Abstract
Type I IFNs exert diverse effector and regulatory functions in host immunity to viral and nonviral infections; however, the role of endogenous type I IFNs in leishmaniasis is unclear. We found that type I IFNR-deficient (IFNAR−/−) mice developed attenuated lesions and reduced Ag-specific immune responses following infection with Leishmania amazonensis parasites. The marked reduction in tissue parasites, even at 3 d in IFNAR−/− mice, seemed to be indicative of an enhanced innate immunity. Further mechanistic analyses indicated distinct roles for neutrophils in parasite clearance; IFNAR−/− mice displayed a rapid and sustained infiltration of neutrophils, but a limited recruitment of CD11b+Ly-6C+ inflammatory monocytes, into inflamed tissues; interactions between IFNAR−/−, but not wild-type (WT) or STAT1−/−, neutrophils and macrophages greatly enhanced parasite killing in vitro; and infected IFNAR−/− neutrophils efficiently released granular enzymes and had elevated rates of cell apoptosis. Furthermore, although coinjection of parasites with WT neutrophils or adoptive transfer of WT neutrophils into IFNAR−/− recipients significantly enhanced infection, the coinjection of parasites with IFNAR−/− neutrophils greatly reduced parasite survival in WT recipients. Our findings reveal an important role for type I IFNs in regulating neutrophil/monocyte recruitment, neutrophil turnover, and Leishmania infection and provide new insight into innate immunity to protozoan parasites.
Type I IFNs exert diverse effector or regulatory functions in innate and adaptive immune responses by activating JAK/STAT signals through the common type I IFNR (IFNAR) (1). Type I IFNs are needed for the control of most, if not all, viral infections, but they can be protective or disease-aggravating in host responses to nonviral infection, depending on the parasitism of invading microbial pathogens (2). For example, type I IFN signaling is crucial for host resistance against extracellular bacteria (e.g., group B streptococci, Streptococcus pneumoniae, and Escherichia coli) (3, 4), extracellular fungal pathogens (e.g., Pneumocystis murina) (5), and intracellular yeast (Cryptococcus neoformans) (6). However, a recent study in mice indicated that the development of type I IFN-mediated antiviral responses can sensitize the hosts to secondary S. pneumoniae infection (7). The detrimental effects of type I IFNs in enhancing host susceptibility to other intracellular bacteria, such as Chlamydia muridarum (8, 9) and Listeria monocytogenes (10–12), have been documented. In contrast, there are controversial results with regard to intracellular mycobacterial infections in mice. Although exogenous type I IFNs can be protective or detrimental (13, 14), the lack of IFNAR signaling has no major effect on host resistance against Mycobacterium tuberculosis in young or aged mice (15).
There have been few reports on the role of type I IFNs in parasitic diseases caused by intracellular protozoan parasites. Infection with Leishmania major can induce the production of IFN-α/β from plasmacytoid, but not myeloid, dendritic cells in a TLR9-dependent manner (16), which is consistent with the findings that L. major-induced IFN-α/β are critical for NO-dependent disease control (17). In addition, a low-dose of exogenous IFN-β given to L. major-infected BALB/c mice can confer long-term protection against progressive leishmaniasis, which is accompanied by increased production of Th1 cytokines and expression of inducible NO synthase (18). In the case of Trypanosoma cruzi infection, a study with IFNAR−/− mice suggested no major role for endogenous type I IFN in host defense (19); however, other studies revealed a protective effect of type I IFNs, especially in the absence of MyD88 signaling (20, 21).
Although exogenous type I IFNs promote the control of L. major infection (17, 18), there is no direct evidence for the role of endogenous type I IFNs in leishmaniasis. To address this issue, we infected IFNAR−/− mice with Leishmania amazonensis, a New World species that can cause nonhealing cutaneous leishmaniasis in the absence of Th2 dominancy in most inbred mouse strains (22, 23). Surprisingly, we found that IFNAR−/− mice developed significantly smaller lesions than did wild-type (WT) controls, which correlated with a reduced, rather than an enhanced, T cell response. Further mechanistic analyses revealed that the IFNAR deficiency was associated with a sustained recruitment of neutrophils (but limited recruitment of monocytes/macrophages [Mϕs]) at the early stage of infection, the enhanced release of neutrophil granular enzymes, fast turnover of neutrophils, and enhanced parasite killing in vitro and in vivo. This study highlights the contribution of innate immunity in parasitic infection and is the first report indicating a detrimental role for endogenous type I IFN signaling in host defense against nonhealing cutaneous leishmaniasis.
Materials and Methods
Mice
Breeding pairs of IFNAR−/− mice from the 129/Sv background were kindly provided by Dr. Herbert Virgin (Washington University School of Medicine, St. Louis, MO) and were bred in our animal facility. WT 129/SvImj and 129/SvEv mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and Taconic (Germantown, NY), respectively, and they exhibited comparable susceptibilities to L. amazonensis parasites. STAT1−/− and WT 129/SvEv mice were purchased from Taconic. BALB/c mice (The Jackson Laboratory)were used to maintain the infectivity of the parasite. All mice were maintained under specific pathogen-free conditions in an accredited animal care facility at the University of Texas Medical branch and used at 8–12 wk of age, according to institutional-approved protocols.
