on an SDS gel and probed with an antibody to EAAT3. AMPH pretreatment significantly decreased biotinylated EAAT3 in lysates of acute slices (Figures 2B and 2C), consistent with a reduction in EAAT3 at the cell surface. We found these results to be consistent across midbrain tissues from both male and female animals (Figure S2B). The DAT antagonist, cocaine, prevented the effects of AMPH on both EAAT3 and DAT, whereas cocaine alone had no effect on membrane localization of either protein. AMPH pretreatment decreased biotinylated EAAT3 and DAT, but it did not affect astrocytic EAAT2 or the glutamate receptor subunits NR1, GluR1 and GluR4 (Figure 2D). We also found that peripheral injection of AMPH led to a significant loss of biotin-accessible EAAT3 in midbrain tissue (Figures S2C), thus establishing that these events occur in vivo. We also assessed EAAT3 membrane localization in slices rostral to the midbrain and found that in this area, which is devoid of DAT(+) cell bodies but contains DAT(+) / EAAT3(+) processes (Figure 2A), AMPH still reduces surface EAAT3 (Figure S2C). Taken together these data suggest that AMPH stimulates internalization of EAAT3 in brain tissues.
Figure 2. Endogenous EAAT3 in DA neurons in brain slices also internalize in response to AMPH.
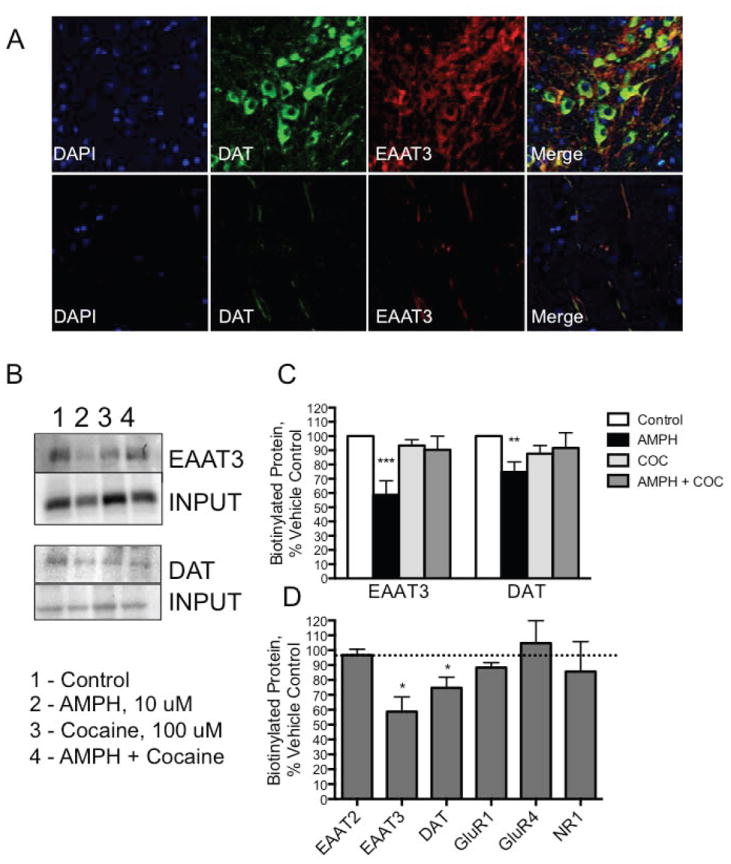
A. DA neurons in midbrain slices express EAAT3 Midbrain sections were immunolabeled for DAT (green) and EAAT3. Soma of DAT(+) neurons in the midbrain were also EAAT3 (top panel). Further out at the processes, these two transporters are still found in the same cells (bottom panel). B. Biotinylated EAAT3 from acute midbrain slices decreases in response to AMPH Acute midbrain slices from adult mice were exposed to AMPH, cocaine, or AMPH and cocaine together. All membrane proteins were biotinylated and analyzed by SDS-Page gel. Probing for EAAT3 indicates a loss of membrane accessible protein in response to AMPH that is prevented by cocaine. DAT protein demonstrates the same sensitivity. C. Quantification of changes in membrane expression of DAT and EAAT3 Densitometry of EAAT3 and DAT shows significant internalization of both carriers that is blocked by cocaine. (*p<0.05 and ***p<0.001 by 2-way ANOVA). D. Quantification of changes in membrane expression of several membrane proteins in response to AMPH Glutamate receptor subunits GluR1, GluR4 and NR1 remain at the cell membrane following exposure to AMPH. The glutamate transporter EAAT2 is also not mobilized by AMPH. EAAT3 and DAT data are the same samples shown in 2C (*p<0.05 by Paired t-tests for each protein; see also Figure S2).
AMPH-mediated internalization of EAAT3 depends on DAT
For AMPH-mediated internalization of the DAT, AMPH must be present within the cell (Kahlig et al., 2006). In acute brain slices we observed that cocaine blocks AMPH-mediated internalization demonstrating a role for DAT in EAAT3 internalization (Figure 2C). To directly test whether the DAT was required to initiate the AMPH-mediated events that modulate EAAT3 activity, HEK293 cells were transfected with EAAT3 and DAT or with EAAT3 alone. In these cells AMPH dose-dependently decreased glutamate transport in cells expressing both DAT and EAAT3 (Figure 3A). However, concentrations of AMPH up to 100μM had no significant effect on transport in the cells expressing EAAT3 alone. The decrease in EAAT3-mediated 3H-glutamate transport in EAAT3/DAT-expressing cells could be prevented by co-application of the DAT inhibitor cocaine with the AMPH (Figure 3B). Cocaine alone had no significant effect on EAAT3 transporters in these cells. These results indicate that endocytosis of EAAT3 depends on the transport of AMPH into the cell by the DAT.
Figure 3. EAAT3 sensitivity to AMPH depends on DAT co-expression.
