Abstract
Schizophrenia is a complex disorder characterized by an array of symptoms. This heterogeneity has resulted in a lack of consensus regarding diagnostic criteria, etiology, and pathophysiology, and has complicated efforts to devise effective treatments.
INTRODUCTION
Schizophrenia is a complex, chronic mental health disorder characterized by an array of symptoms, including delusions, hallucinations, disorganized speech or behavior, and impaired cognitive ability. The early onset of the disease, along with its chronic course, make it a disabling disorder for many patients and their families.1 Disability often results from both negative symptoms (characterized by loss or deficits) and cognitive symptoms, such as impairments in attention, working memory, or executive function.2 In addition, relapse may occur because of positive symptoms, such as suspiciousness, delusions, and hallucinations.1,2 The inherent heterogeneity of schizophrenia has resulted in a lack of consensus regarding the disorder’s diagnostic criteria, etiology, and pathophysiology.1,3
This article provides a concise review of schizophrenia and discusses the available treatment options.
PATHOPHYSIOLOGY
Abnormalities in neurotransmission have provided the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid (GABA) as part of the neurochemical imbalance of schizophrenia.1
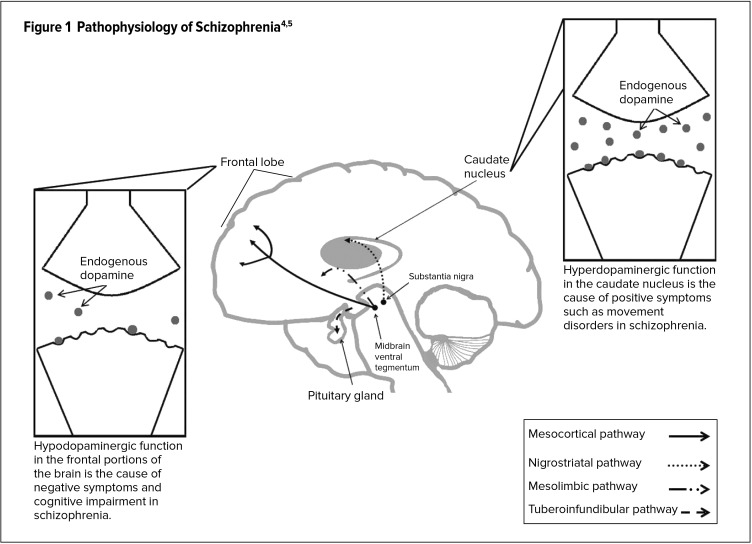
Abnormal activity at dopamine receptor sites (specifically D2) is thought to be associated with many of the symptoms of schizophrenia. Four dopaminergic pathways have been implicated (Figure 1).4,5 The nigrostriatal pathway originates in the substantia nigra and ends in the caudate nucleus. Low dopamine levels within this pathway are thought to affect the extrapyramidal system, leading to motor symptoms.1 The mesolimbic pathway, extending from the ventral tegmental area (VTA) to limbic areas, may play a role in the positive symptoms of schizophrenia in the presence of excess dopamine.1 The mesocortical pathway extends from the VTA to the cortex. Negative symptoms and cognitive deficits in schizophrenia are thought to be caused by low mesocortical dopamine levels. The tuberoinfundibular pathway projects from the hypothalamus to the pituitary gland. A decrease or blockade of tuberoinfundibular dopamine results in elevated prolactin levels and, as a result, galactorrhea, ammenorrhea, and reduced libido.
Figure 1.
The serotonin hypothesis for the development of schizophrenia emerged as a result of the discovery that lysergic acid diethylamide (LSD) enhanced the effects of serotonin in the brain.1 Subsequent research led to the development of drug compounds that blocked both dopamine and serotonin receptors, in contrast to older medications, which affected only dopamine receptors. The newer compounds were found to be effective in alleviating both the positive and negative symptoms of schizophrenia.1
Another theory for the symptoms of schizophrenia involves the activity of glutamate, the major excitatory neurotransmitter in the brain. This theory arose in response to the finding that phenylciclidine and ketamine, two noncompetitive NMDA/glutamate antagonists, induce schizophrenia-like symptoms.6 This, in turn, suggested that NMDA receptors are inactive in the normal regulation of mesocortical dopamine neurons, and pointed to a possible explanation for why patients with schizophrenia exhibit negative, affective, and cognitive symptoms.7
The brain tissue itself appears to undergo detectable physical changes in patients with schizophrenia. For example, in addition to an increase in the size of the third and lateral ventricles, individuals at high risk of a schizophrenic episode have a smaller medial temporal lobe.2
ETIOLOGY
Despite more than a century of research, the precise cause of schizophrenia continues to elude investigators. It is widely accepted, however, that the various phenotypes of the illness arise from multiple factors, including genetic susceptibility and environmental influences.2,8
One explanation for the development of schizophrenia is that the disorder begins in utero.6 Obstetric complications, including bleeding during pregnancy, gestational diabetes, emergency cesarean section, asphyxia, and low birth weight, have been associated with schizophrenia later in life.2 Fetal disturbances during the second trimester—a key stage in fetal neurodevelopment—have been of particular interest to researchers.3 Infections and excess stress levels during this period have been linked to a doubling of the risk of offspring developing schizophrenia.3