Parasite culture and Ag preparation
The infectivity of L. amazonensis (MHOM/BR/77/LTB0016) and L. major (MHOM/IL/80/Friedlin) was maintained by regular passage through BALB/c mice, and that of L. braziliensis (MHOM/BR/79/LTB111) was maintained by regular passage through Syrian golden hamsters (Harlan Sprague Dawley). Promastigotes were cultured at 23°C in Schneider’s Drosophila medium (pH 7) (Invitrogen, Carlsbad, CA) supplemented with 20% FBS (HyClone, Logan, UT), 2 mM l-glutamine, and 50 µg/ml gentamicin. Stationary-stage promastigote cultures of less than five in vitro passages were used in all infection studies. Promastigote lysates were prepared by freeze–thaw cycles of parasites (2 × 108/ml in PBS), followed by a 15-min sonication.
In vivo infection and disease evaluation
Female IFNAR−/− and WT mice (n = 4–5/group) were infected s.c. in the right hind foot with 2 × 106 stationary promastigotes. Lesion size was monitored weekly and expressed as the difference in thickness between the infected and contralateral feet; tissue parasite loads were measured via a limiting dilution assay. At 2, 4, and 8 wk, popliteal draining lymph node (LN) cells (5 × 106/well/ml) from individual mice were restimulated with parasite lysates (equivalent to 5 × 106 parasites). Supernatants were harvested at 72 h to measure the levels of IFN-γ and IL-10 by ELISA. Draining LN cells (1 × 106/ml) at 4 wk were restimulated with PMA/ionomycin/GolgiPlug for 6 h, and intracellular cytokines (IFN-γ and IL-10) gated on CD3+CD4+ T cells were analyzed by FACS.
Serum Ab titer
Serum samples were collected from control and infected mice at 8 wk post infection. To assess parasite-specific Abs, Immulon plates (Thermo Electron, Milford, MA) were coated with promastigote lysates (50 µg/ml) overnight at 4°C. After blocking, plates were incubated with individual serum samples (1:100 dilution) for 1 h at room temperature. Then, plates were incubated with HRP-conjugated goat anti-mouse IgG1 or IgG2a (BD Biosciences, San Jose, CA). Color was developed with the tetramethylbenzidine substrate (BD Biosciences). OD values at 450 nm were measured with a Multiskan Ascent ELISA reader (Labsystems, Helsinki, Finland).
Parasite quantification by real-time PCR
Parasite loads were quantified by measuring the gene of L. amazonensis cysteine proteinase isoform 1 (Llacys1), which is a single-copy gene per haploid genome and is expressed in both developmental stages. Mice were infected intradermally (i.d.) with 1 × 106 promastigotes in the presence or absence of IFN-α (200 U/mouse) in the inside of the ear. At 3, 7, and 14 d, tissues from the inoculation site (~0.5 × 0.5 cm2) were collected for DNA extraction with a DNeasy kit (Qiagen, Valencia, CA). DNA (100 ng) was used for parasite detection by the University of Texas Medical Branch Real-time PCR Core Facility (all reagents were purchased from Applied Biosystems, Foster City, CA). Each sample was run in duplicate and normalized to the amount of total DNA extracted. The number of parasites per sample was calculated based on a standard curve, as described in our previous studies (24).
Tissue section staining
Mice were infected i.d. with 1 × 106 promastigotes in the presence or absence of IFN-α (200 U/mouse) in the ear. At 1, 3, and 7 d, ear tissue was collected, fixed with 10% neutral buffered formalin, and embedded in paraffin. Tissue sections (5-µm thickness) were stained with H&E.
Parasite injection in a peritoneal model
Mice were injected i.p. with promastigotes (2 × 107 in 1 ml). At 6, 24, and 48 h post injection, peritoneal cells were collected and stained with FITC-conjugated anti–Gr-1, PE-conjugated anti-F4/80, and PerCP Cy5.5-conjugated anti-CD11b. Unless specified otherwise, all Abs and isotype controls were purchased from eBioscience (San Diego, CA). For typing Gr-1+ cells, FITC-conjugated anti–Ly-6C and PE-conjugated anti–Ly-6G (BD Biosciences) were used together with PerCP Cy5.5-conjugated anti-CD11b and allophycocyanin-conjugated anti–Gr-1. Cell populations were analyzed by FACSCanto, and data were analyzed by using FlowJo software (Tree Star, Ashland, OR). In some cases, different cell populations were further sorted out and cytospun onto slides. Cell morphology was observed following Diff-Quik staining. Alternatively, single-cell images were visualized by using Amnis Imagestream100 (Amnis, Seattle, WA). For cross-transfer experiments, peritoneal exudates were harvested with 1 ml PBS at 24 h post injection with parasites for the preparation of cell-free supernatants. Recipient mice were injected i.p. with 2 × 107 promastigotes prepared in 1-ml supernatants from WT or IFNAR−/− mice. At 24 h post injection, peritoneal cells were collected for FACS analysis.