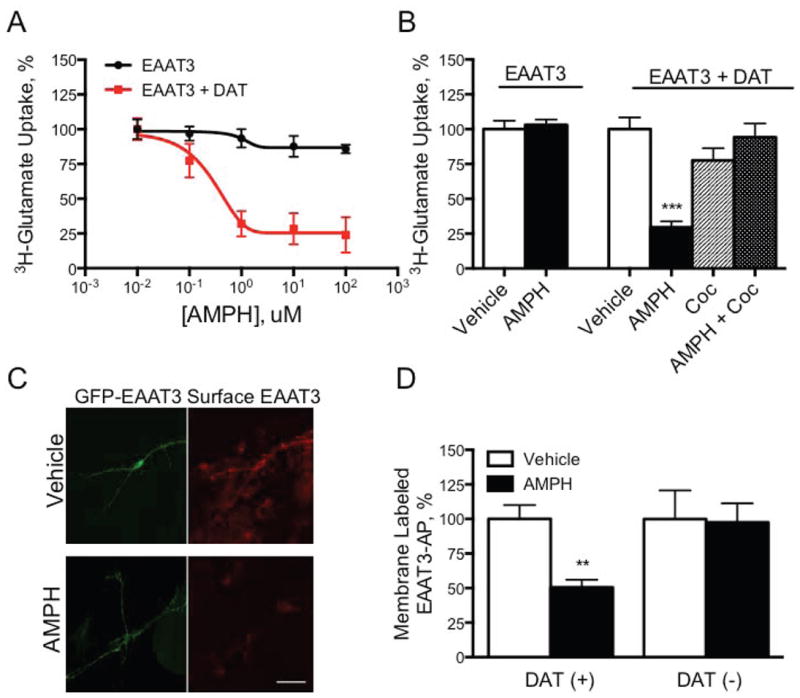
A. Glutamate transport in HEK293 cells expressing EAAT3 or EAAT3 and DAT HEK293 cells were transiently transfected with either EAAT3 or EAAT3 and DAT and exposed to increasing concentrations of AMPH. 3H-glutamate uptake was then measured. Glutamate transport in cells expressing only EAAT3 did not respond to AMPH pretreatment while those expressing both DAT and EAAT3 exhibited a decrease in transport capacity. B. Pharmacology of AMPH-mediated decrease in EAAT3 activity HEK293 cells expressing only EAAT3 were unaffected by AMPH but glutamate transport was greatly decreased in cells expressing both EAAT3 and DAT. Cocaine, 100 μM, co-application was sufficient to block AMPH-mediated effects on these cells but had no effect on its own. (***p<0.001 by 1-way ANOVA). C.Membrane labeling of eGFP-EAAT3-AP in primary neurons Primary neurons were transiently transfected with eGFP-EAAT3-AP constructs, treated with vehicle or AMPH for thirty minutes, and then labeled for surface localized transporter. Total protein (green, GFP) did not differ from vehicle treated control, however the proportion of carrier that could be labeled at the membrane (red, Streptavidin-Alexa Fluor 568) greatly decreased in response to AMPH. D. Quantification of membrane labeled GFP-EAAT3-AP in DAT(+) and DAT(-) neurons Membrane and total (green) expression in both DAT(+) and DAT(-) primary neurons in culture were quantized by photon counting. Data are expressed as a ratio of membrane fraction to total. (**p<0.01 by one-way ANOVA. Scale bars are 50μm.)
To further examine the role of DAT in the action of AMPH on EAAT3, we engineered a functional EAAT3 chimera with a biotin acceptor peptide (AP, GLNDIFEAQKIEWHEAR) inserted in extracellular loop 2 of EAAT2. The AP peptide sequence is recognized and biotinylated specifically by the E. coli enzyme BirA (Howarth and Ting, 2008). We designed a dual expression vector with the eGFP-EAAT3-AP chimera at one site and the BirA enzyme tagged with an ER-retention signal (KDEL) in a second site, thus enabling the co-expression of BirA enzyme and subsequent biotinylation of the eGFP-EAAT3-AP protein as it transits through the ER. Addition of cell-impermeable streptavidin-Alexa-568 to HEK293 cells co-expressing eGFP-EAAT3-AP and KDEL-BirA, efficiently labeled the eGFP-EAAT3-AP protein at the cell surface with only a modest decrease in 3H-glutamate uptake (<15%). AMPH treatment of HEK293 cells transiently expressing eGFP-EAAT3-AP and DAT significantly decreased the Streptavidin-Alexa 568 labeled surface eGFP-EAAT3-AP by 49 ± 6%, similar to results observed after biotinylation of AMPH-treated brain slices (Figure 2B).
Because EAAT3 is broadly expressed in neurons and is not specific to DA neurons, we used the eGFP-EAAT3-AP labeling assay to assess membrane localization of EAAT3 in DAT(+) and DAT(-) neurons in culture. In DAT(+) primary neurons expressing eGFP-EAAT3-AP, extracellular labeling of the glutamate transporter was significantly attenuated (50.6±5%) in AMPH treated cultures compared to those treated with vehicle alone (Figures 3C and D). In contrast, DAT(-) displayed no response to AMPH. These data indicate that the actions of AMPH on EAAT3 in neurons depend on DAT, similar to observations in HEK293 cells.
The mechanism of AMPH mediated EAAT3 internalization
To confirm that the observed changes in cellular distribution of EAAT3 were due to an increase in the rate of internalization of eGFP-hEAAT3-AP and not to a decrease in rate of insertion at the membrane, we used a pH-sensitive dye, CypHer5, coupled to streptavidin to monitor internalization. CypHer5 fluoresces maximally at infrared wavelengths under acidic conditions and is ideally suited for monitoring surface protein internalization into acidic endosomal compartments (Adie et al., 2003). CypHer5 streptavidin binds to the biotin-tagged eGFP-hEAAT3-AP at the surface, and fluoresces only after it is internalized and enters an acidic compartment. Using this method to monitor internalization we observed that AMPH application resulted in a significant increase in intracellular CypHer5 labeling within 10 minutes when compared to vehicle-treated controls (Figures 4A and B). These data indicate that biotinylated eGFP-hEAAT3-AP labeled at the cell surface with the CypHer5-Streptavidin internalized and appears in cytosolic compartments in response to AMPH. Similar results were observed with AP-tagged DAT following exposure to AMPH (Figures S3A and S3B).
Figure 4. Mechanism of AMPH-Mediated EAAT3 internalization.

A. AMPH stimulated movement of CypHer5-labled EAAT3 protein into intracellular vesicles HEK293 were transiently transfected with DAT and eGFP-EAAT3-AP. The uniquely biotinylated eGFP-EAAT3-AP was labeled with streptavidin conjugated to the pH-sensitive dye, CypHer5. Over 10 minutes under vehicle conditions, cells did not exhibit robust CypHer5 labeling. However, 10 minutes of AMPH, 10 μM, lead to an increase in CypHer5 fluorescence. B. Quantification of CypHer-5 labeling at 10 minutes following vehicle or AMPH treatment (*p<0.05 by Paired t-test.) C. AMPH-Induced EAAT3 internalization is dynamin-mediated 3H-glutamate uptake in HEK293 cells transiently transfected with DAT and EAAT3 and pretreated with AMPH were unaffected by pretreatment with brefeldin A. However the dynamin inhibitor Dynasore prevented AMPH-mediated internalization of EAAT3. D. EAAT3 internalization by AMPH is RhoA mediated Co-transfection of EAAT3 and DAT with wildtype RhoA did not alter EAAT3 sensitivity to AMPH. However, coexpression of the dominant negative T17N attenuated EAAT3 AMPH-mediated endocytosis. The constitutively active mutant, V14, also exhibited a diminished effect of AMPH pretreatment on 3H-glutamate uptake, however the baseline transporter capacity of these cells was significantly diminished initially under vehicle control conditions. E. Coexpression of the exotoxin, C3, also blocked AMPH sensitivity in these cells. (*p<0.05, ***p<0.001 and **** p<0.0001 by one-way ANOVA; see also Figure S3).
