Abstract
Pancreatic cancer is a lethal disease for which only a small number of risk factors have been identified. In addition to older age, male gender, and black race, risk factors include smoking, obesity, long-standing diabetes and pancreatitis, and heavy alcohol use; allergies such as hay fever are related to lowered risk. Several genetic syndromes increase risk of pancreatic cancer. Work on more common genetic variants promises to reveal more potentially important genetic associations.
Keywords: epidemiology, smoking, obesity, diabetes, allergies, genetic syndromes
Incidence and Mortality
In the USA, nearly 44,000 new cases of pancreatic cancer are expected in 2012 [1]; the age-adjusted incidence rate in 2008 was 12.0 per 100,000, reflecting a slight but statistically significant increase in the 2000–2008 period [2]. Mortality is almost as high as incidence, 10.9 per 100,000 [3], with more than 37,000 deaths expected in 2012 [1]. In the USA, pancreatic cancer ranks fourth in causes of death from cancer. In other developed countries, incidence rates are similar to those in the USA, while they are considerably lower in less developed countries: With the world age distribution used for standardization, age-adjusted rates are 7.0 per 100,000 in the USA, 6.7 in other developed countries, and 2.4 in less developed countries (Fig. 1, [4]).
Fig. 1.
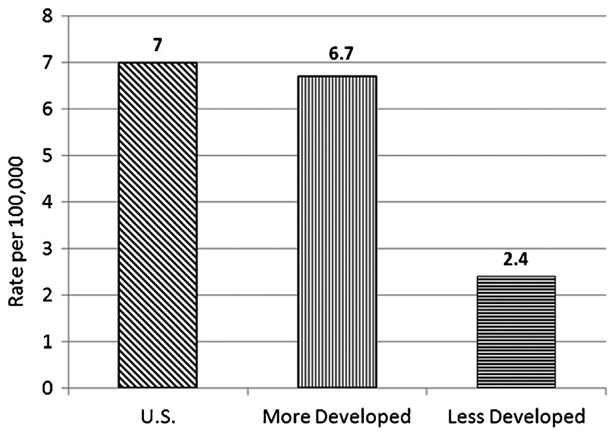
International Incidence Rates. Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. GLOBOCAN 2008v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr, accessed on 10/April/2012.
Risk Factors for Pancreatic Cancer
Age, Gender, and Race
The strongest risk factor for pancreatic cancer is increasing age. In the USA, the median age at diagnosis is 72 [2]; no other cancer has a higher median age. Risk is low (10.4 per 100,000) in those aged 50–54, increasing sharply to 73.5 per 100,000 in those aged 75–79 [3] and continuing to increase in older individuals (Fig. 2). Risk is higher in men (13.5 per 100,000) than in women (10.8 per 100,000) (Fig. 3); the difference between men and women probably reflects smoking habits. Risk is considerably higher in blacks than in non-Hispanic whites in the USA (15.8 vs. 12.0 per 100,000) and somewhat lower in Asians (9.5 per 100,000) and Hispanics (10.7 per 100,000) [3]. Because of different age distributions in the USA for racial and ethnic groups, the actual number of minority patients, including blacks, with pancreatic cancer is quite small [5], making it difficult to study risk factors or outcomes separately in subgroups. Examination of possible reasons for the racial discrepancy in incidence indicated that reasons differed for men and women, but that most of the difference between blacks and whites were explained by established or suspected risk factors [6].
Fig. 2.
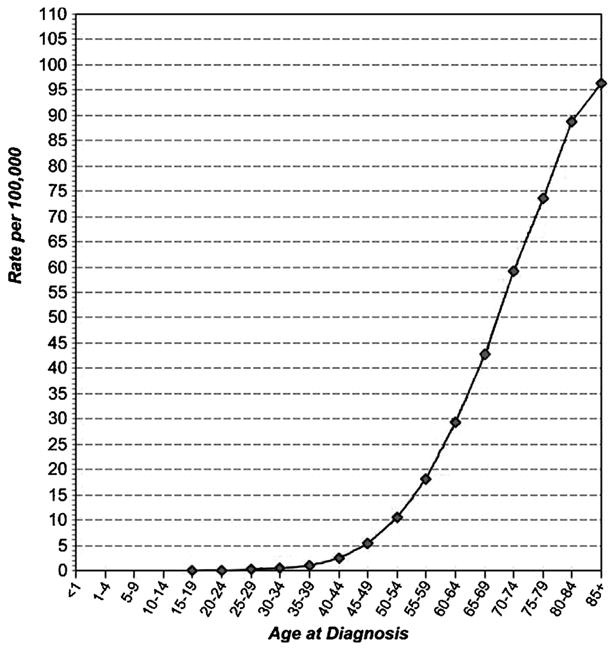
Age-Specific (Crude) SEER Incidence Rates By Cancer Site, All Ages, All Races, Both Sexes, 2000-2008 (SEER 17). Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats. (Accessed on 4-10-2012).