Ear cell staining and real-time PCR
At 1, 2, or 7 d, the infected ears were excised and split into dorsal and ventral halves. Dermal sheets were placed on complete IMDM medium containing 1 mg/ml collagenase/dispase and 50 U/ml DNase I (Roche Diagnostics, Indianapolis, IN) and incubated at 37°C for 1 h. The treated ear sheets were passed through 70-µm cell strainers (BD Biosciences), and the resulting cell suspensions were washed and stained with Abs specific to Ly-6C, Ly-6G, CD11b, and CD45. Cell populations gated on CD45+ cells were analyzed by FACSCanto, and data were analyzed by using FlowJo software. For gene expression in ear tissues, total RNA was extracted by an RNeasy Mini Kit (Qiagen) from the lesion areas at 1, 2, or 7 d, and cDNA was synthesized by the SuperScript III first-strand synthesis system (Invitrogen). Real-time PCR was performed on cDNA samples by using TaqMan probes for mouse Spp1, Il1b, Cxcl2, Ccl2, and Ccl5 genes (all from Applied Biosystems). The relative quantity value is expressed as 2−ΔCT, where ΔCT is the difference between the mean cycle threshold value of duplicates of the sample and of the endogenous 18S control.
Isolation of bone marrow- or peritoneal-derived neutrophils
Bone marrow cells were collected from the femurs using ice-cold RPMI 1640 containing 5% FCS and treated with an RBC lysis buffer. Peritoneal exudate cells were obtained 6 h after injection with 1 ml 3% thioglycollate (TG; Sigma-Aldrich, St. Louis, MO). The isolation of bone marrow and peritoneal neutrophils was accomplished via density gradient centrifugation by using stepwise gradients of 55, 65, and 75% Percoll (Sigma-Aldrich). After centrifugation at 1500 × g for 30 min at 4°C, the band between 65 and 75% of Percoll was collected. The purity of neutrophils (>95%) was validated by FACS and examination of morphology after staining; cell viability was routinely >95%, as monitored by trypan blue exclusion.
Neutrophil and Mϕ coculture
Peritoneal Mϕs were obtained 5 d after injection of 3% TG and seeded in 24-well plates (2–3 × 105 per well). After 4 h of incubation and extensive washing, adherent cells were infected with 2 × 106 promastigotes in the absence or presence of LPS (100 ng/ml) plus IFN-γ (100 ng/ml) at 33°C for 3 d. For neutrophil–Mϕ coculture, TG-elicited purified peritoneal neutrophils (2 × 106/ml) were preinfected with promastigotes (1:5 cell/parasite ratio) at 33°C for 4 h. Preinfected neutrophils were washed and added to a Mϕ monolayer (1:10 Mϕ/neutrophil ratio). After incubation at 33°C for 3 d, DNA samples were extracted for parasite load analysis by real-time PCR.
Neutrophil elastase and myeloperoxidase activity assay
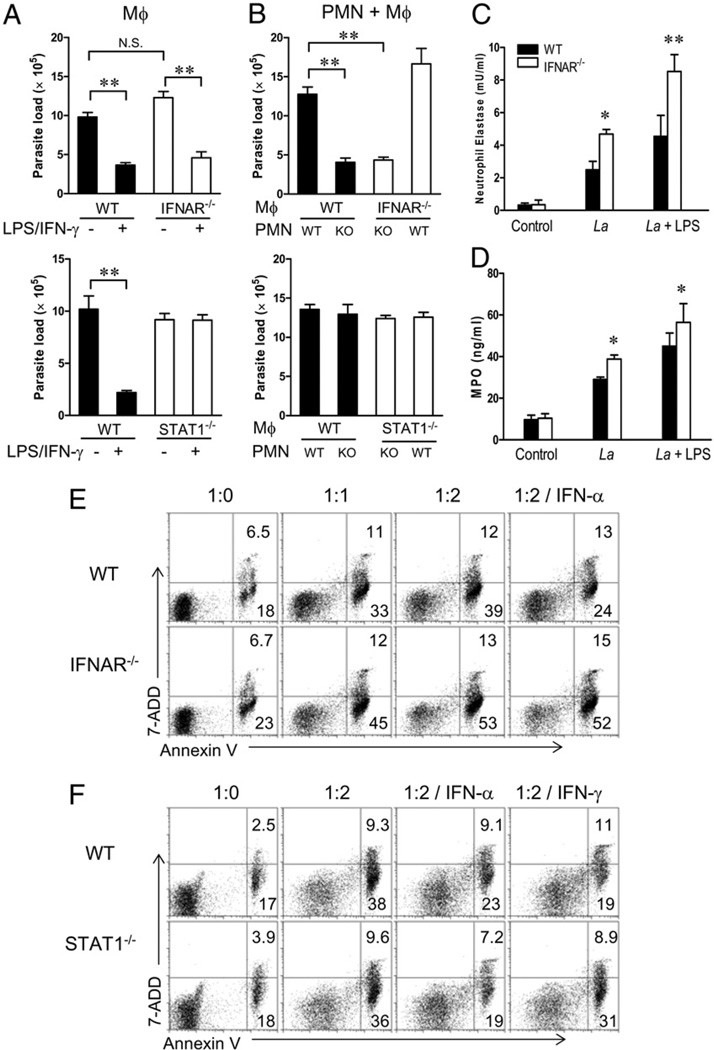
TG-elicited purified peritoneal neutrophils (1 × 107/ml in sera-free medium) from WT and IFNAR−/− mice were infected with promastigotes at a 1:2 cell/parasite ratio at 33°C in the absence or presence of LPS (20 ng/ml) for 6 h. The activities of neutrophil elastase (NE) and myeloperoxidase (MPO) were measured by EnzChek activity assay kits (Molecular Probes, Eugene, OR).