We next sought to define the endocytic mechanism for EAAT3 internalization. Dynamin is a GTPase that plays a prominent role in multiple types of endocytosis (see Mayor and Pagano, 2007 for review). Dynasore, a cell-permeable inhibitor of dynamin reduced the AMPH-mediated decrease in glutamate uptake in HEK293 cells transfected with both EAAT3 and DAT demonstrating that the process is dynamin-dependent (Figure 4C).
Several dynamin-dependent endocytic pathways can mediate the internalization of plasma membrane proteins; one pathway, which is clathrin-independent, depends on the activation of the small GTPase, RhoA. To address whether RhoA activation was required we examined the effects of inhibiting or enhancing RhoA activity on AMPH-induced EAAT3 internalization. In HEK293 cells co-expressing EAAT3 and DAT, AMPH-mediated EAAT3 internalization was inhibited by expression of either the clostridial exotoxin C3, a selective Rho inhibitor (Figure 4D) or the dominant negative mutant, RhoA-T17N (Figure 4E). In contrast, as shown in Figure S3C, a dominant negative version of Rac1, another small GTPase, does not block the AMPH-induced endocytosis of EAAT3. Cells expressing a constitutively active mutant, RhoA-V14 (Figure 4E) had no response to AMPH, but the baseline 3H-glutamate uptake was greatly decreased (43±5.05%) indicating that constitutive activation of RhoA alone decreases EAAT3 surface expression and precludes further downregulation by AMPH. Taken together the results of these studies indicate that AMPH-mediated internalization of EAAT3 proceeds through a RhoA-dependent, dynamin-dependent mechanism.
A unique sequence in the C-terminus of EAAT3 is important for sensitivity to AMPH
AMPH appears to act selectively on trafficking of EAAT3, but not EAAT1 or EAAT2, suggesting the presence of a distinct motif in EAAT3 that confers sensitivity to AMPH. We initially focused on the cytoplasmic C-terminus of EAAT3 to identify a potential signal sequence for AMPH sensitivity of EAAT3. Because EAAT2 localization and transport is insensitive to AMPH, we engineered chimeras in which varying lengths of the C-terminus of EAAT3 were added to the full length EAAT2 protein (Cheng et al., 2002) and assessed the ability of these constructs to transport glutamate. Chimeras that included the entire cytoplasmic C-terminus of EAAT3, E2+E3 (469-525) or a shorter segment of the C-terminus, E2+E3 (498-525) displayed a decrease in glutamate transport after AMPH treatment (Table 1). However, a construct with a smaller segment of the C-terminus, E2+E3 (511-525) was not sensitive to AMPH. All three chimeras displayed baseline transport of 3H-glutamate comparable to that of the parent EAAT2 (Table S1) and showed normal surface expression as assessed by confocal microscopy, indicating that the results are not an artifact of variations in expression. These data suggest that a unique sequence between residues 498 and 511 of EAAT3 confers its sensitivity to AMPH.
Table 1. Mutational analysis of EAAT3 carboxy-terminus and sensitivity to AMPH pretreatment (See also Table S1).
Alanine scanning of C-terminus of EAAT3 for sensitivity to AMPH
| Construct | Sequence | Glu uptake after AMPH treatment, % (SEM) | |||
|---|---|---|---|---|---|
| EAAT3 | … | DTKKSYVNGGFAVDKSDTISFTQTSQF | 52.80% | (8.42%) ** | |
| EAAT2 | … | DECKVTLAANGKSADCSVEEEPWKREK | 94.17% | (7.50%) | |
| E2+E3 (469-525) | EAAT2….EK | AGSA | ELEQMDVSSEV…GGFAVDKSDTISFTQTSQF | 49.46% | (10.74%) **** |
| E2+E3 (498-525) | EAAT2….EK | AGSA | DTKKSYVNGGFAVDKSDTISFTQTSQF | 49.44% | (12.07%) *** |
| E2+E3 (511-525) | EAAT2….EK | AGSA | VDKSDTISFTQTSQF | 93.91% | (7.80%) |
| E2+E3 (Y503A) | EAAT2….EK | AGSA | DTKKSAVNGGFAVDKSDTISFTQTSQF | 57.85% | (3.95%) ** |
| E2+E3 (504-5 AA) | EAAT2….EK | AGSA | DTKKSYAAGGFAVDKSDTISFTQTSQF | 81.21% | (4.12%) |
| E2+E3 (506-7 AA) | EAAT2….EK | AGSA | DTKKSYVNAAFAVDKSDTISFTQTSQF | 94.93% | (2.65%) |
| E2+E3 (508-9 AA) | EAAT2….EK | AGSA | DTKKSYVNGGAAVDKSDTISFTQTSQF | 101.02% | (8.37%) |
| E2+E3 (A509S) | EAAT2….EK | AGSA | DTKKSYVNGGFSVDKSDTISFTQTSQF | 42.83% | (3.18%) **** |
| E2+E3(304-8A5) | EAAT2….EK | AGSA | DTKKSYAAAAAAVDKSDTISFTQTSQF | 91.70% | (3.16%) |
| EAAT3 (504-8 A5) | EAAT3 | … | DTKKSYAAAAAAVDKSDTISFTQTSQF | 96.32% | (7.06%) |
indicates p<0.01 by one-way ANOVA compared to vehicle treated control
indicates p<0.001 by one-way ANOVA compared to vehicle treated control
indicates p<0.001 by one-way ANOVA compared to vehicle treated control
To further resolve the motif required for the actions of AMPH on EAAT3, we examined the effect of a series of pairs of alanine substitutions (504-505, 506-507, and 508-509) on internalization (Table 1). Each pair of substitutions attenuated the sensitivity of the E2+E3 (498-525) chimera to AMPH suggesting a role for this entire domain in EAAT3 sensitivity to AMPH. An E2+E3 chimera containing a single amino acid substitution mutant E2+E3 (A509S) retained sensitivity to AMPH and thus further delineated the motif to the sequence VNGGF. A tyrosine based motif (YVN) at 503-506 in EAAT3 has been implicated in constitutive, clathrin-mediated endocytosis of the carrier (D’Amico et al., 2010). We examined the Y503A mutation in the E2+E3 chimera and found that it still internalized in response to AMPH suggesting that constitutive clathrin-dependent cycling is not directly involved in AMPH-mediated EAAT3 internalization. Alanine substitution of residues 504-508 in the wild type EAAT3 (E3 (504-508 A5) eliminated the AMPH response (Table 1), further resolving the motif to the region comprised of the sequence VNGGF.