Fig. 3.
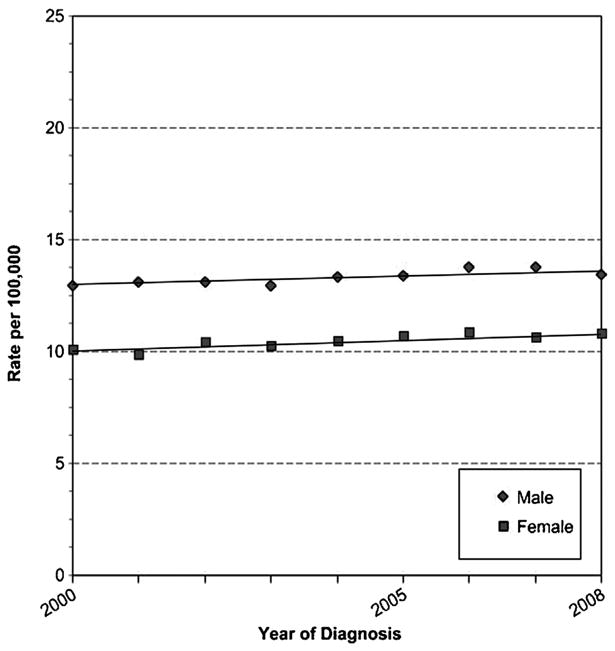
Age-Adjusted SEER Incidence Rates By Sex, Pancreas, All Ages, All Races, 2000-2008 (SEER 17). Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute; http://seer.cancer.gov/faststats. (Accessed on 4-10-2012).
Smoking
Cigarette smoking is the strongest environmental risk factor. A recent meta-analysis of 82 studies, including both case-control and cohort designs, reported a 75% increased risk of pancreatic cancer in current smokers (OR = 1.74, 95% CI 1.61–1.87) [7]. The same result was found in a pooled analysis of 13 studies, including 12 cohorts [8] while somewhat higher risk was found in an analysis of 12 case-control studies, with a more than twofold increase in risk for current smokers [9]. Results from case-control studies are more likely than those from cohort studies to reflect smoking status at the time of diagnosis, since smoking cessation reduces risk and those who smoked at baseline in cohort studies may have quit at a later time. Studies have shown varying results with respect to the length of time required after smoking cessation for risk to match that of those who have never smoked, with most studies finding periods of 10 [7,10] to 15 or more years [8,9] required. Passive smoking (environmental tobacco smoke) among those who have never smoked appears to have little influence on risk [10,11], although some studies have noted a positive association [12].
Obesity
The association between overweight and obesity and pancreatic cancer was highlighted in the landmark 2003 study from the American Cancer Society's prospective Cancer Prevention Study II on overweight and obesity and risk of death from cancer [13]. With 16 years of follow-up, during which 3,358 deaths due to pancreatic cancer were identified in cohort members, risks increased steadily for both men and women as baseline body mass index (BMI) increased above the normal range. In men, death due to pancreatic cancer was 50% higher among those with class II obesity (BMI ≥35). Among women, risk was strongly increased among those with class III obesity (BMI >40): RR = 2.76, 95% CI 1.74-4.36). A more recent analysis, with 2,135 cases pooled from 14 cohort studies [14], supported these findings, with the obese 47% more likely to be diagnosed with pancreatic cancer. Weight gain between early adulthood and cohort entry was also related to increased risk. An association with weight in early adulthood was also reported in a large case-control study [15]. From pooled analyses of data from cohort studies, there is some evidence that central adiposity is also associated with greater risk [14,16]. Some large studies and pooled analyses have suggested that risk associated with high BMI is stronger in non-smokers [13,14,16,17], but other studies have not found this relationship [15]. Although physical activity is related to obesity, the literature on association of physical activity with risk of pancreatic cancer is heterogeneous and there is no clear association [18].