Neutrophil apoptosis
TG-elicited purified peritoneal neutrophils from WT, IFNAR−/−, or STAT1−/− mice were infected with promastigotes at the indicated ratios at 33°C in the absence or presence of IFN-α (200 U/ml) or IFN-γ (100 ng/ml). Eighteen hours later, neutrophils were stained with PE-conjugated anti–Gr-1, FITC-conjugated Annexin V, and 7-aminoactinomycin D (7-AAD) (BD Biosciences). The percentages of apoptotic Gr-1+ neutrophils were analyzed based on positive-staining for Annexin V but negative-staining for 7-AAD− by FACS.
Neutrophil coinjection and adoptive cell transfer
TG-elicited purified peritoneal neutrophils (2 × 106) from WT or IFNAR−/− mice were coinjected with 1 × 106 promastigotes in the ear of recipient mice. Parasite loads at 2 wk were determined by real-time PCR. For adoptive cell transfer, neutrophils were purified from bone marrow and adoptively transferred into recipients through the tail vein (5 × 106 cells in 200 µl HBSS). One day later, recipient mice were infected with 1 × 106 promastigotes in the ear. Parasite loads at 1 and 2 wk were determined by real-time PCR.
Statistical analysis
The comparison between two different groups was determined by using the two-tailed Student t test. The tests were performed by using GraphPad Prism, version 4.00, for Windows (GraphPad Software, San Diego, CA).
Results
IFNAR−/− mice develop an attenuated cutaneous leishmaniasis
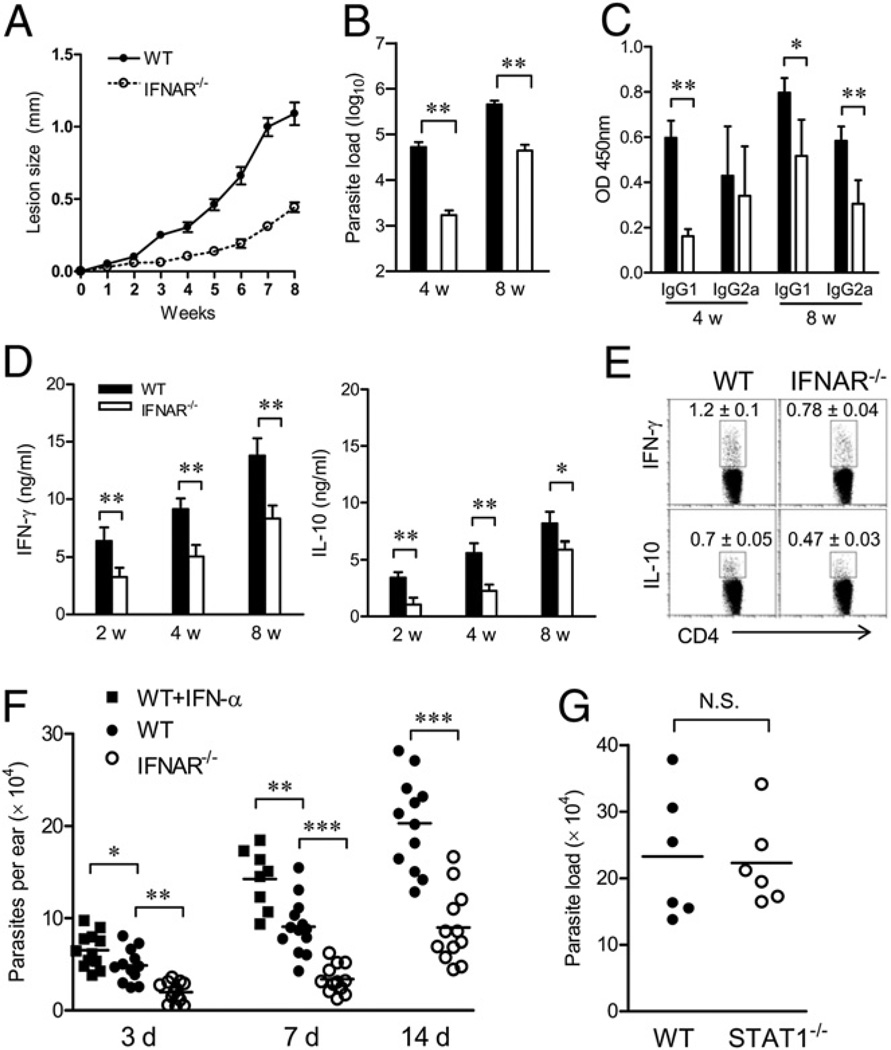
Although type I IFNs play a protective role in the control of L. major infection (17, 18), there is no direct evidence for the role of endogenous type I IFNs in leishmaniasis. In this study, we used IFNAR−/− mice to investigate the role of type I IFN signaling in cutaneous leishmaniasis induced by L. amazonensis parasites. Surprisingly, we found that L. amazonensis-infected IFNAR−/− mice developed an attenuated disease, with significantly smaller lesions and lower parasite loads in foot tissues, compared with WT controls (Fig. 1A, 1B). Serum parasite-specific IgG1 titers at 4 and 8 wk and IgG2a titers at 8 wk from IFNAR−/− mice were also lower than those from WT controls (Fig. 1C). To ascertain whether this attenuated disease in IFNAR−/− mice was due to an enhanced Th1 response, we evaluated cytokine production from draining LN cells following Ag restimulation in vitro. As shown in Fig. 1D, the production of IFN-γ and IL-10 in LN cells of IFNAR−/− mice was significantly lower than that of WT mice at 2, 4, and 8 wk. The similar results were confirmed by intracellular cytokine production at the single-cell level (Fig. 1E). Therefore, the attenuated disease in L. amazonensis-infected IFNAR−/− mice correlated to a reduced, rather than enhanced, adaptive response. In self-healing cutaneous leishmaniasis models caused by L. braziliensis or L. major infection, infected IFNAR−/− mice also developed smaller lesions that healed faster than did those in WT controls (Supplemental Fig. 1). Together, these results suggest an attenuated cutaneous leishmaniasis in IFNAR−/− mice.