A cell permeable peptide including the C-terminal motif occludes AMPH-induced trafficking of EAAT3
To further examine the role of the VNGGF peptide in AMPH-mediated internalization of EAAT3, we designed a TAT fusion peptide containing this sequence (Figure 5A). The TAT domain facilitates entry of peptides into cells and we hypothesized that the introduction of an excess of the TAT-EAAT3 fusion peptide could compete with the endogenous EAAT3 motif and interfere with the formation of AMPH-induced regulatory complexes. HEK293 cells were transiently transfected with EAAT3 and DAT and treated with either the TAT-EAAT3 (504-509, VNGGFA) sequence or a TAT-peptide with a scrambled version of the sequence (TAT-scrambled, GVNAGF) for 30 minutes and then treated with AMPH or vehicle for thirty minutes. Addition of the TAT-EAAT3 peptide had no effect on baseline 3H-glutamate uptake, but blocked the ability of AMPH to decrease transport activity (Figure 5B). The scrambled TAT peptide had no effect on glutamate uptake or on the AMPH induced decrease in transport.
Figure 5. Introduction of the EAAT3 C-terminal motif disrupts the effect of AMPH on EAAT3.
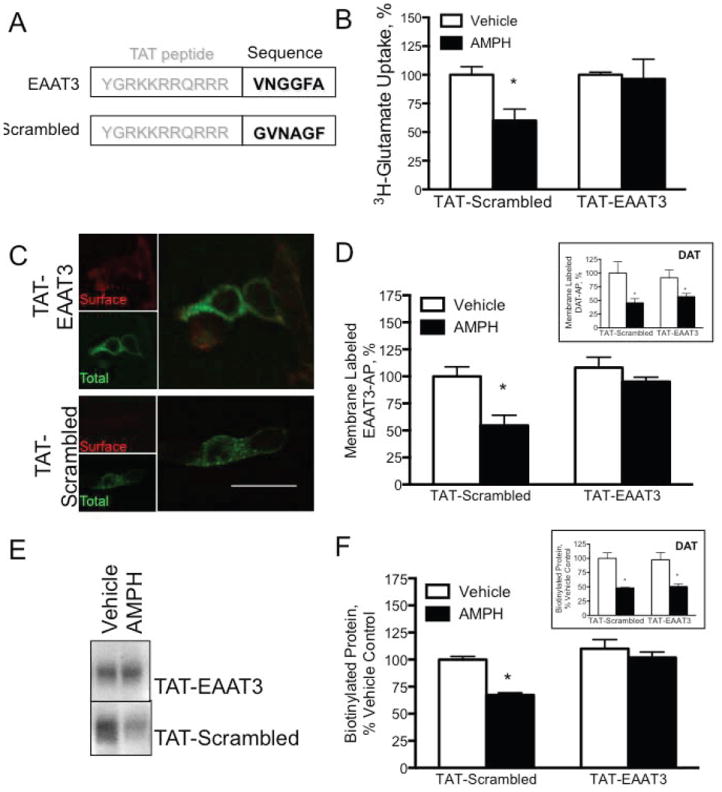
A. Schematic of TAT-peptide constructs B. Responses to AMPH in HEK293 cells exposed to TAT-EAAT3 HEK293 cells transfected with DAT and EAAT3 were assessed for EAAT3 activity by 3H-glutamate uptake. Cells treated with TAT-scrambled were still sensitive to AMPH, but TAT-EAAT3 abolished the AMPH-mediated decrease in carrier activity. C and D. EAAT3 internalization is blocked by TAT-EAAT3 in HEK293 cells C. HEK293 cells were transiently transfected with DAT and eGFP-EAAT3-AP, treated with TAT-EAAT3 (top panel) or TAT-scrambled (bottom panel), and then AMPH. Total eGFP-EAAT3-AP expression was not altered (green), but the surface localized transporters labeled with streptavidin-Alexa568 were decreased only in cells exposed to the TAT-scrambled peptide. TAT-EAAT3 treated cells still had robust surface localized eGFP-EAAT3-AP. D. Quantification of vehicle treated TAT-EAAT3 or TAT-scrambled eGFP-EAAT3-AP membrane localization demonstrates no baseline effect on the transporter. The response to AMPH, however, is blocked in cells treated with TAT-EAAT3 only. Inset In parallel assays, DAT membrane expression is decreased in response to AMPH with both the scrambled as well as the EAAT3 TAT peptides. E and F. Biotinylation of endogenous EAAT3 in an acute brain slice Western blot of biotinylated EAAT3 in acute brain slices treated with TAT-E3 (top panel) or TAT-scrambled (bottom panel) demonstrate EAAT3 trafficking induced by AMPH in the TAT-scrambled treated slices only. TAT-EAAT3 abolished the AMPH-mediated internalization of EAAT3. F. Quantification of biotinylated EAAT3 in acute brain slices. Inset DAT membrane expression is decreased in response to AMPH under both conditions. (Scale bar represents 25μm for merged images, 50μm for single color images. * p<0.05 by one-way ANOVA.)
To confirm that the TAT-EAAT3 peptide was altering AMPH-induced trafficking of EAAT3, we co-transfected HEK293 cells with the eGFP-EAAT3-AP construct and DAT. Assays of EAAT3 surface expression using extracellular Streptavidin-Alexa568 labeling demonstrated that the TAT-EAAT3 peptide could prevent AMPH induced endocytosis (Figures 5C and D). The TAT-scrambled peptide had no effect on AMPH-mediated internalization of the eGFP-EAAT3-AP protein. Parallel assays performed with a DAT-AP construct revealed that AMPH-induced DAT internalization was not affected by co-application of either the TAT-EAAT3 or TAT-scrambled peptide (Figure 5D, inset).
The ability of the VNGGF motif to interfere with AMPH-mediated trafficking of the endogenous EAAT3 was also examined in acute midbrain slices from adult mice. Using cell surface biotinylation of slices to assess changes in EAAT3, we observed an AMPH-induced decrease in surface EAAT3 in tissue incubated with the TAT-scrambled peptide (comparable to the AMPH effect in slices shown in Figure 2C). In contrast, the TAT-EAAT3 peptide prevented AMPH induced internalization of EAAT3 (Figures 5E and F). These effects appeared to be selective for EAAT3 since AMPH-induced DAT internalization remained robust in these lysates (Figure 5F, inset). These data are similar to observations in transfected HEK293 cells with the TAT peptides where the EAAT3 specific peptide blocked EAAT3, but had no effects on the membrane localization or function of DAT.