Diabetes
While an association between presence of diabetes and risk of pancreatic cancer is a consistent finding in epidemiologic studies, the results differ markedly according to the length of time between diagnosis of diabetes and diagnosis of pancreatic cancer, with the highest association for diabetes diagnosed close to the time of cancer diagnosis, and only moderately increased risk with longer duration of diabetes. Two recent meta-analyses, one including 35 cohort studies [19] and the other including 19 cohort and 17 case-control studies [20] observed an increased risk of almost 2 overall (RR = 1.94, 95% CI 1.66-2.27; RR = 1.82 (95% CI 1.66-1.99, respectively). In both analyses, for those studies that evaluated risk according to the timing of diabetes and cancer, risk was increased about 50% for those with diabetes diagnosed ≥10 years earlier. Similar results were found in a pooled analysis of three large case-control studies [21], with threefold increased risk for diabetes diagnosed within 2 years before cancer diagnosis, and 30-40% increased risk for long-term diabetes (>10 years). Although the association between diabetes and pancreatic cancer could be confounded by the effects of high BMI and smoking, this does not appear to explain the results [19,21]. The relatively high-risk estimates for diabetes diagnosed close to the time of cancer diagnosis is likely to represent reverse causality, with cancer-related alterations to pancreatic function leading to diabetes. A study in the Mayo clinic population showed an increase in prospectively measured plasma glucose beginning 24 months before diagnosis [22], indicating that new-onset diabetes is likely to be caused by the tumor and could potentially be a means of identifying early disease. A small number of studies have reported that use of metformin in those with diabetes is related to reduced risk of pancreatic cancer [23-25]; the most recent study [25] found the effect only among women, for reasons that are unclear.
Pancreatitis
Pancreatitis is both an established risk factor for pancreatic cancer and an early indicator of the presence of cancer. Studies that have investigated the timing of pancreatitis relative to that of cancer have found that risk is highest in the year before cancer diagnosis, suggesting that the cancer may be initially diagnosed as pancreatitis. At the same time, studies that have investigated risk with many years between diagnoses have also found that risk is increased [26]. Increased risk for long-term disease has also been found in retrospective cohorts of individuals with pancreatitis [27-29]. In spite of this association, the prevalence of pancreatitis in the population is very low, making it unlikely to that many cases of pancreatic cancer are attributable to this condition. Hereditary pancreatitis attributable to known genetic mutations is discussed below in the section on family history.
Alcohol
Light and moderate consumption of alcohol has not been found to be related to increased risk of pancreatic cancer, while heavy consumption is likely to increase risk [30]. Pooled analyses of 10 case-control studies and 14 cohort studies resulted in ORs of 1.5 (95% CI 1.2-1.8) for consumption of ≥6 drinks per day (≥84 g per day) [31] and 1.22 (95% CI 1.03-1.45) for consumption of ≥30 g of alcohol per day [32], respectively. Alcohol consumption is associated with cigarette smoking; in addition, heavy alcohol consumption is a common cause of pancreatitis, possibly making it difficult to disentangle the effects of these exposures on risk.
Allergies
A number of epidemiologic studies have investigated associations between self-reported allergies and various types of cancer [33,34]; reduced risk for pancreatic cancer is among the most consistent findings. A meta-analysis of 14 pancreatic cancer studies showed a 30% reduced risk in those with any allergies and a 45% reduced risk in those with respiratory allergies such as hay fever in studies with direct interviews rather than proxies [35]. Since the publication of the meta-analysis, three new case-control studies [36-38], one expanded case-control study [39], and a cohort study [40] have supported these results. A recent review of 11 published studies that reported on risks associated with any allergy [26] found statistically significantly reduced risk in most of the studies, and consistently reduced risk in those with respiratory allergies such as hay fever and allergies to plants or pollen. Other allergies, such as those to foods and medications, have been less well studied and associations with risk are unclear. Although asthma is often found in conjunction with respiratory allergies, it is not consistently associated with risk and several studies have shown no association [26,35].
In contrast, studies conducted in Sweden [41,42] and the USA [43] in cohorts of people with allergies have not found evidence that self-reported allergies, skin prick tests, or IgE levels were associated with decreased risk of cancer overall or with specific cancers, including pancreatic cancer. Results from these cohort studies remain inconclusive because the patients studied were young and follow up was only for up to 13 years, resulting in only a handful of cases; in addition, data were not always available to adjust for potential confounding factors such as smoking.