Scientific evidence supports the idea that genetic factors play an important role in the causation of schizophrenia; 2 studies have shown that the risk of illness is approximately 10% for a first-degree relative and 3% for a second-degree relative.9 In the case of monozygotic twins, the risk of one twin having schizophrenia is 48% if the other has the disorder, whereas the risk is 12% to 14% in dizygotic twins.9 If both parents have schizophrenia, the risk that they will produce a child with schizophrenia is approximately 40%.9
Studies of adopted children have been conducted to determine whether the risk of schizophrenia comes from the biological parents or from the environment in which the child is raised. These investigations have tended to show that changes in the environment do not affect the risk of developing schizophrenia in children born to biological parents with the illness.3,6 A genetic basis for schizophrenia is further supported by findings that siblings with schizophrenia often experience onset of the disorder at the same age.2
Environmental and social factors may also play a role in the development of schizophrenia, especially in individuals who are vulnerable to the disorder.1 Environmental stressors linked to schizophrenia include childhood trauma, minority ethnicity, residence in an urban area, and social isolation.1 In addition, social stressors, such as discrimination or economic adversity, may predispose individuals toward delusional or paranoid thinking.1
EPIDEMIOLOGY
The prevalence of schizophrenia is between 0.6% and 1.9% in the U.S. population.10 Moreover, a claims analysis has estimated that the annual prevalence of diagnosed schizophrenia in the U.S. is 5.1 per 1,000 lives.11 The prevalence of the disorder seems to be equal in males and females, although the onset of symptoms occurs at an earlier age in males than in females.2 Males tend to experience their first episode of schizophrenia in their early 20s, whereas women typically experience their first episode in their late 20s or early 30s.12
Research into a possible link between the geography of birth and the development of schizophrenia has provided inconclusive results. A collaborative study by the World Health Organization in 10 countries found that schizophrenia occurred with comparable frequencies across the various geographically defined populations.13 On the other hand, a more recent review, which included data from 33 countries, concluded that the incidence of schizophrenia varied by geographic location.14
CLINICAL PRESENTATION
Schizophrenia is the most common functional psychotic disorder, and (as noted previously) individuals with the disorder can present with a variety of manifestations. Contrary to portrayals of the illness in the media, schizophrenia does not involve a “split personality.” Rather, it is a chronic psychotic disorder that disrupts the patient’s thoughts and affect. The illness commonly interferes with a patient’s ability to participate in social events and to foster meaningful relationships.2
Social withdrawal, among other abnormal (schizoid) behaviors, typically precedes a person’s first psychotic episode; however, some individuals may exhibit no symptoms at all.2 A psychotic episode is characterized by patient-specific signs and symptoms (psychotic features) that reflect the “false reality” created in the patient’s mind.2,15
As noted earlier, the symptoms of schizophrenia are categorized as positive, negative, or cognitive. Each symptom is vitally important as the clinician attempts to distinguish schizophrenia from other psychotic disorders, such as schizoaffective disorder, depressive disorder with psychotic features, and bipolar disorder with psychotic features.12
Positive symptoms are the most easily identified and can be classified simply as “psychotic behaviors not seen in healthy people.”15 Such symptoms include delusions, hallucinations, and abnormal motor behavior in varying degrees of severity.12
Negative symptoms are more difficult to diagnose but are associated with high morbidity as they disturb the patient’s emotions and behavior.12,15 The most common negative symptoms are diminished emotional expression and avolition (decreased initiation of goal-directed behavior). Patients may also experience alogia and anhedonia. It is important to understand that negative symptoms may be either primary to a diagnosis of schizophrenia or secondary to a concomitant psychotic diagnosis, medication, or environmental factor.12,16