EAAT3 internalization mediated by AMPH potentiates synaptic neurotransmission
Although endogenous EAAT3 appears to be internalized in response to AMPH, it was unclear to what extent the resulting changes in glutamate clearance would alter excitatory signaling. We assessed AMPH’s effects on evoked glutamatergic synaptic currents (eEPSCs) in DA neurons within the substantia nigra using whole-cell patch-clamp recordings. To resolve the selective effects of AMPH on excitatory signaling, experiments were done in the presence of DA receptor blockers (see Experimental Procedures). We found that AMPA receptor-mediated eEPSCs were potentiated to 134±6% (Figure 6A; n=5) and NMDA-mediated eEPSC amplitudes were increased by 150±10% following superfusion of AMPH (10μM; Figure 6B; n=6). DA neurons in the VTA also demonstrated an increase in glutamatergic synaptic response following AMPH superfusion (158±20%). Preincubation of the slices with the selective DAT inhibitor, GBR12909 (1μM) blocked the AMPH-induced increase in NMDA-mediated eEPSCs (Figure S4) suggesting that DAT is necessary for the AMPH effect on these synaptic currents.
Figure 6. AMPH potentiates glutamatergic synaptic transmission.

A. Averaged AMPA-mediated evoked excitatory postsynaptic currents (eEPSCs) are increased following AMPH (10μM) superfusion. B. Averaged NMDA-mediated eEPSCs are also potentiated by AMPH. C. Bar graphs with compiled data for AMPA and NMDA-mediated eEPSCs in control and in the presence of AMPH (10μM). D. The effect of AMPH on AMPA-mediated eIPSCs was abolished with the EAAT3 peptide in the intracellular solution compared to a scrambled peptide. E. Bar graph showing the AMPH-induced potentiation normalized to baseline AMPA-mediated eIPSCs (*p<0.05 by student’s t-test, see also Figure S4).
The EAAT3 peptide motif also prevents the potentiation of glutamatergic synaptic responses by AMPH
We next tested whether the selective EAAT3 inhibitor peptide could block the AMPH-mediated increase in synaptic responses (Figures 6D and E ). The scrambled peptide (GVNAGF) or the EAAT3 inhibitor peptide (EAAT3 (504-509, VNGGFA)) were added to the whole-cell pipette solution and allowed to perfuse into the neurons through the patch-clamp electrode for 15 minutes prior to eliciting stable baseline synaptic currents. AMPH produced an increase in the synaptic-mediated currents in the presence of the scrambled peptide (n=4) but this effect was blocked in cells pretreated with the EAAT3 inhibitor peptide (n=7). The EAAT3 interfering peptide also blocked glutamatergic synaptic potentiation in DA neurons in the VTA. These data directly implicate EAAT3 in the potentiation of glutamatergic signaling by AMPH in midbrain DA neurons.
Pharmacological inhibition of EAAT3 in DA neurons mimics the effects of AMPH
DL-threo-β-Benzyloxyaspartic acid (TBOA) is a non-transported inhibitor of EAATs that can block the carriers from the inside (Watzke and Grewer, 2001). We used this inhibitor or the vehicle (DMSO) in the intracellular pipette solution to determine if selective blockade of EAAT3 in the recorded neuron was sufficient to potentiate eEPSCs. Intracellular DMSO did not alter eEPSCs and superfusion of AMPH significantly increased eEPSCs from -168±14 to -307±15pA (n=8; Figures 7A and C), similar to previous observations (Figure 6). Administration of intracellular TBOA (200μM) during whole-cell recordings slowly increased basal eEPSCs from -165±12pA to -228±28pA and occluded the effect of AMPH (-224±42pA; n=10; Figures 7B and C). There is not a statistically significant difference between TBOA and AMPH treated eEPSCs. These data indicate that blockade of EAAT3 activity is sufficient to alter eEPSCs, similar to treatment with AMPH.
Figure 7. AMPH modulation of EAAT3 is involved in the potentiation of NMDA EPSCs by AMPH.

A. Amplitudes of NMDA-mediated EPSCs (every 30s) were measured over time after breaking into whole-cell mode (t=0 min) with pipette solutions containing vehicle (DMSO) control (n=8). AMPH was superfused during the time denoted by bar. B. Time course after breaking into whole-cell mode (t=0 min) with pipette solutions containing TBOA (200 μM; n = 10). C. Bar graph showing the changes in eEPSC amplitude with either vehicle (DMSO) or TBOA added to the intracellular recording solution (Two way Repeated Measures ANOVA, F(2,16) = 8.548, p<0.05). AMPH increased the amplitude of NMDA eEPSCs in cells recorded with vehicle but had no effect in cells recorded with intracellular TBOA. In those cells, TBOA alone significantly increased eEPSCs (*p<0.05).
Discussion
We report here that AMPH potentiates the action of glutamate on DA neurons through a mechanism involving RhoA-dependent endocytosis of the neuronal glutamate transporter, EAAT3. We demonstrate that this AMPH-induced internalization of EAAT3 is of sufficient magnitude to enhance glutamatergic responses in DA neurons. DA neurons receive glutamatergic inputs from several sources including the globus pallidus, subthalamic nucleus, striatum, amygdala, thalamus, hypothalamus, superior colliculus, reticular formation and the periaqueductal gray, indicating multiple distinct circuits that could be significantly affected by internalization of EAAT3 in DA neurons.
Although DA neurons are characterized by their ability to synthesize, package and release DA, several recent studies have shown that DA neurons also express the vesicular glutamate transporters, vGLUT2, which allow glutamate to be packaged in synaptic vesicles and released during neurotransmission (Hnasko et al., 2012; Kawano et al., 2006; Stuber et al., 2010; Trudeau, 2004). In fact, vGLUT2-mediated glutamate release from DA neurons has been found to be essential to AMPH-mediated behavioral responses (Birgner et al., 2009). Elevations in extracellular glutamate concentrations after AMPH treatment have been observed in the striatum (Del Arco et al., 1999; Mora and Porras, 1993), nucleus accumbens (Dalia et al., 1998; Xue et al., 1996) and prefrontal cortex (Del Arco et al., 1998) supporting a role for AMPH-mediated EAAT3 modulation at post-synaptic targets of DA and glutamate release from dopaminergic neurons. Indeed, our biotinylation data addressing EAAT3(+) processes in slices more rostral to the midbrain suggests that AMPH-mediated endocytosis also occurs in terminal fields of DA projections (Figure S2C).
Relationship between AMPH-mediated internalization of EAAT3 and the DAT
AMPH-mediated internalization of EAAT3 depends upon DAT co-expression. Primary midbrain neurons that did not express DAT showed no downregulation of EAAT3 after AMPH treatment, whereas DAT(+) neurons in the same culture displayed robust AMPH-mediated internalization of EAAT3. Although there could be other proteins expressed by DAT(+) neurons which contribute to AMPH-mediated trafficking changes in EAAT3, additional data underscore the importance of the DAT for this effect. For example, in transiently transfected HEK293 cells, which neither synthesize nor release DA, AMPH-induced EAAT3 internalization exhibits the same dependence upon DAT. Previous work has shown that AMPH also stimulates internalization of the DAT itself through a mechanism that is dependent upon AMPH transport through the carrier and into the cytosol (Kahlig et al., 2006). As was observed for AMPH-induced DAT trafficking, the effects of AMPH on EAAT3 internalization were blocked by cocaine, consistent with the idea that AMPH acts intracellularly after entry through DAT. Moreover, we also found that the DAT-specific antagonist GBR12909 blocked AMPH’s ability to potentiate synaptic responses. Notably, AMPH actions on EAAT3 do not depend on increases in DA or DA receptor activation, because they occur even in the absence of DA synthesis and in the presence of DA receptor blockers. Taken together these data indicate that AMPH’s actions within the cell not only enhance DA signaling by blocking DA uptake and increasing release, but also directly affect glutamatergic neurotransmission by prolonging the action of glutamate released from or onto DA neurons.