Genetic Polymorphisms
Several known genetic mutations leading to inherited susceptibility to pancreatic cancer are discussed in the section below on family history. In addition to these established factors that confer high risk but are rare in the population, recent genome-wide association studies (GWAS) have been successful in identifying more common germline variants that are related to risk of pancreatic cancer. In the first of the PanScan studies conducted by the National Cancer Institute with DNA from several cohort and case-control studies [44], the strongest association was with variants that determine ABO blood type. Increased risk for non-O blood types had been noted in earlier studies [45,46], and recent work based on genotypes has confirmed that risk increases with each non-O allele: Risk is higher for the AA genotype than for AO, and higher for BB than for BO [47]. An interaction between ABO blood type and presence of H pylori [48] has been reported; there have been mixed results among studies that have investigated H pylori and risk, but the weight of evidence is that this exposure is related to increased risk [49].
GWAS studies in Caucasian [44,50] and Asian populations [51,52] also identified several loci at or near genes that had not previously been implicated in pancreatic cancer risk. A study using a pleiotropy approach, including only SNPs previously found to be associated with other cancers or diseases, identified risk associated with HNF1A, a gene related to diabetes and other traits [53]. The influence of genetic variants on risk of pancreatic cancer is an area of active research, with ongoing studies including pathways analyses and functional studies as well as new GWAS.
Family History and Pancreatic Cancer Risk
For several decades the inherited susceptibility to pancreatic cancer has been described in multiple case reports. Clustering of pancreatic cancer in families is illustrated by an early report of the occurrence of pancreatic cancer in three women in successive generations who, over 11 years, developed pancreatic cancer each at a younger age [54]. The authors speculated that inheritance may play a role in some pancreatic cancers. In 1985 Lynch et al. [55] originally described five generations of a family with early age of onset of multiple right-sided colon cancers and pancreatic cancer. The occurrence of pancreatic cancer in the family was felt to be “enigmatic.” This family was felt to represent hereditary non-polyposis colon cancer and this report was an early description of what we now believe to be a specific genetic susceptibility to pancreatic cancer.
Familial Pancreatic Cancer
It is now felt that two broad categories of hereditary risk for pancreatic cancer can be defined. Familial pancreatic cancer is defined as an inherited predisposition based on family clustering in families in which there are multiple first and second degree relatives with ductal pancreatic adenocarcinoma in the absence of a known genetic susceptibility syndrome. A family history of pancreatic cancer is seen in between 5–10% of individuals with pancreatic cancer [56]. As pancreatic cancer is a relatively common cancer, more than one family member may be affected solely by chance alone. There also may be common environmental factors, such as smoking or high BMI, or indeed, an as yet unidentified common genetic factor. Having multiple family members with pancreatic cancer increases the risk of non-affected family members developing the disease. This is especially true when multiple first-degree relatives have pancreatic cancer. When two or more first-degree relatives are affected, one study showed the odds ratio to be 4.26 (95% CI = 0.48–37.79) [57]. Scientists at Johns Hopkins using their National Familial Pancreas Tumor Registry found that when at least one pair of first degree relatives were affected with pancreatic cancer, the risk of developing pancreatic cancer was 18 times that for relatives of an individual with only one sporadic pancreatic cancer. In those pancreatic cancer kindreds with three or more affected family members there was a 57-fold (95% CI = 12.4–175) increased risk of pancreatic cancer [58]. It has become clear that in these familial pancreatic cancer families, the risk of developing pancreatic cancer is closely tied to the number of first-degree relatives in the family with the disease.
Germline Mutations and Pancreatic Cancer
Pancreatic cancer also occurs in the setting of well defined cancer syndromes, so-called cancer susceptibility syndromes, where an identifiable germline mutation may lead to the development of pancreatic adenocarcinoma.
