Abstract
Immune complex accumulation in the kidney is the hallmark of lupus nephritis and triggers a series of events that result in kidney inflammation and injury. Cytotoxic agents and corticosteroids are standard of care for lupus nephritis treatment, but are associated with considerable morbidity and suboptimal outcomes. Recently, there has been interest in using novel biologic agents and small molecules to treat lupus nephritis. These therapies can be broadly categorized as anti-inflammatory (laquinamod, anti–tumor necrosis factor–like weak inducer of apotosis, anti-C5, and retinoids), antiautoimmunity (anti-CD20, anti–interferon α, and costimulatory blockers), or both (anti–interleukin 6 and proteasome inhibitors). Recent lupus nephritis clinical trials applied biologics or small molecules of any category to induction treatment, seeking short-term end points of complete renal response. These trials in general have not succeeded. When lupus nephritis comes to clinical attention during the inflammatory stage of the disease, the autoimmune stage leading to kidney inflammation will have been active for some time. The optimal approach for using novel therapies may be to initially target kidney inflammation to preserve renal parenchyma, followed by suppression of autoimmunity. In this review, we discuss novel lupus nephritis therapies and how they fit into a combinatorial treatment strategy based on the pathogenic stage.
Keywords: Lupus nephritis, systemic lupus erythematosus (SLE), novel therapies, biologics, small molecules
BACKGROUND
Corticosteroids plus cytotoxic agents have been the de facto standard of care for treatment of proliferative lupus nephritis for decades. Cyclophosphamide use became prevalent after a landmark National Institutes of Health trial demonstrated superiority over corticosteroids alone in preventing renal flares and kidney failure during long-term follow-up.1 By contrast, for the first 3-5 years after treatment initiation, the study showed that corticosteroids and cyclophosphamide were equally effective. Due to concerns related to cyclophosphamide toxicity, especially premature ovarian failure and predisposition to future malignancies, alternative lupus nephritis treatment regimens were designed using low-dose cyclophosphamide,2 substituting mycophenolate mofetil (MMF) for cyclophosphamide,3 or combining a calcineurin inhibitor with MMF and corticosteroids.4 Trials of these regimens compared short-term complete and partial remission rates to standard-dose cyclophosphamide. They did not evaluate long-term kidney survival, the outcome for which cyclophosphamide had been shown to be beneficial. Low-dose cyclophosphamide and MMF were found to be equivalent to standard cyclophosphamide, whereas multitarget therapy with cyclosporine, MMF, and corticosteroids appeared to be superior to cyclophosphamide for short-term remission induction. However, before they can be generally recommended, multi-target therapy and low-dose cyclophosphamide will have to be verified in multiracial/ethnic populations because the original trials included Asian and mainly white participants, respectively.
Long-term follow-up studies demonstrated good preservation of kidney function with low-dose cyclophosphamide.5 A 3-year follow-up of the original MMF trial, comparing MMF and azathioprine as maintenance therapies, showed a nonsignificant tendency for patients who underwent induction with cyclophosphamide to have had fewer long-term adverse kidney end points than those who underwent induction with MMF, regardless of the choice of maintenance immunosuppression.6 Of considerable concern is the fact that all of these regimens continue to have a disappointing complete remission rate.7
Recently, there has been excitement surrounding the development and implementation of biologics and small molecules for the treatment of lupus nephritis. The expectation has been that these therapies would target specific disease pathways, increasing treatment efficacy while decreasing undesirable side effects. To date, these expectations have not been realized in lupus nephritis trials. For example, the addition of rituximab or abatacept to MMF and corticosteroids did not improve the complete renal response rates of 25%-30% at 1 year compared to MMF and steroids alone.8,9
A number of factors may have confounded the clinical trials of new lupus nephritis therapies. For example, there is no standard definition of complete renal response. Although all trials assess similar clinical variables, such as proteinuria and kidney function, variations in how these variables are used in renal response criteria can profoundly affect the interpretation of trial results.9
Another concern regarding trials of novel therapeutics is whether trial outcomes were anticipated correctly. Lupus nephritis reaches clinical attention only after a threshold of glomerular and tubulointerstitial damage from intrarenal inflammatory processes has been reached. These inflammatory processes are due to autoimmune mechanisms that are set into motion well before the clinical diagnosis of lupus nephritis is established. We suggest that short-term kidney responses will be improved with anti-inflammatory therapies (Fig 1). In contrast, therapeutics that target the autoimmune mechanisms leading to kidney inflammation would be expected to prevent future lupus nephritis flares and preserve kidney function (Fig 1). To exemplify, a therapy designed to eliminate autoreactive B cells and decrease autoantibody production would not be anticipated to directly affect established kidney inflammation during a current flare and so should not improve the complete renal response rate at 6 or 12 months. In contrast, removing autoreactive B cells, and thus the source of autoantibodies, from the kidney interstitium or circulation would be expected to decrease the likelihood of future lupus nephritis activity. If these issues are taken into account during trial design, the response rate to novel therapeutics should improve. This review examines where novel biologic and small-molecule therapies fit into such a paradigm.
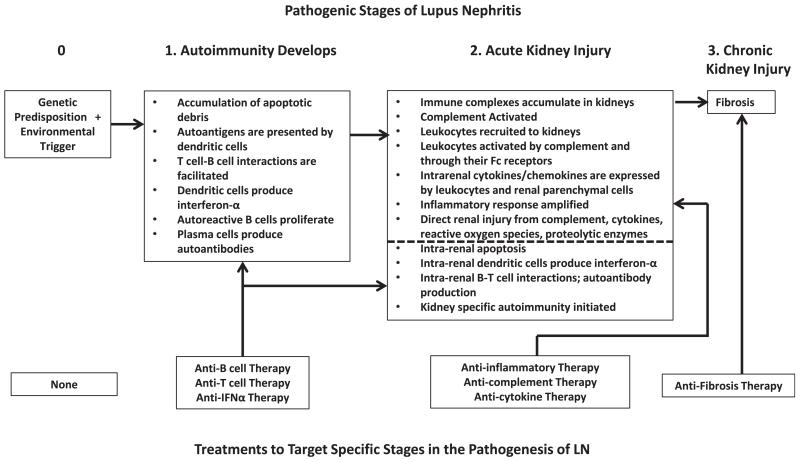
Figure 1.
The pathogenic stages of lupus nephritis (LN) as a guide to therapy. Systemic lupus erythematosus and LN (upper boxes) occur in patients with a genetic predisposition to autoimmunity and presumably an environmental trigger to initiate disease (stage 0). Because these patients cannot be identified with accuracy in the general population, there currently are no therapies (lower boxes) that can be applied at this stage. After initiation, autoimmunity develops as the pathogenic processes listed under stage 1 occur. This would be an ideal point to intervene with drugs that target these pathways, such as B- and T-cell–directed therapies, but patients usually do not have clinical manifestations during stage 1 and the disease is relatively silent. Kidney involvement reaches clinical attention during stage 2, and clinical manifestations are due primarily to inflammatory processes initiated within the kidney (shown in the stage 2 box, above the dotted line). The induction therapy of LN therefore must control inflammation. In addition to intrarenal inflammation, the autoimmune processes of stage 1 likely are still active and kidney-specific autoimmunity may be developing (stage 2 box, below the dotted line). Thus, in addition to anti-inflammatory therapy, the antiautoimmune therapies that were applied to stage 1 also can be used in stage 2. These therapies would contribute less to controlling inflammation, but ideally would prevent further LN flares. Active inflammation also can lead to scarring of the kidneys. Addition of an antifibrotic agent, especially if interstitial fibrosis or glomerulosclerosis were confirmed by biopsy or novel biomarker, could stabilize kidney function and decrease the rate of chronic kidney disease progression. Abbreviation: IFNα, interferon α.