FIGURE 1.
Attenuated disease in L. amazonensis-infected IFNAR−/− mice. WT and IFNAR−/− mice (n = 3–4 per group) were infected s.c. with 2 × 106 promastigotes in the right hind foot. A, Lesion sizes were monitored weekly. B, Parasite loads at 4 and 8 wk were measured by a limiting dilution assay. C, Ag-specific IgG1 and IgG2a in sera at 8 wk were determined by ELISA. D, Draining LN cells at 2, 4, and 8 wk were restimulated with parasite lysates for 72 h. IFN-γ and IL-10 in the supernatant were measured by ELISA. Results shown are pooled from three independent repeats. E, Intracellular cytokines (IFN-γ and IL-10) from CD4+ T cells in draining LN cells at 4 wk were measured by ex vivo restimulation with PMA/ionomycin. F, WT and IFNAR−/− mice (n = 12) were injected i.d. in the ear with 1 × 106 promastigotes in the absence or presence of IFN-α (200 U/mouse). Parasite numbers per ear at indicated days postinfection were estimated by real-time PCR. *p < 0.05; **p < 0.01; ***p < 0.001. G, WT and STAT1−/− mice (n = 6) were similarly infected. Parasite numbers per ear were estimated at 14 d postinfection by real-time PCR.
Limited parasite growth at very early stages in IFNAR−/−, but not STAT1−/− mice
We showed that the early events at the inoculation site determine parasite survival and disease outcome in Leishmania-infected mice (23). To define the contribution of innate immunity, we used an ear infection model to quantify tissue parasite loads and host responses during the first few days. As shown in Fig. 1F, even by day 3, IFNAR−/− mice had significantly lower parasite loads in ear tissues than did the WT controls (p < 0.01); however, the coinjection of IFN-α with parasites significantly enhanced tissue parasite loads in WT mice at days 3 (p < 0.05) and 7 (p < 0.01). Parasite loads in IFNAR−/− mice were consistently lower than those in their WT controls at days 7 and 14 (p < 0.001). Because STAT1 is critical for types I and II IFN signaling, we infected STAT1−/− mice and found no major differences in tissue parasite loads compared with those in WT mice at day 14 (Fig. 1G). These results suggest that the reduced parasite growth in IFNAR−/− mice is mediated by a STAT1-independent mechanism.