BRCA 1 and BRCA2
Perhaps one of the most well known of the cancer predisposition syndromes is the Hereditary Breast and Ovarian Cancer Syndrome. Women who carry gene mutations of the tumor suppressor genes, BRCA1 or BRCA2, have very high lifetime risks for breast and ovarian cancers. The risk of cancers other than breast and ovarian cancer was investigated by a consortium of 20 centers in Europe and North America, in which 173 breast-ovarian cancer families with BRCA2 mutations were identified. Several cancers were found to have a statistically significant increased risk. These included prostate, gallbladder, bile duct, stomach, and pancreas. For pancreatic cancer the relative risk was 3.51 (95% CI = 1. 87–6.58) [59]. Using direct sequencing of constitutional DNA, Murphy et al. [60] analyzed samples from patients with pancreatic cancer who were enrolled in their Familial Pancreatic Tumor Registry for mutations in four tumor suppressor candidate genes including BRCA2. Samples were taken from families in which three or more family members were affected with pancreatic cancer, and at least two were first-degree relatives. BRCA2 gene sequencing identified five mutations believed to be deleterious (17.2%). Three patients harbored the 6174delT frameshift mutation. These findings confirm the increased risk of pancreatic cancer in individuals with BRCA2 mutations. The authors also concluded that germline BRCA2 mutations are the most common inherited genetic alteration in familial pancreatic cancer. In a study by Lynch et al. [61], pancreatic cancer was seen in nine of 15 families where the BRCA1 mutation was either confirmed or inferred. Thompson et al. [62] analyzed data on 11,847 individuals from 699 families. The overall increased risk of cancer in those with deleterious BRCA1 mutations at sites other than breast and ovary is small. BRCA1 mutations may confer increased risks of other abdominal cancers in women and increased risks of pancreatic cancer in men and women.
PALB2
Rahman et al. [63] demonstrated that truncating mutations in PALB2 (partner and localizer of BRCA2) in individuals with familial breast cancer confer a 2.3-fold higher risk of the disease [64]. The group at Johns Hopkins identified such a mutation in a patient with familial pancreatic cancer and found an additional three patients with pancreatic cancer and PALB2 mutations in 96 studied [65].
Lynch Syndrome—Hereditary Nonpolyposis Colon Cancer (HNPCC)
Lynch syndrome is an autosomal dominant condition caused by mutations in the mismatch repair genes MLH1, MSH2, MSH6, and PMS2. Colorectal and endometrial cancers are the most commonly described cancers in this syndrome [66]. Studies of the risk of pancreatic carcinoma in Lynch syndrome patients when compared to the general population have produced variable results. Pancreatic cancer has been included as an HNPCC-associated tumor in the revised Bethesda guidelines [67]. Geary et al. [68] reviewed the family histories of 130 individuals with documented mismatch repair gene mutations. They found 22 cases of pancreatic cancer; half were in proven or obligate carriers. The risk as defined by their study was about seven times expected. Young age of onset was also characteristic, with 14 of 20 with a known age being under the age of 60. Kastrinos et al. [69] found 31 out of 147 families with mismatch repair gene mutations where there was at least one reported case of pancreatic cancer. The cumulative risk of pancreatic cancer in their families with gene mutations was calculated to be 1.31% (0.31–2.32%) in young individuals up to age 50. This rose to 3.68% (1.45–5.88%) up to age 70, an 8.6-fold increase over the general population. It has been suggested that pancreatic cancer occurring in individuals with Lynch Syndrome may have a somewhat better prognosis. Yamamoto et al. [70] described three Lynch syndrome patients with high levels of microsatellite instability in their pancreatic cancers. These individuals had a significantly longer overall survival than pancreatic cancer patients with low tumor levels of microsatellite instability or patients with microsatellite stable tumors.
Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM)
The familial atypical multiple mole melanoma syndrome is caused by a germline mutation in the tumor suppressor gene CDKN2A (p16) on chromosome 9. Mutations in CDKN2A occur in about 20% of familial melanoma families in North America, Asia, Australia, and Europe [71]. Several studies have demonstrated an increased risk of pancreatic cancer among CDKN2A melanoma-prone families [72,73]. In the Dutch FAMMM registry, pancreatic cancer was found to be the second most common cancer in mutation carriers after melanoma [74]. Vasen et al. [75] reported on a surveillance program for individuals with germline mutations of p16-Leiden. It is estimated that carriers of the mutated gene have a lifetime risk of 15–20% of developing pancreatic cancer. Using MRI and MRCP as the surveillance tools, approximately 9% of the 79 people studied were found to have developed pancreatic cancer.