CASE VIGNETTE
An 18-year-old African American woman was given a diagnosis of systemic lupus erythematosus (SLE) at age 16 years after developing a malar rash, polyarthritis, and leukopenia. She was found to have elevated antinuclear antibody and anti–double-stranded DNA (anti-dsDNA) titers and was treated with hydroxychloroquine and prednisone. Her serum creatinine level increased from 0.8 mg/dL (estimated glomerular filtration rate [eGFR], 125 mL/min/1.73 m2 using the CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] equation) to 1.5 mg/dL (eGFR, 59 mL/min/1.73 m2), and she developed new proteinuria with protein excretion of 3.5 g/d. Urinalysis showed acanthocytes and a few red blood cell casts. Kidney biopsy was interpreted as class IV-G (A) lupus nephritis with diffuse endocapillary proliferation, 30% cellular crescents, and severe tubulointerstitial inflammation, but no chronic lesions. She was treated with intravenous methylprednisolone, 1 g/d, for 3 days and then started on treatment with 1 mg/kg/d of prednisone and 1.5 mg/kg/d of oral cyclophosphamide. After 3 months, prednisone dosage was tapered to 10 mg/d, cyclophosphamide therapy was discontinued, and 2,000 mg/d of MMF was started. At month 6, serum creatinine level improved to 1.2 mg/dL (eGFR, 77 mL/min/1.73 m2), proteinuria decreased to protein excretion of 1.5 g/d, anti-dsDNA antibody levels decreased, and complement levels normalized. The patient remained on treatment with MMF and prednisone, and at month 12, serum creatinine level was 0.9 mg/dL (eGFR, 109 mL/min/1.73 m2), and protein excretion was 200 mg/d. Although still on maintenance MMF treatment at month 18, the patient reported fatigue, lower-extremity swelling, and foamy urine. Her proteinuria had increased to protein excretion of 3.0 g/d and serum creatinine level was 1.6 mg/dL (eGFR, 54 mL/min/1.73 m2). Kidney biopsy again showed diffuse proliferative lupus nephritis with active lesions, but new glomerulosclerosis and tubulointerstitial fibrosis was present, and the biopsy was now classified as IV-G (A/C) lupus nephritis.
This case illustrates that despite the use of the best available current lupus nephritis therapies and achieving an excellent clinical response, the kidney often sustains long-term damage (Fig 1; stage 2 of lupus nephritis) and maintenance of remission remains difficult.
PATHOGENESIS
The earliest step in the development of lupus nephritis is immune complex accumulation in the kidneys of patients with SLE, especially within glomeruli. These immune complexes initiate a series of events that result in kidney inflammation and kidney injury. Several mechanisms have been proposed to account for glomerular immune complex accumulation, including deposition of preformed circulating immune complexes, autoantibodies directed against intrinsic glomerular basement membrane proteins, and in situ formation of immune complexes between circulating antichromatin antibodies and extracellular glomerular chromatin derived from apoptotic kidney cells. Recent evidence to support the latter mechanism has been found in both murine10,11 and human lupus nephritis.12-15
In addition to circulating autoantibodies, autoantibodies may be produced within the kidneys in lupus nephritis. T and B cells form aggregates and, in some cases, germinal centers in the tubulointerstitial compartment. Interstitial plasma cells within these aggregates appear to produce antibodies in a clonally restricted fashion.16,17 This may be a mechanism of generating kidney-specific autoimmunity.
Kidney-specific autoimmunity may be facilitated by intrarenal interferon α (IFN-α) expression.18 IFN-α drives the maturation of conventional dendritic cells into potent antigen-presenting cells,19 induces B-cell differentiation to plasma cells,20 and contributes to the development of CD4 helper T (TH) cells21 and CD8 central memory T cells.22 IFN-α–inducible genes are upregulated in peripheral-blood leukocytes of many patients with SLE, referred to as the IFN-α signature.23,24 The major source of IFN-α is the plasmacytoid dendritic cell (pDC). IFN-α is induced after engagement of pDC endosomal Toll-like receptors 7 and 9 (TLR7 and TLR9) by nucleic acids.25,26 In lupus nephritis, pDCs leave the circulation and accumulate in the kidneys, where they conceivably can interact with renal immune complexes and increase IFN-α levels within the kidneys.27,28
Intrarenal immune complexes, especially those containing immunoglobulin G1 (IgG1) and IgG3 autoantibodies, are inflammatory because they activate complement and initiate renal recruitment of leukocytes through the chemotactic complement fragments C3a and C5a. The complement system also can injure the kidney directly through the formation of the membrane attack complex C5b-9.29 Complement-mediated kidney damage, especially through the alternative pathway, has been demonstrated in murine and human lupus nephritis.30-40
Neutrophil and macrophage recruitment to the kidney can cause direct injury through secretion of oxygen radicals and proteolytic enzymes. Dying neutrophils may contribute further to lupus nephritis by releasing neutrophil extracellular traps, which are chromatin structures that can bind autoantigens.41-43 Neutrophil extracellular traps also stimulate IFN-α secretion from dendritic cells,41 thereby amplifying intrarenal autoimmunity.
Leukocytes and intrinsic kidney cells produce proinflammatory cytokines and chemokines in response to immune complexes and complement fragments.44-46 Box 1 lists several of the cytokines and chemokines known to be upregulated in lupus nephritis kidneys.47-56
Box 1. Mediators of Kidney Injury in Lupus Nephritis.
Cytokines
IFN-α, IFN-γ, TNF-α, TWEAK-Fn14, IL-6, IL-1β, IL-18, IL-10, IL-17
Chemokines
IL-8, MCP-1, RANTES, IP-10, MIP-1
Growth factors
TGFβ, BAFF
Adhesion molecules
ICAM-1, VCAM-1
Signal transduction/transcription/costimulation
P38 MAPK, NF-κB, CD40/CD40L
Abbreviations: BAFF, B-cell activating factor; Fn14, fibroblast-growth factor inducible 14; ICAM-1, intercellular adhesion molecule 1; IFN-α, interferon α; IL, interleukin; IP-10, interferon γ–induced protein; MCP-1, monocyte chemoattractant protein 1; MIP-1, macrophage inflammatory protein 1; NF-κB, nuclear factor-κB; P38 MAPK, P38 mitogen-activated protein kinases; RANTES, regulated on activation normal T cell expressed; TGFβ, transforming growth factor β; TNF-α, tumor necrosis factor α; TWEAK, tumor necrosis factor–like weak inducer of apoptosis; VCAM-1, vascular cell adhesion molecule 1.
In lupus nephritis kidneys, TH type 1 (TH1) T-cell cytokines, including interleukin 12 (IL-12), IL-18, and IFN-γ, tend to be overexpressed compared to TH2 cytokines, although some TH2 cytokines, such as IL-10, also are increased.49,52,53,57 TH1 cytokines are associated with activated macrophages and with the production of immunoglobulins that can initiate complement and FcγR pathways, further amplifying kidney inflammation. TH17 and CD4−CD8 T cells express the proinflammatory cytokine IL-1758-60 in the lupus nephritis kidney.54 In addition to mediating inflammation, IL-17 may shift intrarenal T cells away from a regulatory phenotype (Treg) that is capable of attenuating immune responses, particularly autoantibody production.61-65 Although the role of Tregs in human lupus nephritis is not clear, several studies of humans have described lower circulating levels of Tregs in SLE (reviewed in63,66), suggesting that restoring Treg levels may be beneficial.