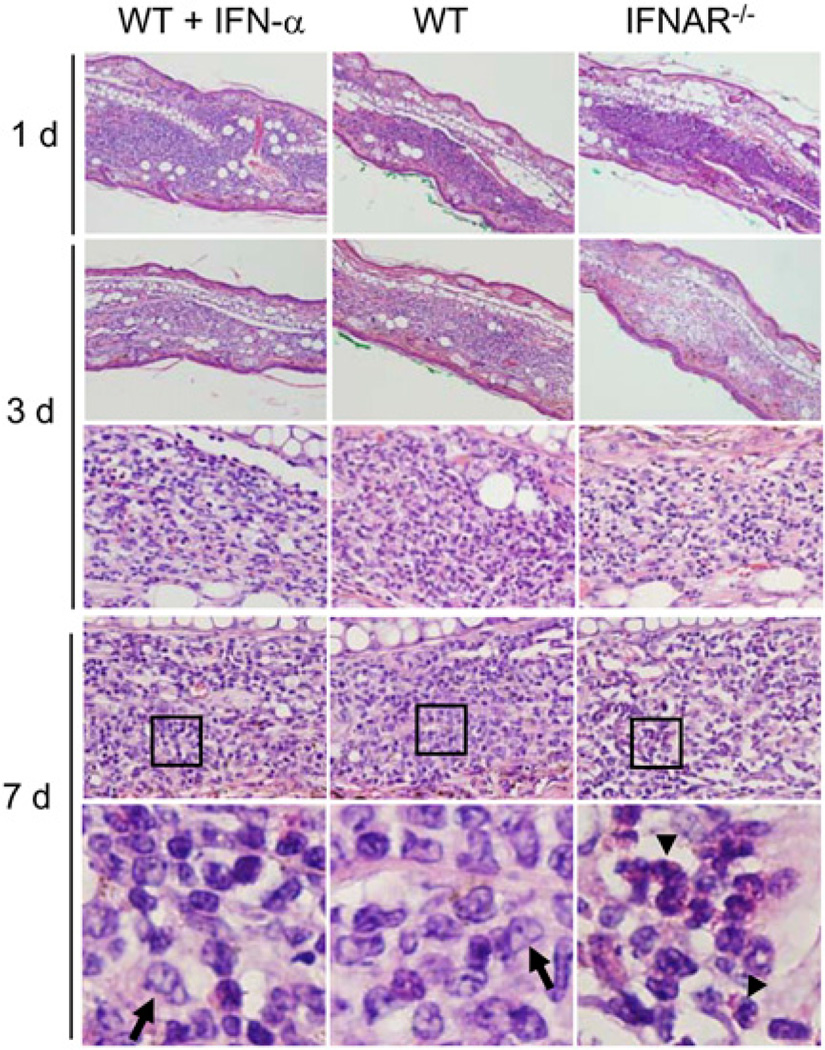
We also examined histological changes in ear tissues and in situ cytokine/chemokine gene expression by microarray analysis. As shown in Fig. 2, except for a more extensive cellular influx at day 1, IFN-α–treated mice displayed comparable tissue responses compared with those in WT controls during our observation period. Of note, although IFNAR−/− mice exhibited comparable cellular infiltration at days 1 and 2, cellular infiltrates in these mice seemed to be milder at days 3 and 7 (Fig. 2, data not shown). In contrast to the prominent Mϕ infiltration in the other two groups at day 7, neutrophil clusters were readily detected in IFNAR−/− mice (Fig. 2, bottom panel). These pathological changes may be related to the differential expression of cytokine/chemokine genes (Supplemental Fig. 2). For example, IFNAR−/− mice expressed relatively high levels of secreted phosphoprotein 1 (Spp1), TNF-α, IL-1β, CXCL2, and CCL4 at day 1, but favored the expression of CXCL9, CCR2, IFN-γ, IL-10, and TGF-β1 at day 3.
FIGURE 2.
Cellular infiltration in infected ear tissues at very early stages. WT and IFNAR−/− mice were injected i.d. in the ear with 1 × 106 promastigotes in the absence or presence of IFN-α (200 U/mouse). Tissue sections were collected at the indicated days post infection and stained with H&E. The lower panels for day 7 represent an enlarged view of the boxed areas. The arrows point to Mϕs, and the arrowheads point to a cluster of neutrophils. Upper two panels, original magnification ×100; middle two panels, original magnification ×200; lower panel, original magnification ×400. Representative results from one of three independent repeats are shown.
IFNAR−/− mice display a unique profile for neutrophil and monocyte/Mϕ recruitment
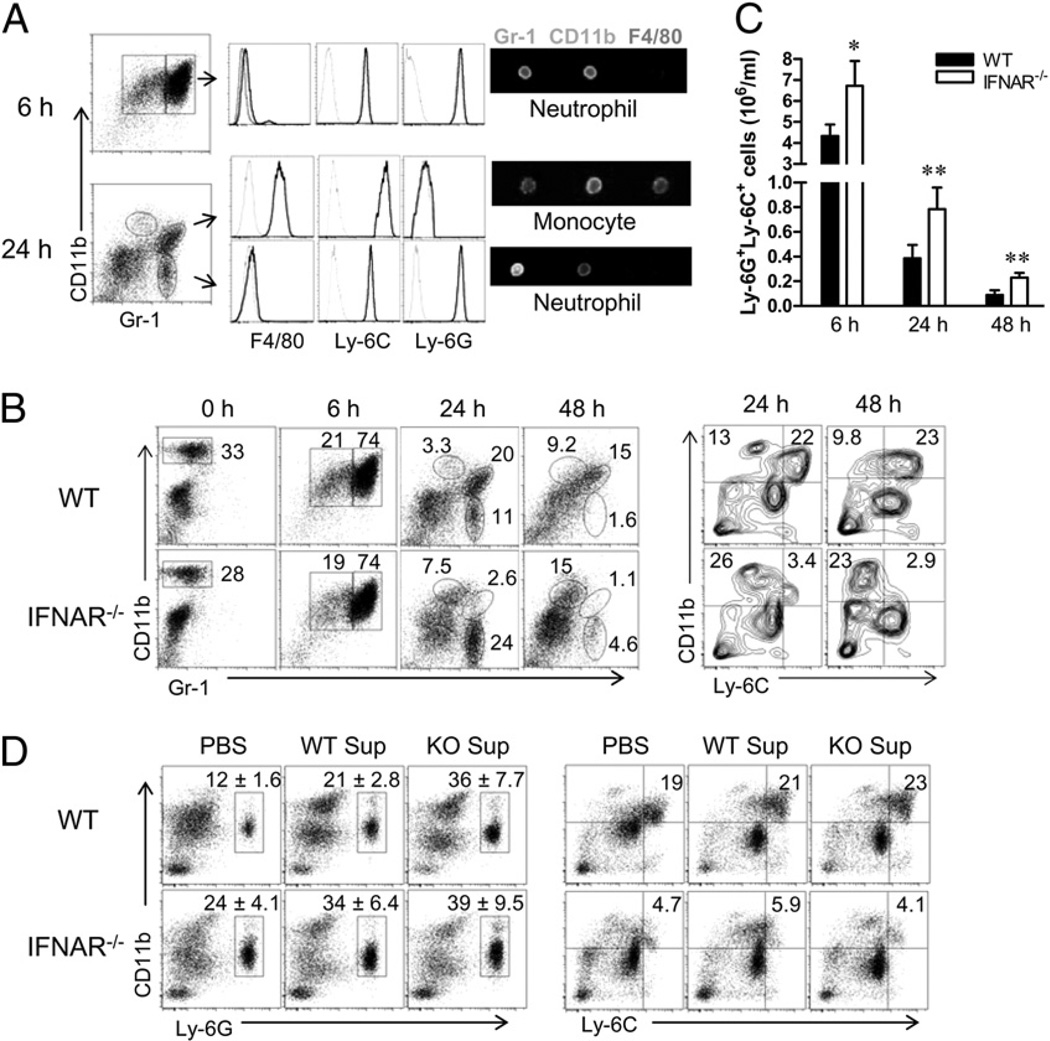
Neutrophils are the first line of host defense and are rapidly recruited to the site of Leishmania inoculation (25). For the ease of defining the phenotype of early infiltrates, we first used a peritoneal injection model, in which 2 × 107 promastigotes were injected. At 6 h postinjection, WT mice predominantly recruited CD11b+Gr-1+ neutrophils that were also positive for Ly-6C and Ly-6G (Fig. 3A). However, at 24 h, two distinct Gr-1+ cell populations were detected: CD11b+Gr-1+F4/80+Ly-6C+Ly-6G− inflammatory monocytes and CD11bdimGr-1+F4/80−Ly-6C+Ly-6G+ neutrophils. A third population (CD11b+Gr-1− cells) represented the residential Mϕs, as previously reported (26). The surface