The dependence of EAAT3 internalization on the DAT also suggests that the two transporters might be internalized together. We found that EAAT3 and DAT are expressed in the same cells, as well as in axons and dendrites. However, the subcellular co-localization of the two neurotransmitter transporters remains to be established definitively by high resolution electron microscopy. One intriguing finding is that the C-terminal peptide motif in EAAT3 that interferes with AMPH-induced EAAT3 internalization did not affect DAT internalization (Figure 5). Moreover, efforts to co-immunoprecipitate EAAT3 and DAT under control or AMPH-stimulated conditions produced negative results and imply that they may not be physically linked. However, these results do not rule out the possibility that AMPH regulates the two carriers in a coordinated fashion. One intriguing possibility is that they may both be associated with the same AMPH-sensitive, macro-molecular regulatory machinery, but interact with this complex through different sites.
Endocytic mechanism involved in EAAT3 internalization by AMPH
We propose that internalization of EAAT3 by AMPH proceeds by a specialized clathrin-independent and dynamin-mediated internalization pathway that requires the activation of the small GTPase, RhoA (Figure 4). There are three sub-families of Rho proteins (Rho, Rac and CDC42), which all play a major role as regulators of actin cytoskeletal remodeling and this function of Rho family GTPases likely plays an important role during RhoA-mediated endocytosis. Internalization of the interleukin-2 receptor has been characterized as clathrin-independent, dynamin-dependent and mediated by RhoA activation (Lamaze et al., 2001) and a number of observations support the idea that AMPH-mediated effects on EAAT3 trafficking occur through similar RhoA-mediated internalization processes. Experiments using co-expression of a dominant negative RhoA construct or a Rho-inhibiting exotoxin C3 confirm the dependence on activated RhoA. In addition, as noted for other RhoA dependent endocytic processes, the effects of AMPH on EAAT3 surface expression are blocked by inhibition of dynamin activity.
The mechanism by which AMPH activates RhoA-dependent internalization remains unclear, although RhoA can be activated through several pathways, a few of which are particularly intriguing candidates for mediating the intracellular effects of AMPH. RhoA-mediated changes in dendritic spine morphology are initiated by an influx of calcium through NMDA receptors and subsequent activation of calmodulin/CamKII (Murakoshi et al., 2011). AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). G12/13 coupled G-protein coupled receptors (GPCRs) can also activate RhoA (Buhl et al., 1995) and AMPH may stimulate an intracellular GPCR found in DA neurons. The process of RhoA activation by AMPH that leads to EAAT3 internalization may parallel one or more of these previously described pathways.
A C-terminal peptide, VNGGF, interferes with AMPH-induced EAAT3 endocytosis
The identification of a small peptide segment, VNGGF, in the EAAT3 cytosolic C-terminus required for EAAT3 sensitivity to AMPH was the basis for generating a cell-permeant fusion protein that interferes with EAAT3 internalization. This provided a selective tool to explore the effects of AMPH on EAAT3 membrane localization and on excitatory neurotransmission in brain slices. We hypothesize that this sequence in the C-terminus of EAAT3 is either a direct recognition signal for AMPH-induced RhoA-dependent endocytosis or that it facilitates interaction with anchoring machinery that can be disrupted by the signaling mechanisms that are activated when AMPH enters the cell. Interestingly, this sequence overlaps a motif that was previously shown to be required for the polarized sorting of EAAT3 to the apical membrane of polarized MDCK cells and to dendrites of hippocampal neurons (Cheng et al., 2002). How, or if, polarization and AMPH sensitivity are related is unclear. We did not observe a clearly polarized localization of EAAT3 in midbrain TH(+) neurons suggesting distinct roles for this sequence in hippocampal neurons and DA neurons. The binding partners of this sequence, VNGGF, have not been identified and further investigation will reveal additional proteins essential for trafficking of EAAT3.
Modulation of glutamatergic synaptic responses
We observed potentiated AMPA- and NMDA-mediated evoked synaptic currents in the presence of AMPH. This could be due to either enhanced glutamate release or reduced glutamate uptake. Incorporation of the specific EAAT3 peptide that prevented EAAT3 internalization into the intracellular pipette solution blocked the AMPH-mediated potentiation of glutamatergic currents. These results indicate that modulatory actions of AMPH on AMPA and NMDA receptors are postsynaptic. Because both AMPA and NMDA receptor responses are potentiated, the simplest explanation would be that extracellular glutamate concentrations increase with AMPH. However, the relatively slow transport kinetics of EAAT3 suggest that EAAT3 has a modest, if any, direct role for EAAT3 in shaping acute synaptic responses (Diamond and Jahr, 1997). The perisynaptic localization of EAAT3 (Danbolt, 2001) may facilitate regulation of glutamate concentrations that activate extrasynaptic glutamate receptors indicating that AMPH-induced EAAT3 internalization could potentiate synaptic glutamatergic responses by regulating glutamate spillover. To date these studies examining the physiological influence of EAAT3 on glutamate signaling have emphasized major glutamatergic neuronal groups and pathways. Our work examines how postsynaptic modulation of EAAT3 activity might shape the signal from excitatory inputs onto DA neurons, which have highly unique structural and physiological properties.
Another potential mechanism by which AMPH-induced EAAT3 internalization could alter glutamatergic synaptic responses is through changes in trafficking and surface localization of glutamate receptors (Mao et al., 2011), similar to the adaptations in AMPA and NMDA receptor surface distribution seen with cocaine administration and withdrawal (Brown et al., 2011; Ghasemzadeh et al., 2009; Schumann and Yaka, 2009). We did not detect a change in membrane expression of AMPA subunits GluR1 or GluR2 or the NMDA subunit NR1 with biotinylation assays, consistent with prior reports (Nelson et al., 2009). However, the number of receptors that would be needed to potentiate synaptic responses may be below the level of detection of a biotinylation assay. Modulation of glutamate receptors may also be a function of lateral movement of receptors from perisynaptic sites into the synapse or functional modifications, such as phosphorylation. Nevertheless, the specificity of the interfering peptide for EAAT3 internalization and the ability of the blocking peptide to inhibit the AMPH-mediated increase in glutamatergic synaptic currents strongly implicates internalization of EAAT3 as paramount to any subsequent AMPH-evoked events in increasing synaptic activity.