Chemokines can amplify kidney inflammation by recruiting more leukocytes to the kidney in a positive-feedback loop. Cytokines can damage kidney parenchyma directly. For example, in murine lupus nephritis, exogenous IL-6 accelerated the progression of kidney injury, whereas treatment with an anti–IL-6 monoclonal antibody mitigated disease.56 Cytokines also can mediate deleterious processes such as mesangial and glomerular epithelial cell proliferation, glomerulo-sclerosis, tubular atrophy, and interstitial fibrosis. As an example, tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK), a member of the TNF superfamily, promotes glomerular epithelial cell proliferation, inflammation, and apoptosis.4,67 Additionally, transforming growth factor β (TGFβ) promotes scarring in injured glomeruli and tubulointerstitium through accelerated matrix deposition.68 The role of specific cytokines and chemokines in lupus nephritis has been assessed by observing the effects of neutralization or genetic deletion on disease activity and histologic and clinical markers of kidney injury in murine lupus nephritis models.4,56,67-73 These experiments provide the rationale for therapeutically targeting cytokines and chemokines in human lupus nephritis.
In summary (see also Fig 1), active lupus nephritis can be characterized as an inflammatory response to immune complexes in the kidneys. Mediators of inflammation, including complement, infiltrating leukocytes, and cytokines derived from leukocytes and intrinsic kidney cells, injure renal parenchyma and network to amplify inflammation through a series of positive-feedback loops. Inflammatory kidney injury results in the local release of kidney antigens through apoptosis and necrosis. These antigens, in the presence of antigen-presenting dendritic cells, T cells, and B cells that have infiltrated the kidney and been conditioned by IFN-α and other cytokines, may result in intrarenal production of kidney-specific autoantibodies. This organ-specific autoimmunity may perpetuate kidney inflammation and facilitate future lupus nephritis flares. Therefore, treatment should focus on attenuating inflammation for current relief of lupus nephritis and addressing organ-specific autoimmunity for preventing reactivation of lupus nephritis.
RECENT ADVANCES
Patients who come to clinical attention and require treatment for lupus nephritis are most commonly in stage 2 of the kidney injury pathway (Fig 1). Some patients also may be on the way to stage 3, and that will be evident by the extent of glomerulosclerosis and interstitial fibrosis on kidney biopsy. We therefore suggest that for most patients, the ideal initial therapy of lupus nephritis would quickly and completely abrogate kidney inflammation. The history of lupus nephritis treatment suggests that this approach works. As discussed previously, anti-inflammatory doses of corticosteroids alone were as effective as corticosteroids plus cyclophosphamide in the early phase of lupus nephritis treatment.1,74 However, over time, corticosteroids alone proved to be inferior because patients had progressive kidney failure and more frequent lupus nephritis flares.1,74 In retrospect, this outcome is not surprising because focusing on inflammation alone probably does not adequately address the processes of systemic or kidney-specific autoimmunity (stage 1; Fig 1) that may be ongoing or poised to recur. The addition of cyclophosphamide to corticosteroid therapy likely attenuates some of these autoimmune processes. However, complete renal response rates to cyclophosphamide and other immunosuppressive agents remain fairly low.7 Recent data suggesting that cyclophosphamide promotes autoimmune mechanisms may at least partially explain this. In oncology patients, a single 3- to 4-g dose of intravenous cyclophosphamide had the expected cytotoxic effects, but also induced a type 1 interferon gene signature in peripheral-blood mononuclear cells, similar to that seen in SLE, and increased levels of the B-cell survival factor (B-cell activating factor [BAFF]).75 Similarly, after cyclophosphamide-induced lymphocyte depletion in mice, reconstituting B cells were exposed to high levels of BAFF, and this was associated with anti-DNA autoreactivity and the appearance of circulating anti-DNA autoantibodies.76 Thus cyclophosphamide may contribute to autoimmunity, although in patients with lupus nephritis, concomitant corticosteroid treatment may mitigate the proinflammatory effects of cyclophosphamide. We therefore suggest that concurrent with or shortly after anti-inflammatory therapy for lupus nephritis is initiated, novel therapies that target mechanisms of autoimmunity should be administered. It is expected that this approach will prevent lupus nephritis flares over the long term and thus limit progressive kidney failure.77 This treatment paradigm also implies that trials to examine the efficacy of novel therapies need to collect data for long-term outcomes and not only short-term renal response rates, as most have done recently.
Novel therapeutics are summarized in Table 1 and discussed in detail next.
Table 1.
Novel Therapies for Lupus Nephritis
| Drug | Type | Target | Clinical Trial Status |
ClinicalTrials.gov Study ID |
|---|---|---|---|---|
| Rituximab | Monoclonal antibody | CD20 (B cells) | Phase 3 completed with results | NCT00282347 |
| Belimumab | Monoclonal antibody | BLyS (B cells) | Phase 3 underway, FDA approved for nonrenal SLE |
NCT01639339 |
| Epratuzumab | Monoclonal antibody | CD22 (B cells) | Phase 3 underway for nonrenal SLE | NCT01262365 |
| Abatacept | CTLA4-Ig | CTLA4-B7 interaction | Phase 3: 1 completed, 1 underway |
NCT00430677 NCT00774852 |
| Anti-CD40 ligand | Monoclonal antibody | CD40 ligand | Phase 2 in lupus nephritis stopped early; thromboembolic events |
NCT00001789 |
| Fresolimumab | Monoclonal antibody | TGFβ1 | Phase 2 in FSGS underway, no studies in lupus nephritis |
NCT01665391 |
| Anti-TWEAK | Monoclonal antibody | TWEAK | Phase 2 underway | NCT01499355 |
| Laquinamod | Small molecule | Inflammation | Phase 2 in SLE completed | NCT01085097 |
| Tocilizumab | Monoclonal antibody | IL-6 receptor | Phase 1 completed | Illei et al100 |
| Sirukumab | Monoclonal antibody | IL-6 | Phase 2 underway | NCT01273389 |
| Tamibarotene | Small molecule | Retinoic acid receptor α/β |
Phase 2 underway | NCT01226147 |
| Bortezomib | Proteasome inhibitor | Plasma cells | Phase 4 stopped | NCT01169857 |
| Sifalimumab | Monoclonal antibody | INF-α | Phase 2 in SLE completed | NCT01283139 |
| Medi-546 | Monoclonal antibody | INF-α receptor | Phase 2 in SLE underway | NCT01559090 |
| Rontazilumab | Monoclonal antibody | INF-α | Phase 2 underway | NCT00962832 |
| Eculizumab | Monoclonal antibody | Complement component C5 |
Phase 1 completed | Barilla-Labarca et al85 |
Abbreviations: FDA, Food and Drug Administration; FSGS, focal and segmental glomerulosclerosis; Ig, immunoglobulin; IL, interleukin; INF-α, interferon α; SLE, systemic lupus erythematosus; TGFβ1, transforming growth factor β1; TWEAK, tumor necrosis factor–like weak inducer of apoptosis.
Anti-inflammatory Therapies
Because lupus nephritis is a severe manifestation of SLE and the implications of therapeutic failure include end-stage kidney disease and renal replacement therapy, new therapies almost always will be trialed initially as add-ons to corticosteroids plus an immunosuppressive. In this context, several novel biologics or small molecules may be useful in controlling inflammation more rapidly and/or more completely than current induction therapies. However, the possibility that some of these therapies could replace corticosteroids on the basis of efficacy and/or safety should not be overlooked. This would require a shift from add-on clinical trial design to substitution clinical trial design.