markers and morphology of these cell populations were confirmed by using Image Stream analysis (Fig. 3A) and Diff-Quik staining (Supplemental Fig. 3), respectively. Using these markers, we then examined cellular recruitment in WT and IFNAR−/− mice. As shown in Fig. 3B, both mouse strains contained ~30% of CD11b+Gr-1−F4/80+ residential Mϕs prior to parasite injection, as well as comparable percentages (~74%) of CD11b+Gr-1+ (Ly-6C+Ly-6G+) neutrophils at 6 h post injection. However, IFNAR−/− mice recruited significantly higher percentages of CD11bdimGr-1+ (F4/80−Ly-6C+Ly-6G+) neutrophils than did WT mice at 24 h (24% versus 11%) and at 48 h (4.6% versus 1.6%), a finding suggestive of a strong and sustained recruitment of neutrophils in IFNAR−/− mice. This conclusion was supported by the significantly high numbers of Ly-6G+Ly-6C+ neutrophils recovered from IFNAR−/− mice at 6–48 h (Fig. 3C). In contrast, WT mice recruited 6–8-fold more CD11b+Ly-6C+Ly-6G− inflammatory monocytes than did the IFNAR−/− mice (Fig. 3B, right panel). The similar trends in the recruitment of neutrophils and inflammatory monocytes were observed following i.p. injection of L. major promastigotes (Supplemental Fig. 4) and L. braziliensis promastigotes (data not shown).
FIGURE 3.
Neutrophil and monocyte recruitment following injection with parasites. WT and IFNAR−/− mice (n = 3 per group) were injected i.p. with 2 × 107 promastigotes. At the indicated time points, peritoneal cells were collected, stained with different markers, and analyzed by FACS. A, The expression of F4/80, Ly-6C, and Ly-6G was analyzed on Gr-1+ populations from WT mice at 6 and 24 h. B, The percentages of peritoneal subsets from WT and IFNAR−/− mice were examined by staining with CD11b and Gr-1 (left panel), and the percentages of CD11b+Ly-6C+ inflammatory monocytes and CD11b+Ly-6C− monocytes at 24 and 48 h were confirmed by FACS (right panel). C, The numbers of peritoneal Ly-6G+Ly-6C+ neutrophils were calculated at the indicated time points. Results shown are pooled from three independent repeats. *p < 0.05; **p < 0.01. D, Peritoneal exudates were collected at 24 h post injection, and cell-free supernatants were injected together with promastigotes into WT or IFNAR−/− (KO Sup) mice; 24 h later, the percentages of neutrophils and monocytes were analyzed by FACS. The percentages of neutrophils are shown as mean ± SD from three repeats, and the representative results for inflammatory monocytes are from one of three independent repeats.