AMPH-mediated internalization of EAAT3 may modulate DA neuron firing patterns
Several studies describe AMPH-mediated increases in DA neuron firing that are, similar to EAAT3 trafficking, dependent upon DAT expression. There are several mechanisms by which AMPH modulates phasic firing rates of DA neurons including activation of autoreceptors on DA neurons, activation of local interneurons with reciprocal connections onto DA neurons, as well as DAT-mediated conductances that are stimulated by AMPH (Branch and Beckstead, 2012; Ingram et al., 2002). Glutamatergic afferents are critical for the shift from tonic to phasic firing in DA neurons and, intriguingly, in vivo studies demonstrate that inactivation of the laterodorsal tegmentum, a glutamatergic afferent to VTA DA neurons, blocks burst firing (Lodge and Grace, 2006). We predict that AMPH-mediated EAAT3 internalization and glutamatergic synaptic potentiation would contribute to AMPH-induced modulation of phasic firing in DA neurons.
Glutamate plays a role in acute and addictive behaviors associated with AMPH
AMPH administration increases extracellular glutamate concentrations that are associated with the development of behavioral sensitization (Ferrario et al., 2011; Zhang et al., 2001). Both AMPA and NMDA receptor inhibitors have been shown to decrease AMPH-induced locomotor sensitization (Karler et al., 1989; Vezina and Queen, 2000; Wolf and Khansa, 1991) and self-administration (Karler et al., 1989; Suto et al., 2003; Vezina and Queen, 2000; Wolf and Khansa, 1991) suggesting that activation of glutamate receptors is necessary for the behavioral effects of AMPH. Modulation of glutamate neurotransmission has been clearly linked to the actions of AMPH, however these actions have previously been thought to be indirect through the actions of DA to shape activity within the limbic circuitry. Here we present evidence that AMPH can directly modulate not only dopaminergic, but also glutamatergic signaling. Beyond the more widely appreciated effects of AMPH to potentiate biogenic amine signaling, we show here a distinct action of AMPH to increase the internalization of EAAT3 and potentiate glutamatergic synaptic activity in midbrain DA neurons. This dual action of AMPH predicts that EAAT3 downregulation will underlie some of the behavioral effects of AMPH and is consistent with previous findings implicating glutamate transporters in AMPH-mediated behaviors. For example, sensitization to AMPH is enhanced by blocking glutamate transporters (Aked et al., 2005) while acute responses (Rasmussen et al., 2011) and reinforcing effects (Fujio et al., 2005; Rawls et al., 2008) of AMPH are attenuated by increasing the expression of glutamate transporters.These data underscore the importance of glutamate transporters and the processes that govern their activity to the behavioral responses to AMPH.
Experimental Procedures
Cell culture
HEK293 cells (ATCC) were maintained in DMEM with 5% FBS and Pen/Strep. Cells were transiently transfected with Translt-LT1 transfection reagent (Mirus). All cell culture assays were performed on cultures from at least three different plating procedures. Where possible, experiments were performed in a blind fashion.
Primary midbrain cultures
All animal-related protocols were approved by the University of Pittsburgh, Oregon Health Sciences University IACUCs or the NIMH ACUC. Primary cultures were derived from midbrains of E15 Swiss Webster mice. Tissue was dissociated and plated at a density of 3 midbrains per 6×25mm or 12×12mm poly-D-lysine coated glass coverslips (Warner Instruments). Cultures were treated with AraC to inhibit astrocyte overgrowth and maintained in DMEM supplemented with 5% horse serum and 5% calf serum for two to four weeks. Neurons were transiently transfected with NeuroMag according to manufacturers instructions (Oz Biosciences).
3H-Glutamate uptake assays
Pretreatments with AMPH, cocaine or TAT-fusion peptides were performed in DMEM for 30 minutes at 37°C in 5% CO2. Glutamate transport activity was assessed with 250μM cold glutamate and 100 nM 3H-glutamate in PBS with 100 μM CaCl2 and 1mM MgCl2 for ten minutes at room temperature. Cultures were then washed with ice cold PBS, solublized in scintillation fluid and radioactivity was measured by a 1250 Microbeta Wallac Liquid Scintillation Counter. Radioactivity into wells treated similarly but with non-transfected cells were used as background measurement.
Acceptor peptide labeling
Cells were chilled to 4° C to minimize further protein trafficking, non-specific binding was blocked with 5% milk in PBS and membrane proteins were probed with Streptavidin conjugated to Alexa Fluor-568, washed with PBS and fixed with 4% paraformaldehyde.
Acute Substantia Nigra slices
Male Swiss Webster mice were deeply anesthetized with isoflurane and the brains were rapidly removed and placed in ice cold buffer containing (in mM): 75 NaCl, 2.5 KCl, 0.1 CaCl2, 6 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 2.5 D-dextrose, 50 sucrose. Midbrain horizontal slices (~1-230μm) were cut in 95% O2 and 5% CO2 oxygenated buffer and warmed to room temperature in artificial cerebrospinal fluid (ACSF) containing kynurenic acid (50μM) for at least 30 minutes.
Electrophysiology
Patch clamp recordings were made from visually identified substantia nigra pars compacta (SNc) neurons and further characterized by the presence of a hyperpolarization–activated membrane rectification (Ih current) and post-hoc immunohistochemical staining for tyrosine hydroxylase (TH). Patch pipettes had a resistance of 2-4 MOhm when filled with the intracellular solution containing (in mM): 130 CsCl, 5.4 KCl, 0.1 CaCl2, 2MgCl2, 10 HEPES, 1.1 EGTA, 30 D-dextrose, 2 MgATP, 0.3 NaGTP; pH 7.3. Cells were voltage-clamped at −70mV, currents were filtered at 2 kHz and digitized at 5 kHz (Multiclamp 700A, Molecular Devices, USA). Synaptic currents were evoked with bipolar stimulating electrodes placed ~ 200-300 μm distally from the recorded cell. Stimulation pulses were delivered at 0.03 Hz and NMDA-mediated evoked excitatory postsynaptic currents (eEPSCs) were recorded in Mg2+-free ACSF containing NBQX (5 μM) and glycine (10μM). AMPA-mediated eEPSCs were isolated in APV (50μM), kynurenic acid (1mM) and cyclothiazide (100μM) to decrease maximal activation by synaptic glutamate. Inhibitors of DA receptors and α-adrenergic were included in the ACSF recording solution: DA-D1: SCH 23390, 1μM; DA-D2: raclopride 10μM; α-AR: prazosin 1μM. Bicuculline (10μM) and strychnine (1μM) were also included in the bath to eliminate GABAA and glycine receptor contamination. During each experiment, a voltage step of -10mV from the holding potential was applied periodically to monitor cell capacitance and access resistance. Recordings in which access resistance or capacitance changed by >10% during the experiment were excluded in data analysis. Electrophysiology data were acquired and analyzed using AxoGraph X software (Axograph, Sydney AUSTRALIA).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde, permeablized with 1% triton x-100, blocked with 5% NGS or milk, and incubated in primary antibody at 1:1000 overnight. Appropriate secondary Alexa Fluor conjugated antibodies were applied at 1:10,000 for one hour and coverslips were then mounted in AntiFade (Invitrogen).