Laquinimod
Laquinimod is a small-molecule derivative of quinolone-3-carboxamide. It has been studied extensively as therapy for multiple sclerosis and just completed a phase 2 trial in lupus nephritis (clinical trial NCT01085097 at www.clinicaltrials.gov), with results expected in early 2014. In experimental autoimmune encephalitis, a model of multiple sclerosis, laquinimod behaves as an anti-inflammatory agent, decreasing infiltration of the central nervous system by monocytes and reducing proinflammatory cytokine and transcription factor expression, such as MCP-1 (monocyte protein 1) and NF-κB (nuclear factor-κB), respectively.78,79 Laquinimod also appears to modulate the inflammatory environment by polarizing T cells toward Tregs and away from TH1 and TH17 phenotypes.79,80 Human leukocytes or dendridic cells treated with laquinimod or taken from patients with multiple sclerosis treated with laquinimod show suppression of inflammatory cytokine and chemokine genes and decreased expression of NF-κB.80,81 Laquinimod also may have a role in modulating autoimmunity (stage 1; Fig 1) because it can decrease the expression of genes involved in antigen presentation and reduces pDCs.80,81
Synthetic Retinoids
Small-molecule synthetic retinoids that are ligands only for α/β retinoic acid receptors have been used to treat experimental autoimmune uvoretinitis and experimental autoimmune encephalitis.82,83 A trial of one such agent, tamibarotene, in SLE is planned (clinical trial NCT01226147, www.clinicaltrials.gov). In experimental inflammatory diseases, the retinoid AM80 decreased central nervous system and retinal infiltration by leukocytes, prevented retinal granuloma formation, and increased gene expression of an NF-κB repression factor.82,83 AM80 also may increase Tregs and decrease TH17 cells. Some potential concerns with AM80 include its downregulation of the anti-inflammatory cytokine IL-10 and an inability to prevent chronic inflammation in experimental autoimmune encephalitis.83
Eculizimab
Eculizumab is a recombinant fully humanized IgG2/IgG4 monoclonal antibody directed at human complement component C5. Eculizumab inhibits the conversion of C5 to C5a and C5b, thus preventing formation of the membrane attack complex (C5b-9) and the chemotactic fragment C5a. Eculizumab interacts with the complement system distal to classical pathway components and should not interfere with beneficial immune complex clearance activities of the classical pathway.35 It is conceivable that in lupus nephritis, eculizumab could prevent direct complement-mediated injury to intrinsic glomerular cells and attenuate kidney inflammation by reducing renal leukocyte recruitment. Although eculizumab currently is approved only for treatment of atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria,84 it was shown to be safe and well-tolerated in a phase 1 trial in SLE.85
Anti-TWEAK
TWEAK and its receptor fibroblast growth factor inducible 14 (FN14) are expressed at low levels in healthy adult kidneys.86 Under stress, including the immunologic injury of lupus nephritis,4,87-89 renal TWEAK and FN14 are upregulated, and binding of TWEAK to FN14 activates NF-κB in renal tubular cells. Through NF-κB, TWEAK induces renal tubular cell expression of several cytokines and chemokines, including MCP-1, IL-6, IP-10 (IFN-γ–induced protein 10), MIP-1α (macrophage inflammatory protein 1), ICAM-1 (intercellular adhesion molecule 1), VCAM-1 (vascular cell adhesion molecule 1), and RANTES (regulated on activation normal T cell expressed).67,90 Sustained NF-κB activation by TWEAK promotes renal tubular epithelial cell proliferation, inflammation, and apoptosis.87 Neutralization of TWEAK in lupus nephritis thus would be expected to decrease kidney inflammation and injury, an outcome that has been demonstrated in several animal models of kidney disease.91-93 Anti-TWEAK currently is being evaluated in a phase 3 lupus nephritis trial (clinical trial NCT01499355, www.clinicaltrials.gov).
Anti–IL-6
IL-6 is a multifunctional cytokine produced by leukocytes and intrinsic kidney cells that affects inflammation, increases mesangial cell proliferation, and also contributes to autoimmunity by stimulating terminal B-cell differentiation, autoantibody secretion, and T-cell differentiation.94,95 IL-6 works in synergy with IL-1 and TNF-α to promote inflammation, but also can be anti-inflammatory by termination of IL-1– and TNF-α–mediated inflammatory cascades.96 In murine and human SLE, IL-6 levels are increased in serum, urine, and glomeruli and correlate with disease activity.97,98 Neutralizing IL-6 or blocking the IL-6 receptor mitigates disease in murine models of lupus nephritis.72,95,99
Toculizumab is a humanized monoclonal antibody that blocks IL-6 from binding to its receptor. A phase 1 clinical trial of toculizumab in SLE demonstrated safety and tolerability (clinical trial NCT00046774, www.clinicaltrials.gov). In this trial, 5 of 12 patients with lupus nephritis did not have a change in level of proteinuria during the 12-week treatment period, but urine sediment activity improved and anti-dsDNA titers were reduced.100 Sirukumab is a humanized monoclonal antibody against IL-6 and presently is undergoing a phase 2 clinical trial to assess its safety and efficacy in lupus nephritis (clinical trial NCT01273389, www.clinicaltrials.gov).
Because IL-6 affects inflammation, cell proliferation, and autoimmunity, it is difficult to know its proper place as a therapeutic target within the paradigm presented in Fig 1. Although discussed under anti-inflammatory therapies, results of the current lupus nephritis trial will help in understanding how and when to best use IL-6 inhibition.
Antiautoimmunity Therapies
A number of recently introduced biologics that have been or will be tested in lupus nephritis are directed at the autoimmune mechanisms that predispose the kidney to inflammatory injury. Most of these studies examined short-term (6- to 12-month) renal response rates when added to conventional therapy. However, because these therapies attenuate autoimmune mechanisms, their role in lupus nephritis treatment may be to prevent recurrent disease activity (flares) and thereby the accumulation of organ damage (chronic or end-stage kidney disease). It is conceivable that antiautoimmunity therapeutics may decrease or eliminate the need for maintenance immunosuppressive therapy in lupus nephritis.
Rontalizumab and Sifalimumab
Rontalizumab and sifalimumab are humanized antibodies to IFN-α. Given the critical role of IFN-α in autoimmunity, neutralizing its activity would appear to be a good strategy in preventing further disease flares after kidney inflammation has been controlled. Some indirect clinical data support this approach. In 49 patients with SLE, 22% were found to have naturally occurring anti–IFN-α antibodies and a lower IFN-α gene signature than patients without such antibodies.101 These antibody-positive patients also tended to have a lower SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) score, fewer positive lupus serologic test results, and higher complement levels than patients with the high IFN-α signature. However, ~35% of this cohort were low IFN-α signature but did not have anti–IFN-α autoantibodies, suggesting that not all patients will benefit from exogenous anti–IFN-α therapy. On the positive side, IFN-α gene signature may be a good biomarker for deciding which patients will benefit most from anti–IFN-α therapy.
Rontalizumab and sifalimumab already have been tested for safety in 3 small phase 1 studies, although most patients had only mild to moderate disease, were receiving no more than 20 mg of prednisone per day and no immunosuppressive agents, and did not have active lupus nephritis.102-105 In these studies, 50%-75% of patients demonstrated an IFN-α gene signature, and in general, rontalizumab and sifalimumab inhibited the gene signature in a dose-dependent fashion, although inhibition was short lived and did not completely reverse the expression of IFN-α–activated genes. For example, sifalimumab caused an average IFN-α signature inhibition of 39%.105 Despite decreasing the gene signature, rontalizumab did not decrease protein levels of IFN-α–activated genes or the patients’ autoantibody levels.103 Unexpectedly, a preliminary report of a phase 2 study of rontalizumab in SLE showed more benefit in patients who were low IFN-α signature, suggesting that higher antibody doses may be required to attenuate IFN-α activity in patients with a high gene signature.102
Another approach to attenuating the effects of IFN in lupus nephritis is by blocking the IFN-α receptor. This may be more effective than directly blocking IFN-α because there are several type 1 IFN isotypes that all work through the same receptor. Medi-546 is a humanized anti–IFN-α receptor monoclonal antibody that currently is under investigation (clinical trials NCT01559090, NCT00930683, and NCT01753193; www.clinicaltrials.gov).