Cognitive symptoms are the newest classification in schizophrenia. These symptoms are nonspecific; therefore, they must be severe enough for another individual to notice them. Cognitive symptoms include disorganized speech, thought, and/or attention, ultimately impairing the individual’s ability to communicate.12,16
Patients with symptoms of schizophrenia may experience additional limitations and negative conditions. Substance-abuse disorders occur most often among these patients; these disorders can involve a variety of substances, including alcohol, tobacco, and prescription medications.12,16 Anxiety, depression, panic, and obsessive-compulsive disorder are also prominent in patients with schizophrenia and can exacerbate the symptoms of their disorder.12,16 These patients also have a general lack of awareness of their illness. This mindset has been linked to high rates of nonadherence, relapse, poor psychosocial function, poor hygiene, and worse disease outcomes.2,12
The primary symptoms and comorbid conditions associated with schizophrenia may ultimately lead to social and occupational dysfunction.12 Functional consequences include an inadequate or incomplete education, which may affect the patient’s ability to obtain and hold a stable job. Patients with schizophrenia typically cultivate few social relationships and need daily support to manage relapses and recurring symptoms.12,16
The prognosis for patients with schizophrenia is generally unpredictable.2 Only 20% of patients report favorable treatment outcomes.12 The remaining patients experience numerous psychotic episodes, chronic symptoms, and a poor response to antipsychotics.2
DIAGNOSIS
As described earlier, schizophrenia is a chronic disorder with numerous symptoms, where no single symptom is pathogenic. A diagnosis of schizophrenia is reached through an assessment of patient-specific signs and symptoms, as described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).12 The DSM-5 states that “the diagnostic criteria [for schizophrenia] include the persistence of two or more of the following active-phase symptoms, each lasting for a significant portion of at least a one-month period: delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms.”12 At least one of the qualifying symptoms must be delusions, hallucinations, or disorganized speech.12
Moreover, the DSM-5 states that, to warrant a diagnosis of schizophrenia, the patient must also exhibit a decreased level of functioning regarding work, interpersonal relationships, or self-care.12 There must also be continuous signs of schizophrenia for at least six months, including the one-month period of active-phase symptoms noted above.12
A comprehensive differential diagnosis of schizophrenia is necessary to distinguish the disorder from other mental conditions, such as major depressive disorder with psychotic or catatonic features; schizoaffective disorder; schizophreniform disorder; obsessive-compulsive disorder; body dysmorphic disorder; and post-traumatic stress disorder. Schizophrenia can be differentiated from these similar conditions through a careful examination of the duration of the illness, the timing of delusions or hallucinations, and the severity of depressive or manic symptoms.12 In addition, the clinician must confirm that the presenting symptoms are not a result of substance abuse or another medical condition.12
TREATMENT OPTIONS
Nonpharmacological Therapy
The goals in treating schizophrenia include targeting symptoms, preventing relapse, and increasing adaptive functioning so that the patient can be integrated back into the community.2 Since patients rarely return to their baseline level of adaptive functioning, both nonpharmacological and pharmacological treatments must be used to optimize long-term outcomes.2 Pharmacotherapy is the mainstay of schizophrenia management, but residual symptoms may persist. For that reason, nonpharmacological treatments, such psychotherapy, are also important.17
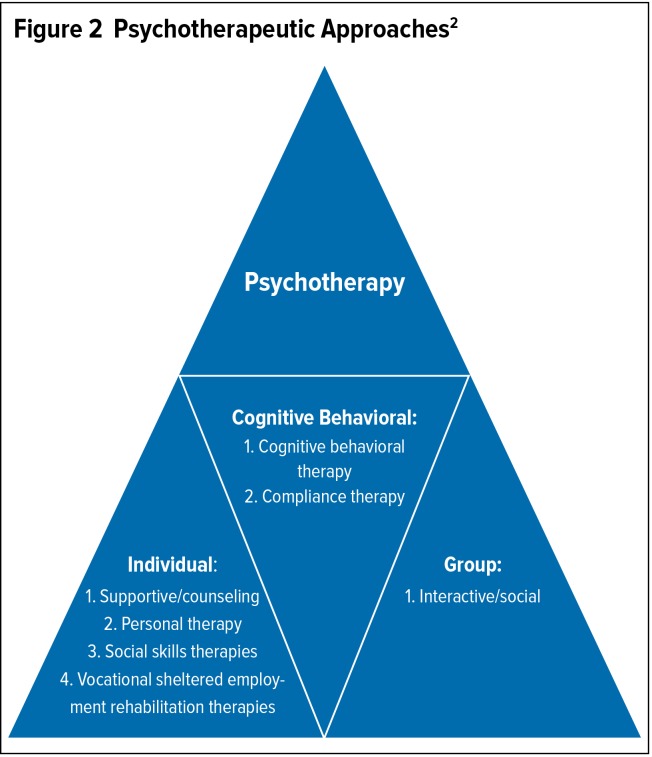
Psychotherapeutic approaches may be divided into three categories: individual, group, and cognitive behavioral (Figure 2).2 Psychotherapy is a constantly evolving therapeutic area. Emerging psychotherapies include meta-cognitive training, narrative therapies, and mindfulness therapy.17 Nonpharmacological treatments should be used as an addition to medications, not as a substitute for them.2
Figure 2.