Antibodies
Rabbit anti-EAAT3 (Alpha Diagnostics), mouse anti-TH (Sigma), rat anti-DAT (Chemicon), rabbit anti-EAAT3 (Alpha diagnostics), rabbit anti-EAAT2 (made inhouse), rabbit anti-GluR4 (Millipore) rabbit anti-GluR1 (Upstate), and mouse anti-NR1 (Millipore),
Microscopy
TIRF microscopy was done on an Olympus 1X71 equipped with a 60x TIRF objective. Olympus Fluoview 1000 was used for confocal imaging and a Nikon Diaphot200 was used for epifluorescent assays. Image analysis was performed with Image J.
Acute midbrain slices
For biochemical analysis, 1mm midbrain slices from 6 week old Swiss Webster mice were prepared with a mouse brain slicer (Zivic). Slices rested for one hour in ACSF containing kynurenic acid prior to each experiment. The ACSF contained (in mM): 126 NaCl, 3.5 KCl, 2 CaCl2, 1.3 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 10 D-dextrose, pH 7.4.
Biotinylation
Cell surface biotinylation was performed in ice cold buffer (in mM: 2 CaCl2, 150 NaCl, and 10 triethanolamine, pH 7.5) with 2 mg/ml sulfosuccinimidyl 2-(biotinamido) methyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin; Pierce, Rockford, IL) for 20 minutes at 4°C. Non-bound biotin was quenched for 20 minutes in glycine and preparations were lysed in buffer comprised of 1% Triton X-100, 150mM NaCl, 5mM EDTA, and 50mM Tris, pH 7.5, containing a protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN)]. Biotinylated proteins were isolated by a 2 hour incubation with Ultralink immobilized NeutrAvidin beads washed with lysis buffer, a highsalt wash buffer (0.1% Triton X-100, 500mM NaCl, 5mM EDTA, and 50mM Tris, pH 7.5) and finally 50mM Tris, pH 7.5.
Reagents
Peptides were ordered from Tien Life Sciences. AP, and BirA constructs from AddGene. All other reagents were from Sigma or Invitrogen.
Supplementary Material
Highlights.
AMPH stimulates dynamin-mediated internalization of the glutamate transporter EAAT3
This event requires AMPH transport into cells by the DAT and RhoA GTPase activation
A unique sequence on the C-terminus of EAAT3 is required for internalization
Glutamatergic synaptic activity is enhanced by AMPH-mediated EAAT3 internalization
Acknowledgments
This work was supported by NIH grant MH080726 (SMU, SGA) and DA024041 (SLI) and by the NIMH Department of Intramural Research (SMU, SGA).
References
- Adie EJ, Francis MJ, Davies J, Smith L, Marenghi A, Hather C, Hadingham K, Michael NP, Milligan G, Game S. CypHer 5: a generic approach for measuring the activation and trafficking of G protein-coupled receptors in live cells. Assay Drug Dev Technol. 2003;1:251–259. doi: 10.1089/15406580360545062. [DOI] [PubMed] [Google Scholar]
- Aked J, Coizet V, Clark D, Overton PG. Local injection of a glutamate uptake inhibitor into the ventral tegmental area produces sensitization to the behavioural effects of d-amphetamine. Neuroscience. 2005;134:361–367. doi: 10.1016/j.neuroscience.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, et al. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 2009;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol. 2012;108:802–809. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- Cheng C, Glover G, Banker G, Amara SG. A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin-Darby canine kidney cells and hippocampal neurons. J Neurosci. 2002;22:10643–10652. doi: 10.1523/JNEUROSCI.22-24-10643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Soragna A, Di Cairano E, Panzeri N, Anzai N, Vellea Sacchi F, Perego C. The surface density of the glutamate transporter EAAC1 is controlled by interactions with PDZK1 and AP2 adaptor complexes. Traffic. 2010;11:1455–1470. doi: 10.1111/j.1600-0854.2010.01110.x. [DOI] [PubMed] [Google Scholar]
- Dalia A, Uretsky NJ, Wallace LJ. Dopaminergic agonists administered into the nucleus accumbens: effects on extracellular glutamate and on locomotor activity. Brain Res. 1998;788:111–117. doi: 10.1016/s0006-8993(97)01518-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Gonzalez-Mora JL, Armas VR, Mora F. Amphetamine increases the extracellular concentration of glutamate in striatum of the awake rat: involvement of high affinity transporter mechanisms. Neuropharmacology. 1999;38:943–954. doi: 10.1016/s0028-3908(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Martinez R, Mora F. Amphetamine increases extracellular concentrations of glutamate in the prefrontal cortex of the awake rat: a microdialysis study. Neurochem Res. 1998;23:1153–1158. doi: 10.1023/a:1020769816332. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, Galli A. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Ting AY. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc. 2008;3:534–545. doi: 10.1038/nprot.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Erichsen MN, Nielsen CW, Stensbol TB, Kehler J, Bunch L. Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1. J Med Chem. 2009;52:912–915. doi: 10.1021/jm8013458. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol. 2006;70:542–548. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Brand U, Menge HG. Effects of chronic amphetamine treatment on the glutamate concentration in cerebrospinal fluid and brain: implications for a theory of schizophrenia. Neurosci Lett. 1981;24:93–96. doi: 10.1016/0304-3940(81)90365-7. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Post-translational modification biology of glutamate receptors and drug addiction. Front Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, Porras A. Effects of amphetamine on the release of excitatory amino acid neurotransmitters in the basal ganglia of the conscious rat. Can J Physiol Pharmacol. 1993;71:348–351. doi: 10.1139/y93-054. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Baron DA, Kim JK, Unterwald EM, Rawls SM. beta-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids. 2011;40:761–764. doi: 10.1007/s00726-010-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2008;107:261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neurosci. 2004;29:296–310. [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology (Berl) 2000;151:184–191. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- Watzke N, Grewer C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 2001;503:121–125. doi: 10.1016/s0014-5793(01)02715-6. [DOI] [PubMed] [Google Scholar]
- Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A, Saunders C. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol Pharmacol. 2007;71:835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Khansa MR. Repeated administration of MK-801 produces sensitization to its own locomotor stimulant effects but blocks sensitization to amphetamine. Brain Res. 1991;562:164–168. doi: 10.1016/0006-8993(91)91202-c. [DOI] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. J Neurochem. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