Rituximab and Other B-Cell–Directed Therapies
Rituximab is a humanized/mouse chimeric monoclonal antibody to CD20, which is present on most B cells but not plasma cells. The rationale for testing rituximab in SLE and lupus nephritis was to deplete autoreactive B cells and thereby attenuate the production of the autoantibodies necessary for disease manifestations. Although there now are a large number of reports on the apparent utility of rituximab in renal and nonrenal lupus, most of these are anecdotal case reports, small uncontrolled case series, or registries.106,107 LUNAR (Lupus Nephritis Assessment with Rituximab) and EXPLORER (Exploratory Phase II/III SLE Evaluation of Rituximab), the 2 large, prospective, placebo-controlled trials, failed to find a benefit of rituximab in renal or nonrenal lupus when added to standard-of-care treatment.8,108 In LUNAR, rituximab plus corticosteroids and MMF did not increase complete renal responses at 1 year compared to placebo plus corticosteroids and MMF.
The reasons for the failure of LUNAR continue to be debated. However, in hindsight and in the context of the paradigm presented in Fig 1, rituximab would be expected to provide long-term kidney protection and not be expected to improve short-term complete response rates. An interesting follow-up to the LUNAR investigation would be to see if complete responders who had received rituximab have had fewer lupus nephritis flares than complete responders who received placebo.
There are several caveats to anti–B-cell therapy with rituximab that should be considered. Because rituximab does not affect plasma cells, it does not cause an immediate decrease in pathogenic autoantibody levels, but instead a decline that depends on how quickly natural or therapeutic elimination of the existing generation of autoreactive plasma cells occurs. Rituximab also may promote autoreactive B cells. As B cells become depleted in response to rituximab, levels of the B-lymphocyte–stimulating factor BAFF increase.109,110 The presence of a high BAFF level as B cells reconstitute may increase the generation of new autoreactive B cells. Finally, human B regulatory cells (Bregs), which appear to attenuate inflammation through an IL-10–dependent mechanism, have been found in patients with SLE.111 It is not known whether rituximab eliminates Bregs in patients, but anti-CD20 antibodies deplete Bregs in mice.112 This could interfere with endogenous anti-inflammatory mechanisms.
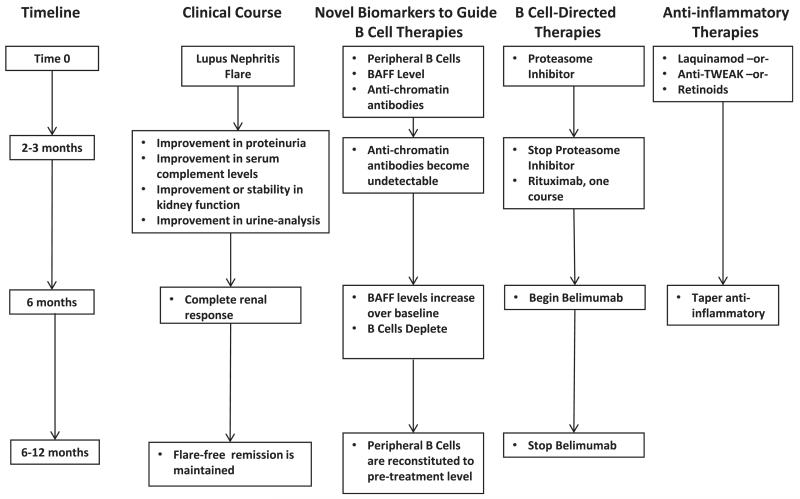
In light of these findings, a combinatorial B-cell–directed approach may be useful in the treatment of lupus nephritis. A testable B-cell–focused trial design based on combinations of B-cell therapies is illustrated in Fig 2. The first goal of a combinatorial approach would be to eliminate plasma cells that are producing pathogenic autoantibodies. This would be done during lupus nephritis induction, followed by rituximab to deplete B cells and prevent the generation of new autoantibody-producing plasma cells. Eliminating plasma cells may give rituximab a clean-slate environment (with no autoantibodies) in which to work. In a mouse model of autoimmune diabetes, anti-CD20 antibodies were most effective in preventing diabetes if given before insulin autoantibodies appeared,113 suggesting that a clean antibody slate may be important. Also, not adding rituximab during acute kidney inflammation may prevent the depletion of Bregs at a time when they may be most effective. To prevent the reconstitution of B cells in a BAFF-enriched environment, BAFF inhibition would be initiated with rituximab.
Figure 2.
A model of combinatorial B-cell–directed therapy for lupus nephritis. This algorithm represents a hypothetical approach to using B-cell–directed therapies in a lupus nephritis trial. The algorithm matches therapies to the pathogenic stage of the clinical disease and uses novel biomarkers of B-cell activity to guide initiation and termination of specific drugs. The timeline provided is a (non–evidence-based) guide to the desired or ideal clinical milestones after initiation of treatment. At flare, patients would receive a proteasome inhibitor to reduce/eliminate plasma cells producing autoantibodies and an anti-inflammatory drug to synergize with the anti–NF-κB (nuclear factor κ light-chain enhancer of activated B cells) effects of the proteasome inhibitor and attenuate renal inflammation. Baseline data on B-cell numbers, B-cell activating factor (BAFF) levels, and antichromatin antibodies would be obtained. After 2-3 months, clinical parameters should be improving. If antichromatin antibodies become undetectable, the proteasome inhibitor would be discontinued and rituximab would be initiated to eliminate B cells and prevent repopulation of autoreactive plasma cells that had been killed by the proteasome inhibitor. Because BAFF levels increase as rituximab depletes B cells confounding the antiautoimmune effects of rituximab, belimumab would be started as BAFF levels increase over the time 0 baseline values. B-Cell and BAFF levels would be followed up prospectively, and after reconstitution of B cells in a BAFF-free environment, belimumab could be stopped. At 6 months, clinical parameters should be consistent with a complete renal response. At this time, the anti-inflammatory agents can be tapered off over time. Ideally, because inflammation will have been attenuated and autoreactive B cells eliminated, the patient will remain flare-free on no immunosuppression. Abbreviation: TWEAK, tumor necrosis factor–like weak inducer of apoptosis.
Combinatorial B-cell–directed therapy is feasible with existing biologics and small molecules. The boronic acid derivatives bortezomib, carfilzomib, and delanzomib are proteasome inhibitors used for the treatment of multiple myeloma. These small molecules induce apoptosis in plasma cells because plasma cells are sensitive to intracellular stress.114 Proteasome inhibitors have been found to be effective in 2 murine models of SLE and lupus nephritis and can both prevent lupus nephritis and ameliorate established lupus nephritis.114-116 Importantly, these models provide evidence that the proteasome inhibitors target plasma cells. Lupus mice treated with proteasome inhibitors had significantly lower concentrations of circulating autoantibodies and reduced frequency of splenic autoantibody-secreting cells compared with controls.114,116 Finally, because the transcription factor NF-κB needs a functional proteasome for activation, these drugs block the induction of several NF-κB–dependent proinflammatory cytokines and also appear to increase Tregs in models of lupus.114,115 Thus proteasome inhibitors may have a dual effect in the induction phase of lupus nephritis treatment, acting both as an anti-inflammatory agent and to clear out autoantibodies for rituximab. A study of bortezomib in an experimental model of antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis showed that bortezomib worked well only if started early in the disease course.117 This suggests that proteasome inhibitors may not be sufficient stand-alone anti-inflammatory agents for lupus nephritis that has reached clinical attention and probably will need to be combined with an additional anti-inflammatory agent to fully attenuate intrarenal inflammation (Fig 2). These data also support the idea that B-cell therapies must be applied to lupus nephritis at specific stages of the disease course for efficacy. Finally, some caution with these drugs is necessary because at least bortezomib is known to have important neurotoxicity.118
To counteract the potentially detrimental increase in BAFF levels that occurs as rituximab depletes B cells, belimumab, a monoclonal antibody that neutralizes BAFF, is the last component of combinatorial B-cell–directed therapy.119 A key clinical question will be when to start belimumab treatment. It would not be unreasonable to use circulating BAFF as a biomarker and initiate belimumab therapy when BAFF levels start to increase over baseline. Belimumab therapy would be continued until peripheral B cells have been reconstituted, and then possibly withdrawn. A recent post hoc analysis of phase 3 belimumab trials in nonrenal SLE examined renal outcomes120 and showed that belimumab-treated patients tended to have fewer renal flares, providing some support for the use of B-cell–directed therapies in the maintenance phase of lupus nephritis.