Psychotherapeutic Approaches2
Not only do nonpharmacological therapies fill in gaps in pharmacological treatments; they can help to ensure that patients remain adherent to their medications.18 Nonadherence rates in schizophrenia range from 37% to 74%, depending on the report.19 Individuals with mental disorders tend to be less adherent for several reasons. They may deny their illness; they may experience adverse effects that dissuade them from taking more medication; they may not perceive their need for medication; or they may have grandiose symptoms or paranoia.2
Patients with schizophrenia who stop taking their medication are at increased risk of relapse, which can lead to hospitalization.18 Therefore, it is important to keep patients informed about their illness and about the risks and effectiveness of treatment.20 Some psychotherapies can help educate patients about the importance of taking their medications. These initiatives include cognitive behavioral therapy (CBT), personal therapy, and compliance therapy.17
In addition to focusing on the patient, treatment programs that encourage family support have been shown to decrease rehospitalization and to improve social functioning.2 Family members can be taught how to monitor the patient and when to report adverse effects of treatment to the clinician.20 Most psychotherapies promote family involvement.17
Pharmacological Therapy
In most schizophrenia patients, it is difficult to implement effective rehabilitation programs without antipsychotic agents.16 Prompt initiation of drug treatment is vital, especially within five years after the first acute episode, as this is when most illness-related changes in the brain occur.16,21 Predictors of a poor prognosis include the illicit use of amphetamines and other central nervous system stimulants,22 as well as alcohol and drug abuse.2 Alcohol, caffeine, and nicotine also have the potential to cause drug interactions.2
In the event of an acute psychotic episode, drug therapy should be administered immediately. During the first seven days of treatment, the goal is to decrease hostility and to attempt to return the patient to normal functioning (e.g., sleeping and eating).2 At the start of treatment, appropriate dosing should be titrated based on the patient’s response.2
Treatment during the acute phase of schizophrenia is followed by maintenance therapy, which should be aimed at increasing socialization and at improving self-care and mood.2 Maintenance treatment is necessary to help prevent relapse. The incidence of relapse among patients receiving maintenance therapy, compared with those not receiving such therapy, is 18% to 32% versus 60% to 80%, respectively.16,23 Drug therapy should be continued for at least 12 months after the remission of the first psychotic episode.16,24
According to the American Psychiatric Association, second-generation (atypical) antipsychotics (SGAs)—with the exception of clozapine—are the agents of choice for first-line treatment of schizophrenia.16,25 Clozapine is not recommended because of its risk of agranulocytosis.2 SGAs are usually preferred over first-generation (typical) antipsychotics (FGAs) because they are associated with fewer extrapyramidal symptoms.2 However, SGAs tend to have metabolic side effects, such as weight gain, hyperlipidemia, and diabetes mellitus.26 These adverse effects can contribute to the increased risk of cardiovascular mortality observed in schizophrenia patients.26
The Texas Medication Algorithm Project (TMAP) has provided a six-stage pharmacotherapeutic algorithm for the treatment of schizophrenia. Stage 1 is first-line monotherapy with an SGA. If the patient shows little or no response, he or she should proceed to stage 2, which consists of monotherapy with either another SGA or an FGA. If there is still no response, the patient should move to stage 3, which consists of clozapine monotherapy with monitoring of the white blood cell (WBC) count.24 If agranulocytosis occurs, clozapine should be discontinued. If stage-3 therapy fails to elicit a response, the patient should proceed to stage 4, which combines clozapine with an FGA, an SGA, or electroconvulsive therapy (ECT).24 If the patient still shows no response to treatment, stage 5 calls for monotherapy with an FGA or an SGA that has not been tried.24 Finally, if stage 5 treatment is unsuccessful, stage 6 consists of combination therapy with an SGA, an FGA, ECT, and/or a mood stabilizer.24
Combination therapy is recommended only in the later stages of the treatment algorithm.27 The routine prescription of two or more antipsychotics is not recommended because it may increase the risk of drug interactions, nonadherence, and medication errors.27
Before a new antipsychotic agent is initiated, the patient’s complete medication history should be obtained. Whether the patient has shown a favorable or unfavorable response to previous antipsychotic treatment will help guide the selection of a new medication.2
Long-Acting Injectable Antipsychotic Agents
Long-acting injectable (LAI) antipsychotic medications offer a viable option for patients who are nonadherent to an oral medication.2 Clinicians should determine whether the patient’s nonadherence is due to the adverse effects of treatment. If so, then the clinician should consider an oral medication with a more favorable side-effect profile.2 Before moving to LAI therapy, a short trial should be conducted with the oral counterpart of the LAI to determine tolerability.2
A recent meta-analysis of randomized controlled trials (RCTs) concluded that outcomes with LAIs are similar to those with oral antipsychotics.28 The authors suspected, however, that RCTs might not reflect the “real world” efficacy and safety of LAIs.29 Therefore, they conducted a meta-analysis of 25 mirror-image studies, in which a total of 5,940 subjects served as their own controls in naturalistic settings.29 This analysis demonstrated the superiority of LAIs over oral antipsychotics in preventing hospitalizations (risk ratio [RR] = 0.43) and in reducing the number of hospitalizations (RR = 0.38).29
Treatment-Resistant Schizophrenia
Between 10% and 30% of patients with schizophrenia show little symptomatic improvement after multiple trials of FGAs, and an additional 30% to 60% experience partial or inadequate improvement or unacceptable side effects during antipsychotic therapy.16
Clozapine is the most effective antipsychotic in terms of managing treatment-resistant schizophrenia. This drug is approximately 30% effective in controlling schizophrenic episodes in treatment-resistant patients, compared with a 4% efficacy rate with the combination of chlorpromazine and benztropine.30 Clozapine has also been shown to increase serum sodium concentrations in patients with polydipsia and hyponatremia.31
However, as indicated earlier, clozapine has a problematic safety profile. For example, patients treated with this drug are at increased risk of developing orthostatic hypotension, which can require close monitoring.2 Moreover, high-dose clozapine has been associated with serious adverse effects, such as seizures.2
Augmentation and Combination Therapy
Both augmentation therapy (with ECT or a mood stabilizer) and combination therapy (with antipsychotics) may be considered for patients who fail to show an adequate response to clozapine. Clinicians should observe the following guidelines when administering augmentation therapy:24
The treatment should be used only in patients with an inadequate response to prior therapy.