Peutz–Jeghers Syndrome
Peutz–Jeghers syndrome is an autosomal dominant disease characterized by hamartomatous polyps of the gastrointestinal tract and by mucocutaneous melanin deposits and caused by germline mutations of STK11 (serine threonine kinase 11). These mutations can be found in the majority (66–94%) of Peutz–Jeghers syndrome patients [76]. In 1987, Giardiello et al. [77] described the increased risk of cancer in patients with Peutz–Jeghers syndrome. Relative-risk analysis demonstrated that the development of cancer in Peutz–Jeghers syndrome patients was 18 times greater than expected in the general population, and the cumulative lifetime risk for pancreatic cancer from age 15 to 64 was 36%. Ninety-six cancers were found in a study of 419 individuals with Peutz–Jeghers syndrome of which 297 had documented STK11/LKB1 mutations. The most frequent cancers represented in this analysis were gastroesophageal, small bowel, colorectal, and pancreatic. Hearle et al. [78] found 96 cancers in 297 individuals with Peutz–Jeghers syndrome with germline mutations in STK11/LKB1. Here again, multiple sites of gastrointestinal cancers were seen which included pancreatic cancer. The issue of ascertainment bias inflating the overall cancer risk was raised by Giardeillo et al. [76].
Familial Adenomatous Polyposis (FAP)
Familial adenomatous polyposis is an autosomal dominantly inherited disorder caused by germline mutation of the adenomatous polyposis coli (APC) gene on chromosome 5q. While colorectal cancer is the major cancer risk in affected individuals, the relative risk of other cancers such as thyroid and pancreas has also been described [79]. A study using the Johns Hopkins Polyposis registry found four patients with pancreatic cancer in their cohort of 1,391 patients with FAP. Patients with FAP are now surviving longer due to early identification and management of their colorectal cancer risk; as a result, more frequent diagnoses of extracolonic cancers, such as pancreatic cancer can be expected [80].
Hereditary Pancreatitis
Hereditary pancreatitis is an autosomal dominant disease. Acute pancreatitis begins early and can lead to chronic pancreatitis. The disease has variable expression and penetrance is estimated to be about 80% [81]. In 1996, Whitcomb et al. [82] reported that an arginine–histidine substitution at residue 117 of the cationic trypsinogen gene was likely the cause of hereditary pancreatitis. The gene for hereditary pancreatitis was found to map to chromosome 7q35 [83]. Chronic pancreatitis has been linked to pancreatic cancer, and an international multi-institutional study estimated the risk of pancreatic cancer in affected individuals in hereditary pancreatitis cohorts to be about 40% by age 70 [81].
Management of Individuals With Pancreatic Cancer Risk Due to Family History
Management of high-risk individuals from pancreatic cancer families remains an important issue. A number of familial pancreatic cancer registries have been developed throughout the United States and the world [84]. Screening programs attempting to identify these pancreatic lesions in at-risk healthy family members are underway and several have begun to report their preliminary findings. Canto et al. [85] at Johns Hopkins, utilizing endoscopic ultrasound and endoscopic retrograde cholangiopancreatography (ERCP), initially reported diagnostic yield of 5.3% in a pilot study of 38 patients. One invasive cancer was detected. In a second report from [86], seven high-risk individuals were identified with pathologically confirmed intraductal papillary mucinous neoplasms (IPMNs) and one patient had a pancreatic intraepithelial neoplasm (PanIN) for a diagnostic yield of 10%. In a similar screening program in our Memorial Sloan-Kettering Cancer Center Familial Pancreatic Tumor Registry, utilizing cross-sectional imaging (magnetic resonance cholangiopancreatography (MRCP) and CT), we found IMPNs in eight of 113 healthy at-risk individuals [87]. Langer et al. [88] recently published their results of screening 76 individuals from 34 familial pancreatic cancer families culled from the German National Collection for Familial Pancreatic Cancer. Ten individuals were found to have solitary pancreatic lesions and seven of these underwent surgery. No cancers were identified and only one IPMN was found for a low diagnostic yield of 1.3%.
Recommendations of the Fourth International Symposium of Inherited Diseases of the Pancreas are that screening of familial pancreatic cancer family members is appropriate in the context of a clinical trial [89]. Their recommendation is for screening of healthy individuals with at least three affected first degree relatives and in those individuals with a known BRCA2 mutation and at least one family member with pancreatic cancer. The age when screening should begin is at age 50 or 10 years younger than the youngest affected family member. The frequency of screening remains an unanswered question, but genetic counseling is strongly recommended.