Rituximab recently has been compared to cyclophosphamide in ANCA-associated vasculitis. Although short-term remissions were similar with both therapies, patients in the rituximab arms were not given maintenance immunosuppression and did as well as the cyclophosphamide group, which received maintenance azathioprine.121-123 This finding supports the idea that attenuation of autoimmune mechanisms by B-cell depletion may be very effective at preventing reactivation of kidney diseases that typically relapse.
Abatacept
CTLA4 binds B7 molecules that are on the surface of dendritic cells or B cells and can block the costimulatory interaction between B7.1/B7.2 and CD28 on T cells.124 Abatacept, a fusion protein between CTLA4 and IgG heavy chain components, was developed as a therapeutic agent to target costimulation. Abatacept recently has been tested in 2 large randomized controlled trials in lupus nephritis as an add-on to either low-dose cyclophosphamide (ACCESS [Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis] Trial, clinical trial NCT00774852, www.clinicaltrials.gov) or MMF (clinical trial NCT01714817, www.clinicaltrials.gov). The primary end points were 6- to 12-month complete renal response rates or time to complete renal response. The complete renal response end point was chosen for these trials based on murine studies that showed that abatacept in combination with low-dose cyclophosphamide was able to induce complete lupus nephritis remission in mice with established nephritis.124,125 Unfortunately, abatacept did not improve complete renal response rates in either clinical trial.9,126
Differences in the effects of abatacept in mice and humans may have contributed to the differences between the murine experiments and clinical trial results. For example, kidney inflammation decreased significantly in mice after abatacept treatment.125 However, in patients with rheumatoid arthritis, CTLA4-Ig reduced serum IFN-γ, but not IL-6, VEGF (vascular endothelial growth factor) or TNF-α and had no effect on circulating dendritic cells, indicating perhaps a limited anti-inflammatory effect in humans.127
In the context of the paradigm presented in Fig 1, an ideal use for abatacept in lupus nephritis may be to interrupt interactions between T and B cells in the lymphocyte aggregates found in about half of lupus nephritis biopsy specimens.17 Such an intervention would be expected to decrease kidney-specific autoimmunity and prevent future nephritis activity. Consistent with this idea, abatacept-treated complete responders in the ACCESS trial were not started on maintenance immunosuppression after 6 months of induction therapy. During the following 6 months, 50% of these patients maintained their complete responses with no further immunosuppression.126
Antifibrotic Therapies
As kidney inflammation heals, scars develop in the kidney parenchyma, manifested as glomerular sclerosis and interstitial fibrosis. As in other types of glomerular diseases, patients with lupus nephritis who sustain sufficient chronic tubulointerstitial injury tend to have progressive kidney failure.128 Arresting progressive fibrosis in lupus nephritis kidneys may now be feasible.
Fresolimumab
Fresolimumab is a humanized IgG4 monoclonal antibody that neutralizes all 3 isoforms of TGFβ. It currently is undergoing a trial in primary focal segmental glomerulosclerosis (clinical trial NCT01665391, www.clinicaltrials.gov). A previous safety and pharmacokinetic study of single-dose fresolimumab was done in patients with focal segmental glomerulosclerosis (n = 16) and demonstrated that it was well tolerated and associated with a small decline in proteinuria.129 The relevance of the proteinuria observation is not clear because the real potential of fresolimumab is in long-term preservation of kidney function in patients who have had progressive scarring. It is unlikely that fresolimumab will reverse scarring and therefore significant improvements in proteinuria would not be expected unless the inhibition of TGFβ positively influences remaining podocyte number and function, an effect seen with early but not late TGFβ inhibition in animal models.130,131 We envision fresolimumab being used in patients with lupus nephritis who have ongoing proteinuria and declining kidney function in the absence of (biopsy-proven) continued kidney inflammation (stage 3; Fig 1). An argument could be made that patients who have >25% interstitial inflammation, and thereby a poor prognosis,128 should be treated with an antifibrotic at diagnosis. However, the consequences of blocking TGFβ during active inflammation are difficult to predict and may be detrimental in lupus nephritis because TGFβ has important anti-inflammatory and immune tolerance effects.132 Thus the timing of when to add fresolimumab in lupus nephritis will need to be critically evaluated.
SUMMARY
The goal of treatment in lupus nephritis is to induce remission, prevent disease recurrence, and preserve kidney function while minimizing side effects from therapy. Current standard of care is suboptimal and new agents have been developed that are intended to more specifically target mechanisms involved in the pathogenesis of lupus nephritis. Results of clinical trials of these novel therapies to date have been disappointing. Trial failures can be attributed to multiple variables, including the lack of standardized end points and the limitations associated with studies involving only specific populations. In this review, we have emphasized discordance between clinical expectations of new therapies and expectations based on their mechanisms of action as another factor contributing to trial failures. The clinical goal of improving short-term complete response rates, although important, has resulted in almost all trials of novel therapeutics being designed as add-on to standard-of-care trials.
Moving forward, it will be necessary to standardize clinical trial end points for lupus nephritis therapies. To determine whether a new therapy is equally effective in all races and ethnicities, it would be ideal to foster an international consortium of lupus nephritis investigators to facilitate trials in diverse populations. This is beginning to develop through working groups such as the Lupus Nephritis Trials Network (www.lupusnephritis.org). Finally, clinical trials will need to be designed with end points that better align with a drug’s mechanism of action. Combinatorial therapies targeting both kidney inflammation and intra- and extrarenal autoimmunity offer the potential of abrogating inflammation to improve short-term response rates and abrogating autoimmunity to prevent disease recurrence, chronic injury, and progressive kidney failure.
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Support: The project described was supported by grant UL1TR000090 from the National Center for Advancing Translational Sciences.
Footnotes
Financial Disclosure: Dr Rovin is a member of the medical/scientific advisory boards of Biogen-Idec, Onyx, Lilly, and Genentech and has received grant funding from Teva. Dr Parikh declares that he has no other relevant financial interests.