Augmentation agents are rarely effective for schizophrenia symptoms when given alone.
Patients responding to augmentation treatment usually improve rapidly.
If an augmentation strategy does not improve the patient’s symptoms, then the agent should be discontinued.
Mood stabilizers are common augmentation agents. Lithium, for example, improves mood and behavior in some patients but does not have an antipsychotic effect.23
In combination therapy, two antipsychotic drugs—such as an FGA and an SGA, or two different SGAs—are administered concurrently.2 However, exposure to multiple antipsychotics at the same time may increase the risk of serious side effects.24,25,32
Mechanism of Action
The precise mechanism of action of antipsychotic drugs is unknown, although it has been suggested that these drugs comprise three main categories: 1) typical, or traditional, antipsychotics, which are associated with high dopamine (D2) antagonism and low serotonin (5-HT2A) antagonism; 2) atypical antipsychotics that have moderate-to-high D2 antagonism and high 5-HT2A antagonism; and 3) atypical antipsychotics that demonstrate low D2 antagonism and high 5-HT2A antagonism.2,33,34
At least 60% to 65% of D2 receptors must be occupied to decrease the positive symptoms of schizophrenia, whereas a D2 blockade rate of 77% or more has been associated with extrapyramidal symptoms.33,35
The improvement of negative symptoms and cognition with atypical antipsychotics may be due to 5-HT2A antagonism in combination with D2 blockade, resulting in the release of dopamine into the prefrontal cortex (the area of the brain in which dopaminergic receptors are hypoactive in untreated individuals with schizophrenia).2 Although atypical antipsychotics appear to improve negative symptoms, no approved treatment options are specifically indicated for these symptoms.
Adverse Effects
Typical vs. Atypical Antipsychotics
The adverse effects of schizophrenia medications can involve several organ systems, as discussed below.
Table 1 illustrates the risk of two key adverse effects of anti-psychotic agents: weight gain and extrapyramidal symptoms.2 SGAs are associated with a greater risk of weight gain, whereas FGAs are associated with a greater risk of extrapyramidal side effects. SGAs with the lowest risk of extrapyramidal symptoms include aripiprazole, quetiapine, and clozapine.18,36,37,38
Table 1.
Comparative Risks of Weight Gain and Extrapyramidal Symptoms With Typical and Atypical Antipsychotic Agents2
| Drug | Weight Gain | Extrapyramidal Symptoms |
|---|---|---|
| Typical Antipsychotics (First-Generation Antipsychotics) | ||
| Chlorpromazine (Thorazine) | ++ | +++ |
| Fluphenazine (Prolixin) | + | ++++ |
| Haloperidol (Haldol) | + | ++++ |
| Perphenazine (Trilafon) | + | ++++ |
| Thioridazine (Mellaril) | + | +++ |
| Thiothixene (Navane) | + | ++++ |
| Atypical Antipsychotics (Second-Generation Antipsychotics) | ||
| Aripiprazole (Abilify) | + | + |
| Asenapine (Saphris) | + | ++ |
| Clozapine (Clozaril) | ++++ | + |
| Iloperidone (Fanapt) | ++ | ± |
| Lurasidone (Latuda) | ± | + |
| Olanzapine (Zyprexa) | ++++ | ++ |
| Paliperidone (Invega) | ++ | ++ |
| Quetiapine (Seroquel) | ++ | + |
| Risperidone (Risperdal) | ++ | ++ |
| Ziprasidone (Geodon) | + | ++ |
± = negligible risk; + = low risk; ++ = moderate risk; +++ = moderately high risk; ++++ = high risk
Endocrine System
Hyperprolactinemia can occur in up to 87% of patients treated with risperidone or paliperidone, possibly leading to sexual dysfunction, decreased libido, menstrual irregularities, or gynecomastia.2 Aripiprazole or ziprasidone is a potential treatment option for patients with increased prolactin levels.39
Weight gain is another important side effect in patients receiving antipsychotic drugs.39,40 It can occur in patients treated for their first psychotic episode2 and may eventually lead to nonadherence.41
Along with hyperprolactinemia and weight gain, antipsychotic drugs also can increase the risks of diabetes mellitus and cardiovascular-related mortality.39,42 Olanzapine has the greatest risk of diabetes, followed by risperidone and quetiapine. The latter two agents cause minimal weight gain, however.2
Cardiovascular System
Orthostatic hypotension can occur in up to 75% of patients treated with an antipsychotic agent.43 Patients with diabetes, pre-existing cardiovascular disease, or advanced age appear to have the greatest risk, but all patients receiving antipsychotic medications should be counseled to rise slowly from a sitting position to avoid a hypotensive episode.2
Electrocardiographic changes, especially QTc prolongation, can occur in some patients treated with antipsychotics, including thioridazine, clozapine, iloperidone, and ziprasidone. QTc prolongation should be monitored during therapy, and treatment should be discontinued if this interval consistently exceeds 500 msec.2 Antipsychotic medications should be chosen carefully in patients with pre-existing cardiac or cerebrovascular disease, and in those taking diuretics or medications that prolong the QTc interval.43
Although some studies have shown that the risk of sudden cardiac death in patients treated with FGAs or SGAs is nearly twice that in individuals who do not use antipsychotic medications, more recent findings suggest that both types of drugs have similar cardiac mortality risks.43,44
Lipid Changes
Patients treated with SGAs or phenothiazines tend to show increased concentrations of serum triglycerides and cholesterol.2 SGAs with a lower risk in this regard include risperidone, ziprasidone, and aripiprazole.41,42 In the CATIE trial, olanzapine was shown to have negative effects on cholesterol levels and lipids.45
Central Nervous System
Dystonia is another common side effect of antipsychotic medications. This disorder often results in nonadherence and can be life-threatening.2 Dystonic reactions typically accompany treatment with FGAs and are most common in younger male patients.2 Dystonia may be minimized by using SGAs or by initiating FGAs at lower doses.2
Akathisia (often accompanied by dysphoria) occurs in 20% to 40% of patients treated with high-potency FGAs, such as haloperidol and fluphenazine.36,46 Quetiapine and clozapine appear to have the lowest risk for this side effect.36,37