References
- 1.American Cancer Society. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2011. SEER cancer statistics review, 1975-2008. http://seercancergov/csr/1975_2008/based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Surveillance Research Program. National Cancer Institute; [Accessed on 4-10-2012]. Fast Stats: An interactive tool for access to SEER cancer statistics. http://seer.cancer.gov/faststats. [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Olson SH, Layne TM, Simon JA, et al. Studying cancer in minorities: A look at the numbers. Cancer. 2011;117:2762–2769. doi: 10.1002/cncr.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman DT, Hoover RN, Brown LM, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbeck's archives of surgery/Deutsche Gesellschaft fur Chirurgie. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 8.Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2011 doi: 10.1093/annonc/mdr541. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tranah GJ, Holly EA, Wang F, et al. Cigarette, cigar and pipe smoking, passive smoke exposure, and risk of pancreatic cancer: A population-based study in the San Francisco Bay Area. BMC Cancer. 2011;11:138. doi: 10.1186/1471-2407-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinen MM, Verhage BA, Goldbohm RA, et al. Active and passive smoking and the risk of pancreatic cancer in the Netherlands Cohort Study. Cancer Epidemiol Biomarkers Prev. 2010;19:1612–1622. doi: 10.1158/1055-9965.EPI-10-0121. [DOI] [PubMed] [Google Scholar]
- 12.Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2394–2403. doi: 10.1002/ijc.24907. [DOI] [PubMed] [Google Scholar]
- 13.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 14.Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129:1708–1717. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: A pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21:1305–1314. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedenreich CM, Cook LS, Magliocco AM, et al. Case-control study of lifetime total physical activity and endometrial cancer risk. Cancer Causes Control. 2010;21:1105–1116. doi: 10.1007/s10552-010-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: A pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22:189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318–2325. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 25.Bodmer M, Becker C, Meier C, et al. Use of antidiabetic agents and the risk of pancreatic cancer: A case-control analysis. Am J Gastroenterol. 2012;107:620–626. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 26.Olson SH. Selected medical conditions and risk of pancreatic cancer. Mol Carcinog. 2012;51:75–97. doi: 10.1002/mc.20816. [DOI] [PubMed] [Google Scholar]
- 27.Talamini G, Falconi M, Bassi C, et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253–1260. doi: 10.1111/j.1572-0241.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P, Cavallini G, et al. N Engl J Med. Vol. 328. International Pancreatitis Study Group; 1993. Pancreatitis and the risk of pancreatic cancer; pp. 1433–1437. [DOI] [PubMed] [Google Scholar]
- 29.Karlson BM, Ekbom A, Josefsson S, et al. The risk of pancreatic cancer following pancreatitis: An association due to confounding? Gastroenterology. 1997;113:587–592. doi: 10.1053/gast.1997.v113.pm9247480. [DOI] [PubMed] [Google Scholar]
- 30.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51:40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 31.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23:374–382. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genkinger JM, Spiegelman D, Anderson KE, et al. Alcohol intake and pancreatic cancer risk: A pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–776. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60:1098–1111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner MC, Chen Y, Krewski D, et al. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 35.Gandini S, Lowenfels AB, Jaffee EM, et al. Allergies and the risk of pancreatic cancer: A meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2005;14:1908–1916. doi: 10.1158/1055-9965.EPI-05-0119. [DOI] [PubMed] [Google Scholar]
- 36.Olson SH, Orlow I, Simon J, et al. Allergies, variants in IL-4 and IL-4R alpha genes, and risk of pancreatic cancer. Cancer Detect Prev. 2007;31:345–351. doi: 10.1016/j.cdp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Lo AC, Soliman AS, El-Ghawalby N, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas. 2007;35:120–129. doi: 10.1097/mpa.0b013e318053e7d3. [DOI] [PubMed] [Google Scholar]
- 38.Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control. 2009;20:825–834. doi: 10.1007/s10552-009-9303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maisonneuve P, Lowenfels AB, Bueno-de-Mesquita HB, et al. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann Epidemiol. 2010;20:92–98. doi: 10.1016/j.annepidem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Turner MC, Chen Y, Krewski D, et al. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162:212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 41.Lindelof B, Granath F, Tengvall-Linder M, et al. Allergy and cancer. Allergy. 2005;60:1116–1120. doi: 10.1111/j.1398-9995.2005.00808.x. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson NE, Holmen A, Hogstedt B, et al. A prospective study of cancer incidence in a cohort examined for allergy. Allergy. 1995;50:718–722. doi: 10.1111/j.1398-9995.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 43.McWhorter WP. Allergy and risk of cancer. A prospective study using NHANES I followup data. Cancer. 1988;62:451–455. doi: 10.1002/1097-0142(19880715)62:2<451::aid-cncr2820620234>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macafee AL. ABO blood groups and carcinoma of pancreas. Ulster Med J. 1964;33:129–131. [PMC free article] [PubMed] [Google Scholar]