REFERENCES
- 1.Austin HA, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–619. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- 2.Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 3.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80(7):708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- 5.Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose versus high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 6.Dooley MA, Jayne D, Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–1895. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 7.Rovin BH, Stillman IE. The kidney in systemic lupus erythematosus. In: Lahita RG, editor. Systemic Lupus Erythematosus. 5th ed. Academic Press; London, UK: 2011. pp. 769–814. [Google Scholar]
- 8.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment With Rituximab Study. Arthritis Rheum. 2012;64:1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 9.Wofsy D, Hillson JL, Diamond B. Abatacept for lupus nephritis. Arthritis Rheum. 2012;64:3660–3665. doi: 10.1002/art.34624. [DOI] [PubMed] [Google Scholar]
- 10.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168(6):1779–1792. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalaaji M, Sturfelt G, Mjelle JE, Nossent H, Rekvig OP. Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 2006;54(3):914–926. doi: 10.1002/art.21622. [DOI] [PubMed] [Google Scholar]
- 12.Kalaaji M, Fenton KA, Mortensen ES, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71(7):664–672. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 13.Manson JJ, Ma A, Rogers P, et al. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res Ther. 2009;11(5):R154. doi: 10.1186/ar2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchini M, Antonioli R, Lleo A, et al. HLA class II antigens associated with lupus nephritis in Italian SLE patients. Hum Immunol. 2003;64(4):462–468. doi: 10.1016/s0198-8859(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor KE, Chung SA, Graham RR, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7(2):e1001311. doi: 10.1371/journal.pgen.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinmetz OM, Velden J, Kneissler U, et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74(4):448–457. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 17.Chang A, Henderson SG, Brandt D, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186(3):1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23(2):113–121. doi: 10.1016/j.smim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Majchrzak-Kita B, Fish EN, Gommerman JL. Dynamic accumulation of plasmacytoid dendritic cells in lymph nodes is regulated by interferon-beta. Blood. 2009;114(13):2623–2631. doi: 10.1182/blood-2008-10-183301. [DOI] [PubMed] [Google Scholar]
- 20.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ. Type I interferon (IFN alpha) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol. 2009;183(5):2915–2920. doi: 10.4049/jimmunol.0801607. [DOI] [PubMed] [Google Scholar]
- 22.Ramos HJ, Davis AM, Cole AG, Schatzle JD, Forman J, Farrar JD. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood. 2009;113(22):5516–5525. doi: 10.1182/blood-2008-11-188458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X, Wu H, Grossman JM, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 25.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 27.Kaser A, Kaser S, Kaneider NC, Enrich B, Wiedermann CJ, Tilg H. Interleukin-18 attracts plasmacytoid dendritic cells (DC2s) and promotes Th1 induction by DC2s through IL-18 receptor expression. Blood. 2004;103(2):648–655. doi: 10.1182/blood-2002-07-2322. [DOI] [PubMed] [Google Scholar]
- 28.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58(1):251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 29.Biesecker G, Katz S, Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med. 1981;154(6):1779–1794. doi: 10.1084/jem.154.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164(2):786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 31.Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. 2004;65(1):129–138. doi: 10.1111/j.1523-1755.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 32.Bao L, Haas M, Quigg RJ. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22(2):285–295. doi: 10.1681/ASN.2010060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekine H, Reilly CM, Molano ID, et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol. 2001;166(10):6444–6451. doi: 10.4049/jimmunol.166.10.6444. [DOI] [PubMed] [Google Scholar]
- 34.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol. 2005;16(12):3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- 35.Sekine H, Ruiz P, Gilkeson GS, Tomlinson S. The dual role of complement in the progression of renal disease in NZB/W F(1) mice and alternative pathway inhibition. Mol Immunol. 2011;49(1-2):317–323. doi: 10.1016/j.molimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad U S A. 1996;93(16):8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158(10):4525–4528. [PubMed] [Google Scholar]
- 38.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 39.Birmingham DJ, Irshaid F, Nagaraja HN, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus. 2010;19(11):1272–1280. doi: 10.1177/0961203310371154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekine H, Kinser TT, Qiao F, et al. The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthritis Rheum. 2011;63(4):1076–1085. doi: 10.1002/art.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weidenbusch M, Rommaie C, Schrottie A, Anders HJ. Beyond the LUNAR trial: rituximab for refractory lupus nephritis. Nephrol Dial Transplant. 2013;28(1):106–111. doi: 10.1093/ndt/gfs285. [DOI] [PubMed] [Google Scholar]
- 42.Sanz I, Lee FE-H. B Cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend MJ, Monroe JG, Chan AC. B-Cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CL. Human IgG Fc receptors. Clin Immunol Immunopathol. 1989;53(2, pt 2):63–71. doi: 10.1016/0090-1229(89)90071-8. suppl 2. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Ptacek TS, Brown EE, Edberg JC. Fcgamma receptors: structure, function and role as genetic risk factors in SLE. Genes Immun. 2009;10(5):380–389. doi: 10.1038/gene.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rovin BH. Chemokines as therapeutic targets in renal inflammation. Am J Kidney Dis. 1999;34:761–764. doi: 10.1016/S0272-6386(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 47.Rovin BH. The chemokine network in systemic lupus erythematosis nephritis. Front Biosci. 2007;13:904–922. doi: 10.2741/2731. [DOI] [PubMed] [Google Scholar]
- 48.Peterson KS, Huang JF, Zhu J, et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest. 2004;113(12):1722–1733. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan RW, Lai FM, Li EK, et al. Intrarenal cytokine gene expression in lupus nephritis. Ann Rheum Dis. 2007;66(7):886–892. doi: 10.1136/ard.2006.063123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malide D, Russo P, Bendayan M. Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum Pathol. 1995;26(5):558–564. doi: 10.1016/0046-8177(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 51.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Diaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998;7(3):154–158. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 52.Uhm WS, Na K, Song GW, et al. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology (Oxford) 2003;42(8):935–938. doi: 10.1093/rheumatology/keg255. [DOI] [PubMed] [Google Scholar]
- 53.Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44(9):2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Crispin JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz N, Su L, Burkly L, et al. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 2006;27:242–250. doi: 10.1016/j.jaut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13(5):339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F. Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol. 2008;154(2):247–254. doi: 10.1111/j.1365-2249.2008.03758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge D, You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1(1):1–19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 60.Qiu Z, Dillen C, Hu J, et al. Interleukin-17 regulates chemokine and gelatinase B expression in fibroblasts to recruit both neutrophils and monocytes. Immunobiology. 2009;214(9-10):835–842. doi: 10.1016/j.imbio.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 62.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn A, Beissert S, Krammer PH. CD4(+)CD25(+ ) regulatory T cells in human lupus erythematosus. Arch Dermatol Res. 2009;301(1):71–81. doi: 10.1007/s00403-008-0891-9. [DOI] [PubMed] [Google Scholar]
- 64.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 65.Iikuni N, Lourenco EV, Hahn BH, La Cava A. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183(3):1518–1522. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerli R, Nocentini G, Alunno A, et al. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8(5):426–430. doi: 10.1016/j.autrev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Sanz AB, Justo P, Sanchez-Nino MD, et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19(4):695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Border WA. Transforming growth factor-beta and the pathogenesis of glomerular diseases. Curr Opin Nephrol Hypertens. 1994;3(1):54–58. doi: 10.1097/00041552-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-faslpr mice. J Exp Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez de Lema G, Maier H, Franz TJ, et al. Chemokine receptor CCR2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol. 2005;16:3592–3601. doi: 10.1681/ASN.2005040426. [DOI] [PubMed] [Google Scholar]
- 71.Hasegawa H, Kohno M, Sasaki M, et al. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 2003;48(9):2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- 72.Kiberd BA. Interleukin-6 receptor blockage ameliorates murine lupus nephritis. J Am Soc Nephrol. 1993;4(1):58–61. doi: 10.1681/ASN.V4158. [DOI] [PubMed] [Google Scholar]
- 73.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donadio JV, Holley KE, Ferguson RH, Ilstrup DM. Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med. 1978;23:1151–1155. doi: 10.1056/NEJM197811232992102. [DOI] [PubMed] [Google Scholar]