Pseudoparkinsonism has occurred in patients receiving antipsychotic therapy. The incidence of this disorder has ranged from 15% to 36% in patients treated with FGAs. It occurs more often in females and in older patients.2 The risk of pseudoparkinsonism during treatment with SGAs is generally low, although an increased risk is associated with higher doses of risperidone.2
The risk of tardive dyskinesia has ranged from as low as 0.5% to as high as 62% during treatment with FGAs and is increased in elderly patients.2,37,46 The overall prevalence of the disorder ranges from 20% to 25% among patients receiving long-term FGA therapy.2,37 The risk of tardive dyskinesia is significantly lower with SGAs, and no cases have been reported in patients receiving clozapine monotherapy.2,37
Chlorpromazine, thioridazine, mesoridazine, clozapine, olanzapine, and quetiapine have the highest sedation potential.2 Studies have shown that SGAs offer superior cognitive benefits compared with FGAs, although the CATIE trial found no differences in cognitive improvement among patients treated with SGAs compared with the FGA perphenazine.2,47
All patients treated with antipsychotic agents are at increased risk of seizures. The antipsychotics with the greatest seizure risk are clozapine and chlorpromazine.2 Those with the lowest risk include risperidone, molindone, thioridazine, haloperidol, pimozide, trifluoperazine, and fluphenazine.36
Poikilothermia (the inability to maintain a constant internal body temperature independent of external temperatures) can be a serious side effect of antipsychotic medications.48 In addition, patients may be at increased risk of heat stroke during exercise because of an impaired ability to dissipate excess body heat.2 These side effects most commonly occur during treatment with low-potency FGAs, such as chlorpromazine, but they have also been associated with the SGAs that have more anticholinergic effects, such as clozapine.2
Neuroleptic malignant syndrome (NMS) is a rare but life-threatening side effect of antipsychotic drug therapy, occurring in 0.5% to 1.0% of patients treated with FGAs.2 Since the introduction and increased use of SGAs, however, the treatment-related occurrence of this disorder has diminished.2
Psychiatric side effects, such as delirium and psychosis, can occur with higher doses of FGAs or with combination treatments involving anticholinergics.2 Elderly patients, in particular, are at increased risk of chronic confusion and disorientation during treatment with antipsychotic drugs.2
Miscellaneous Adverse Effects
Schizophrenia medications can cause a variety of other adverse effects, including the following:
Antipsychotic medications with anticholinergic effects have been shown to worsen narrow-angle glaucoma, and patients should be appropriately monitored.49 Chlorpromazine is most commonly associated with opaque deposits in the cornea and lens.2 Because of the risk of cataracts, eye examinations are recommended for patients treated with quetiapine.50 Those using thioridazine at doses exceeding 800 mg daily are at risk of developing retinitis pigmentosa.2
Low-potency FGAs and clozapine have been associated with urinary hesitancy and retention.2 The incidence of urinary incontinence among patients taking clozapine can be as high as 44% and can be persistent in 25% of patients.2,51
FGAs and risperidone have a greater tendency to cause sexual dysfunction compared with SGAs.2,52
Treatment with antipsychotics can cause transient leukopenia.2,53
The three antipsychotics with the greatest risk for hematological complications are clozapine, chlorpromazine, and olanzapine.54 Clozapine is associated with an especially high risk for the development of neutropenia or agranulocytosis.54
On rare occasions, dermatological allergic reactions have occurred at approximately eight weeks after the initiation of antipsychotic therapy.2
Both FGAs and SGAS can cause photosensitivity, leading to severe sunburn.2
Clozapine has been reported to cause sialorrhea in approximately 54% of patients with schizophrenia.2 The mechanism of this effect is unknown.2
The varying safety profiles of antipsychotic medications may be due to their effects on various neuroreceptor systems. 33,34,55
Progress Evaluation
As in other medical specialties, recovery during the treatment of schizophrenia is defined both objectively and subjectively.56
Objective dimensions of recovery include the remission of symptoms and the patient’s return to full-time work or enrollment in college.56 Several tools are available for rating the progress of patients with schizophrenia. The Brief Psychiatric Rating Scale (BPRS) and the Positive and Negative Syndrome Scale (PANSS), for example, were developed as numerical indicators of improvement.57 Clinicians also use quicker four-item instruments such as the Positive Symptom Rating Scale and the Brief Negative Symptom Assessment.24,58
Subjective dimensions of recovery are measured by the patient in terms of his or her life satisfaction, hope, knowledge about his or her mental illness, and empowerment.56
Despite continued therapeutic advances, the life expectancy of patients with schizophrenia is reduced by approximately 10 to 25 years compared with that of healthy individuals.59 The increased mortality among patients with schizophrenia has been attributed to unhealthy lifestyles common among this population (i.e., lack of exercise, unhealthy diet, and excessive smoking and alcohol intake), treatment-related adverse events, the suboptimal treatment of concomitant physical illnesses, and suicide.59
CONCLUSION
Schizophrenia is a complex disorder that requires prompt treatment at the first signs of a psychotic episode. Clinicians must consider the potential for nonadherence and treatment-related adverse effects when developing a comprehensive treatment plan. Although patients can increase adaptive functioning through available pharmacological and nonpharmacological treatment options, it is hoped that future research will address gaps in treatment and potentially a cure for schizophrenia.