- 46.Aird I, Lee DR, Roberts JA. ABO blood groups and cancer of oesophagus, cancer of pancreas, and pituitary adenoma. Br Med J. 1960;1:1163–1166. doi: 10.1136/bmj.1.5180.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolpin BM, Kraft P, Gross M, et al. Pancreatic cancer risk and ABO blood group alleles: Results from the pancreatic cancer cohort consortium. Cancer Res. 2010;70:1015–1023. doi: 10.1158/0008-5472.CAN-09-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Risch HA. Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol Carcinog. 2012;51:109–118. doi: 10.1002/mc.20826. [DOI] [PubMed] [Google Scholar]
- 49.Trikudanathan G, Philip A, Dasanu CA, et al. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis JOP. 2011;12:26–31. [PubMed] [Google Scholar]
- 50.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet. 2012;44:62–66. doi: 10.1038/ng.1020. [DOI] [PubMed] [Google Scholar]
- 52.Low SK, Kuchiba A, Zembutsu H, et al. Genome-wide association study of pancreatic cancer in Japanese population. PLoS Genet. 2010;5:e11824. doi: 10.1371/journal.pone.0011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce BL, Ahsan H. Genome-wide “pleiotropy scan” identifies HNF1A region as a novel pancreatic cancer susceptibility locus. Cancer Res. 2011;71:4352–4358. doi: 10.1158/0008-5472.CAN-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrenthal D, Haeger L, Griffin T, et al. Familial pancreatic adenocarcinoma in three generations. A case report and a review of the literature Cancer. 1987;59:1661–1664. doi: 10.1002/1097-0142(19870501)59:9<1661::aid-cncr2820590923>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 55.Lynch HT, Voorhees GJ, Lanspa SJ, et al. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: A family study. Br J Cancer. 1985;52:271–273. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg. 2010;44:293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: A pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 2010;127:1421–1428. doi: 10.1002/ijc.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–744. [PubMed] [Google Scholar]
- 59.Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 60.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 61.Lynch HT, Deters CA, Snyder CL, et al. BRCA1 and pancreatic cancer: Pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005;158:119–125. doi: 10.1016/j.cancergencyto.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 62.Thompson D, Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 63.Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tischkowitz MD, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–1186. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch HT, Lanspa SJ, Boman BM, et al. Hereditary nonpolyposis colorectal cancer–Lynch syndromes I and II. Gastroenterol Clin N Am. 1988;17:679–712. [PubMed] [Google Scholar]
- 67.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geary J, Sasieni P, Houlston R, et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC) Fam Cancer. 2008;7:163–172. doi: 10.1007/s10689-007-9164-6. [DOI] [PubMed] [Google Scholar]
- 69.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto H, Itoh F, Nakamura H, et al. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001;61:3139–3144. [PubMed] [Google Scholar]
- 71.Goldstein AM. Familial melanoma, pancreatic cancer and germline CDKN2A mutations. Hum Mutat. 2004;23:630. doi: 10.1002/humu.9247. [DOI] [PubMed] [Google Scholar]
- 72.Bergman W, Watson P, de Jong J, et al. Systemic cancer and the FAMMM syndrome. Br J Cancer. 1990;61:932–936. doi: 10.1038/bjc.1990.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergman W, Gruis N. Familial melanoma and pancreatic cancer. N Engl J Med. 1996;334:471–472. [PubMed] [Google Scholar]
- 74.Vasen HF, Gruis NA, Frants RR, et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809–811. [PubMed] [Google Scholar]
- 75.Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850–856. doi: 10.1053/j.gastro.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 76.Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. 2006;4:408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- 78.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 79.Giardiello FM, Offerhaus GJ, Lee DH, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groen EJ, Roos A, Muntinghe FL, et al. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol. 2008;15:2439–2450. doi: 10.1245/s10434-008-9981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 82.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 83.Whitcomb DC, Preston RA, Aston CE, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- 84.Greenhalf W, Malats N, Nilsson M, et al. International registries of families at high risk of pancreatic cancer. Pancreatology. 2008;8:558–565. doi: 10.1159/000159214. [DOI] [PubMed] [Google Scholar]
- 85.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: An EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 86.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 87.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–954. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 89.Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]