- 75.Moschella F, Torelli GF, Valentini M, et al. Cyclophosphamide induces a type I interferon-associated sterile inflammatory response signature in cancer patients’ blood cells: implications for cancer chemoimmunotherapy. Clin Cancer Res. 2013;19(15):4249–4261. doi: 10.1158/1078-0432.CCR-12-3666. [DOI] [PubMed] [Google Scholar]
- 76.Kawabata D, Venkatesh J, Ramanujam M, Davidson A, Grimaldi CM, Diamond B. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One. 2010;5(1):e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parikh SV, Nagaraja H, Hebert LA, Rovin BH. Renal flare as a predictor of incident and progressive chronic kidney disease in patients with Lupus Nephritis [published online ahead of print November 21, 2013] Clin J Am Soc Nephrol. doi: 10.2215/CJN.05040513. http://dx.doi.org/10.2215/CJN.05040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra MK, Wang J, Silva C, Mack M, Yong VW. Kinetics of proinflammatory monocytes in a model of multiple sclerosis and its perturbation by laquinimod. Am J Pathol. 2012;181(2):642–651. doi: 10.1016/j.ajpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Schulze-Topphoff U, Shetty A, Varrin-Doyer M, et al. Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PLoS One. 2012;7(3):e33797. doi: 10.1371/journal.pone.0033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jolivel V, Luessi F, Masri J, et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain. 2013;136(pt 4):1048–1066. doi: 10.1093/brain/awt023. [DOI] [PubMed] [Google Scholar]
- 81.Gurevich M, Gritzman T, Orbach R, Tuller T, Feldman A, Achiron A. Laquinimod suppresses antigen presentation in relapsing-remitting multiple sclerosis: in-vitro high-throughput gene expression study. J Neuroimmunol. 2010;221(1-2):87–94. doi: 10.1016/j.jneuroim.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Keino H, Watanabe T, Sato Y, Okada AA. Oral administration of retinoic acid receptor-alpha/beta-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52(3):1548–1556. doi: 10.1167/iovs.10-5963. [DOI] [PubMed] [Google Scholar]
- 83.Klemann C, Raveney BJ, Klemann AK, et al. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174(6):2234–2245. doi: 10.2353/ajpath.2009.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 85.Barilla-Labarca ML, Toder K, Furie R. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol. 2013;148(3):313–321. doi: 10.1016/j.clim.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 86.Chicheportiche Y, Bourdon PR, Xu H, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272(51):32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 87.Michaelson JS, Wisniacki N, Burkly LC, Putterman C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J Autoimmun. 2012;39(3):130–142. doi: 10.1016/j.jaut.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu ZC, Zhou QL. Tumor necrosis factor-like weak inducer of apoptosis and its potential roles in lupus nephritis. Inflamm Res. 2012;61(4):277–284. doi: 10.1007/s00011-011-0420-8. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz N, Rubinstein T, Burkly L, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao HX, Campbell SR, Burkly LC, et al. TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine. 2009;46(1):24–35. doi: 10.1016/j.cyto.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Z, Burkly LC, Campbell S, et al. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179(11):7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 92.Xia Y, Campbell SR, Broder A, et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145(2):108–121. doi: 10.1016/j.clim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molano A, Lakhani P, Aran A, Burkly LC, Michaelson JS, Putterman C. TWEAK stimulation of kidney resident cells in the pathogenesis of graft versus host induced lupus nephritis. Immunol Lett. 2009;125(2):119–128. doi: 10.1016/j.imlet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Kitani A, Hara M, Hirose T, et al. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin Exp Immunol. 1992;88(1):75–83. doi: 10.1111/j.1365-2249.1992.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitani A, Hara M, Hirose T, et al. Heterogeneity of B cell responsiveness to interleukin 4, interleukin 6 and low molecular weight B cell growth factor in discrete stages of B cell activation in patients with systemic lupus erythematosus. Clin Exp Immunol. 1989;77(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 96.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83(1):113–118. [PubMed] [Google Scholar]
- 97.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147(1):117–123. [PubMed] [Google Scholar]
- 98.Peterson E, Robertson AD, Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5(6):571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- 99.Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ. Interleukin-6 exacerbates glomerulonephritis in (NZB × NZW)F1 mice. Am J Pathol. 1994;144(5):927–937. [PMC free article] [PubMed] [Google Scholar]
- 100.Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62(2):542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morimoto AM, Flesher DT, Yang J, et al. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(8):2407–2415. doi: 10.1002/art.30399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirou KA, Gkrouzman E. Anti-interferon alpha treatment in SLE. Clin Immunol. 2013;148(3):303–312. doi: 10.1016/j.clim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 103.McBride JM, Jiang J, Abbas AR, et al. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 2012;64(11):3666–3676. doi: 10.1002/art.34632. [DOI] [PubMed] [Google Scholar]
- 104.Merrill JT, Wallace DJ, Petri M, et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011;70(11):1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 105.Petri M, Wallace DJ, Spindler A, et al. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65(4):1011–1021. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weidenbusch M, Rommele C, Schrottle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28(1):106–111. doi: 10.1093/ndt/gfs285. [DOI] [PubMed] [Google Scholar]
- 107.Rovin BH. Targeting B-cells in lupus nephritis: should cautious optimism remain? Nephrol Dial Transplant. 2013;28(1):7–9. doi: 10.1093/ndt/gfs319. [DOI] [PubMed] [Google Scholar]
- 108.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pollard RP, Abdulahad WH, Vissink A, et al. Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjögren’s syndrome: data from a placebo-controlled clinical trial. Ann Rheum Dis. 2013;72(1):146–148. doi: 10.1136/annrheumdis-2012-202071. [DOI] [PubMed] [Google Scholar]
- 110.Vallerskog T, Heimburger M, Gunnarsson I, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121(11):4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Serreze DV, Chapman HD, Niens M, et al. Loss of intra-islet CD20 expression may complicate efficacy of B-cell-directed type 1 diabetes therapies. Diabetes. 2011;60(11):2914–2921. doi: 10.2337/db11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seavey MM, Lu LD, Stump KL, Wallace NH, Ruggeri BA. Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int Immunopharmacol. 2012;12(1):257–270. doi: 10.1016/j.intimp.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 115.Weng J, Lai P, Lv M, et al. Bortezomib modulates regulatory T cell subpopulations in the process of acute graft-versus-host disease. Clin Lab. 2013;59(1-2):51–58. doi: 10.7754/clin.lab.2012.120215. [DOI] [PubMed] [Google Scholar]
- 116.Hainz N, Thomas S, Neubert K, et al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron Exp Nephrol. 2012;120(2):e47–e58. doi: 10.1159/000334955. [DOI] [PubMed] [Google Scholar]
- 117.Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R. Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol. 2011;22(2):336–348. doi: 10.1681/ASN.2010010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corthals SL, Kuiper R, Johnson DC, et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica. 2011;96(11):1728–1732. doi: 10.3324/haematol.2011.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stohl W. Biologic differences between various inhibitors of the BLyS/BAFF pathway: should we expect differences between belimumab and other inhibitors in development? Curr Rheumatol Rep. 2012;14(4):303–309. doi: 10.1007/s11926-012-0254-6. [DOI] [PubMed] [Google Scholar]
- 120.Dooley MA, Houssiau F, Aranow C, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. 2013;22(1):63–72. doi: 10.1177/0961203312465781. [DOI] [PubMed] [Google Scholar]
- 121.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 122.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daikh DI, Wofsy D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol. 2001;166(5):2913–2916. doi: 10.4049/jimmunol.166.5.2913. [DOI] [PubMed] [Google Scholar]
- 125.Schiffer L, Sinha J, Wang X, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171(1):489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 126.Rovin BH, Access Investigators Treatment of lupus nephritis with abatacept and low-dose pulse cyclophosphamide: the results of the ACCESS trial [abstract]; Presented at: American Society of Nephrology Meeting; Atlanta, GA. 2013.Nov 4-10, [Google Scholar]
- 127.Marti L, Golmia R, Golmia AP, et al. Alterations in cytokine profile and dendritic cells subsets in peripheral blood of rheumatoid arthritis patients before and after biologic therapy. Ann N Y Acad Sci. 2009;1173:334–342. doi: 10.1111/j.1749-6632.2009.04740.x. [DOI] [PubMed] [Google Scholar]
- 128.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 2011;63(6):865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trachtman H, Fervenza FC, Gipson DS, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79(11):1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Benigni A, Zoja C, Campana M, et al. Beneficial effect of TGFbeta antagonism in treating diabetic nephropathy depends on when treatment is started. Nephron Exp Nephrol. 2006;104(4):e158–e168. doi: 10.1159/000094967. [DOI] [PubMed] [Google Scholar]
- 131.Sam R, Wanna L, Gudehithlu KP, et al. Glomerular epithelial cells transform to myofibroblasts: early but not late removal of TGF-beta1 reverses transformation. Transl Res. 2006;148(3):142–148. doi: 10.1016/j.trsl.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 132.Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006;17(6):487–499. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]