REFERENCES
- 1.Lavretsky H. History of Schizophrenia as a Psychiatric Disorder. In: Mueser KT, Jeste DV, editors. Clinical Handbook of Schizophrenia. New York, New York: Guilford Press; 2008. pp. 3–12. [Google Scholar]
- 2.Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. 1Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014. pp. 1019–1046. [Google Scholar]
- 3.Beck AT, Rector NA, Stolar N, Grant P. Schizophrenia: Cognitive Theory, Research, and Therapy. New York, New York: Guilford Press; 2009. Biological Contributions; pp. 30–61. [Google Scholar]
- 4.Schwartz JH, Javitch JA. Neurotransmitters. In: Kandel ER, Schwartz JH, Jessell TM, et al., editors. Principles of Neural Science. 5th ed. New York, New York: McGraw-Hill; 2013. pp. 289–305. [Google Scholar]
- 5.Stahl SM. Psychosis and Schizophrenia. In: Stahl SM, editor. Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 2000. pp. 365–399. [Google Scholar]
- 6.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SM, Morrissette DA, Citrome L, et al. “Meta-guidelines” for the management of patients with schizophrenia. CNS Spectr. 2013;18(3):150–162. doi: 10.1017/S109285291300014X. [DOI] [PubMed] [Google Scholar]
- 8.Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 9.McDonald C, Murphy KC. The new genetics of schizophrenia. Psychiatr Clin North Am. 2003;26(1):41–63. doi: 10.1016/s0193-953x(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 10.Van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 11.Wu E, Lizheng S, Birnbaum H, et al. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med. 2006;36(11):1535–1540. doi: 10.1017/S0033291706008191. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. Schizophrenia and other psychotic disorders; pp. 89–122. [Google Scholar]
- 13.Satorius N, Jablensky A, Korten A, et al. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO collaborative study on determinants of outcomes of severe mental disorders. Psychol Med. 1968;16(4):909–928. doi: 10.1017/s0033291700011910. [DOI] [PubMed] [Google Scholar]
- 14.McGrath J, Saha S, Welham J, et al. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status, and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute of Mental Health Schizophrenia 2009. Available at: http://www.nimh.nih.gov/health/publications/schizophrenia-easy-to-read/nimh-schizophrenia-quadfold.pdf. Accessed June 20, 2014.
- 16.Lehman AF, Lieberman JA, Dixon LB, et al. American Psychiatric Association Practice Guidelines; Work Group on Schizophrenia. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. (2nd ed) 2004;161(suppl 2):1–56. [PubMed] [Google Scholar]
- 17.Dickerson FB, Lehman AF. Evidence-based psychotherapy for schizophrenia: 2011 update. J Nerv Ment Dis. 2011;199(8):520–526. doi: 10.1097/NMD.0b013e318225ee78. [DOI] [PubMed] [Google Scholar]
- 18.Lindenmayer JP, Liu-Seifert H, Kulkarni PM, et al. Medication non-adherence and treatment outcomes in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70(7):990–996. doi: 10.4088/JCP.08m04221. [DOI] [PubMed] [Google Scholar]
- 19.Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi: 10.1186/1471-244X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rummel-Kluge C, Kissling W. Psychoeducation for patients with schizophrenia and their families. Exp Rev Neurother. 2008;8(7):1067–1077. doi: 10.1586/14737175.8.7.1067. [DOI] [PubMed] [Google Scholar]
- 21.Castle DJ, Buckley PF. Schizophrenia. Oxford, United Kingdom: Oxford University Press; 2008. [Google Scholar]
- 22.Green AL, Canuso CM, Brenner MJ, Wojcik JD. Detection and management of comorbidity in patients with schizophrenia. Psychiatr Clin North Am. 2003;26(1):115–139. doi: 10.1016/s0193-953x(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 23.Leucht S, Barnes TR, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized controlled trials. Am J Psychiatry. 2003;160(7):1209–1222. doi: 10.1176/appi.ajp.160.7.1209. [DOI] [PubMed] [Google Scholar]
- 24.Argo TR, Crimson ML, Miller AL, et al. Schizophrenia Treatment Algorithms Texas Medication Algorithm Project procedural manual. Austin, Texas: Texas Department of State Health Services; 2008. Available at: http://www.dshs.state.tx.us/mhprograms/TIMA.shtm. Accessed June 20, 2014. [Google Scholar]
- 25.Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68(11):1751–1762. doi: 10.4088/jcp.v68n1115. [DOI] [PubMed] [Google Scholar]
- 26.Raedler TJ. Cardiovascular aspects of antipsychotics: Curr Opin Psychiatry. 2010;23(6):574–581. doi: 10.1097/YCO.0b013e32833f46c9. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association Choosing wisely: five things physicians and patients should question. 2013. Available at: http://www.choosingwisely.org/wp-content/uploads/2013/09/102913_F64_46-APA-5things-List_Draft-5.pdf. Accessed June 20, 2014.
- 28.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213. doi: 10.1093/schbul/sbs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- 30.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 31.Spears NM, Leadbetter RA, Shutty MS. Clozapine treatment in polydipsia and intermittent hyponatremia. J Clin Psychiatry. 1996;57(3):123–128. [PubMed] [Google Scholar]
- 32.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of anti-psychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 33.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Meltzer L, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Nyberg S, Eriksson B, Oxenstierna G, et al. Suggested minimal effective dose of risperidone based on PET measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156(6):869–875. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 36.Haddad PM, Dursun SM. Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol. 2008;23(suppl 1):15–26. doi: 10.1002/hup.918. [DOI] [PubMed] [Google Scholar]
- 37.Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf. 2005;28(3):191–208. doi: 10.2165/00002018-200528030-00002. [DOI] [PubMed] [Google Scholar]
- 38.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26(5):649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- 39.Monteleone P, Martiadis V, Maj M. Management of schizophrenia with obesity, metabolic and endocrinological disorders. Psychiatr Clin North Am. 2009;32(4):775–794. doi: 10.1016/j.psc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Correll CU, Manu P, Olshanskiy, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiden PJ, Ross R. Why do patients stop their antipsychotic medications? A guide for family and friends. J Psychiatr Pract. 2002;8(6):413–416. doi: 10.1097/00131746-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JM. Cardiovascular illness and hyperlipidemia in patients with schizophrenia. In: Meyer JM, Nasarallah HA, editors. Medical Illness and Schizophrenia. Washington, D.C: American Psychiatric Press; 2003. pp. 53–80. [Google Scholar]
- 43.Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):3–14. doi: 10.1002/hup.915. [DOI] [PubMed] [Google Scholar]
- 44.Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1–11. doi: 10.1016/j.schres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Nasrallah HA. Metabolic findings from the CATIE trial and their relation to tolerability. CNS Spectr. 2006;11(suppl 7):32–39. doi: 10.1017/s1092852900026663. [DOI] [PubMed] [Google Scholar]
- 46.Caplan JP, Epstein LA, Quinn DK, et al. Neuropsychiatric effects of prescription drug abuse. Neuropsychol Rev. 2007;17(3):363–380. doi: 10.1007/s11065-007-9037-7. [DOI] [PubMed] [Google Scholar]
- 47.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drug use and the risk of heat-related hospitalization. Eur Psychiatry. 2007;22(6):335–338. doi: 10.1016/j.eurpsy.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127–141. doi: 10.2165/00002018-200831020-00003. [DOI] [PubMed] [Google Scholar]
- 50.Fraunfelder FW. Twice-yearly exams unnecessary for patients taking quetiapine. Am J Ophthalmol. 2004;138(5):870–871. doi: 10.1016/j.ajo.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 51.Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging. 2008;25(7):541–549. doi: 10.2165/00002512-200825070-00001. [DOI] [PubMed] [Google Scholar]
- 52.Knegtering H, van der Moolen AE, Castelein S, et al. What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology. 2003;28(suppl 2):109–123. doi: 10.1016/s0306-4530(02)00130-0. [DOI] [PubMed] [Google Scholar]
- 53.Hall RL, Smith AG, Edwards JG. Haematologic safety of antipsychotic drugs. Exp Opin Drug Saf. 2003;2(4):395–399. doi: 10.1517/14740338.2.4.395. [DOI] [PubMed] [Google Scholar]
- 54.Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23(suppl 1):27–41. doi: 10.1002/hup.917. [DOI] [PubMed] [Google Scholar]
- 55.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 56.Lysaker PH, Buck KD. Is recovery from schizophrenia possible? An overview of concepts, evidence, and clinical implications. Prim Psychiatry. 2008;15(6):60–65. [Google Scholar]
- 57.Miller AL, Chiles JA, Chiles JK, et al. The TMAP schizophrenia algorithms. J Clin Psychiatry. 1999;60(10):649–657. doi: 10.4088/jcp.v60n1002. [DOI] [PubMed] [Google Scholar]
- 58.Spina E, de Leon J. Metabolic drug interactions with newer anti-psychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007;100(1):4–22. doi: 10.1111/j.1742-7843.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 59.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]