To confirm these findings and to explore the possible mechanism underlying the differential recruitment of neutrophils and inflammatory monocytes, we generated cell-free supernatants from WT or IFNAR−/− peritoneal exudates and injected them with parasites into two strains of recipients. We found that although both types of supernatants induced a 2–3-fold increase in total peritoneal cells compared with a PBS control at 24 h post transfer (data not shown), the supernatants from IFNAR−/− mice greatly promoted the recruitment of CD11b+Ly-6G+ neutrophils in WT recipients (36% ± 7.7% versus 21% ± 2.8%) (Fig. 3D, left panel). However, no major differences were observed for the recruitment of CD11b+Ly-6C+ inflammatory monocytes in the recipients (Fig. 3D, right panel). These results point to the possibility that enhanced neutrophil recruitment in IFNAR−/− mice is due to the production of soluble factors in the supernatants.
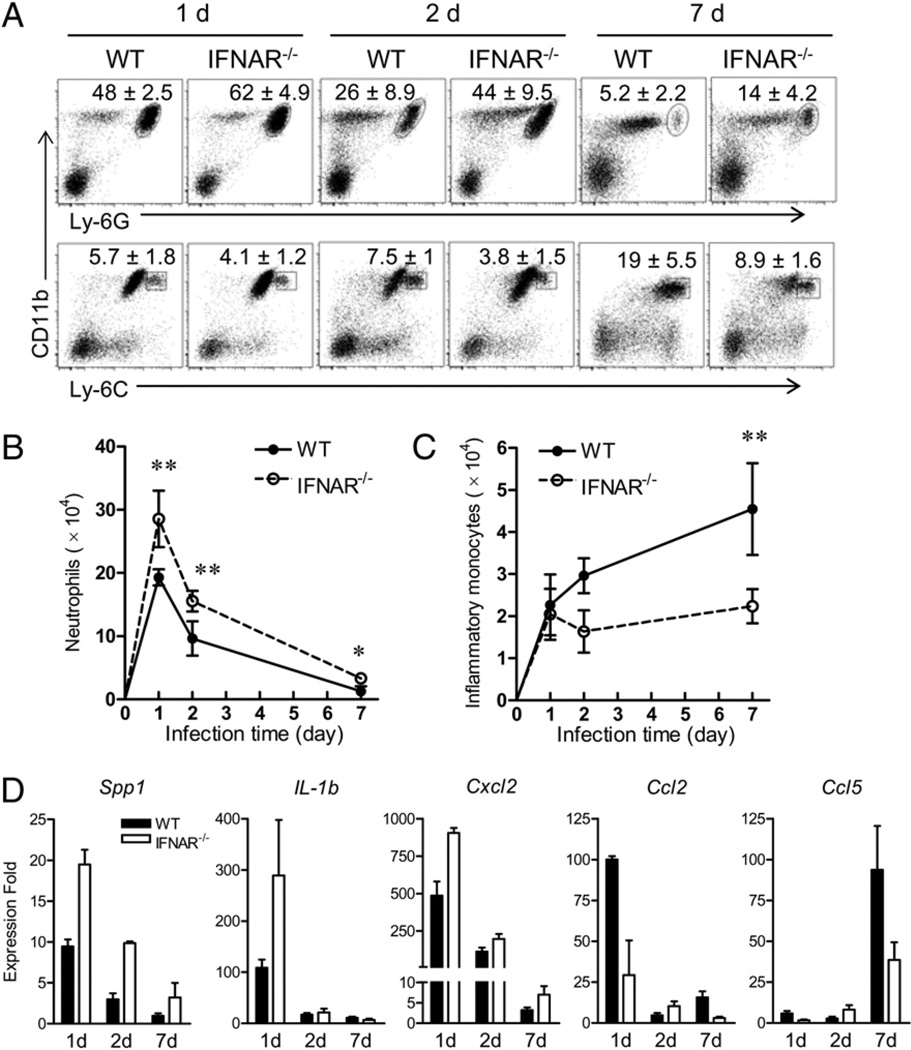
Importantly, our data from the ear infection model validated and extended these findings. At 1, 2, and 7 d, we consistently detected greater percentages of CD11b+Ly-6G+ neutrophils (Fig. 4A, upper row) and significantly greater numbers of neutrophils (Fig. 4B) from the ear tissues of IFNAR−/− mice than from WT controls, although both mouse strains displayed similar kinetics of neutrophil recruitment (Fig. 4B). In sharp contrast to the favored neutrophil recruitment, IFNAR−/− mice were less favored for the recruitment of inflammatory monocytes than were the WT controls, as judged by their low percentages of CD11b+Ly-6C+ cells (Fig. 4A, lower row) and the significantly low numbers of inflammatory monocytes recovered, especially at day 7 (Fig. 4C). Furthermore, following gene-expression analysis of infected tissues, there was an elevated expression of neutrophil chemokine Cxcl2, as well as other inflammatory cytokines, such as Spp1 and IL-1b (Fig. 4D), which correlated to the enhanced neutrophil recruitment at days 1 and 2 in IFNAR−/− mice. However, in WT mice, the expression levels of Ccl2 and Ccl5 at day 7 seemed to correlate with the favored recruitment of inflammatory monocytes (Fig. 4D). In addition, we detected comparable percentages of neutrophils and monocytes in the blood of naive or infected mice and in the draining LNs of naive WT and IFNAR−/− mice (Supplemental Fig. 5A) but a decreased recruitment of inflammatory monocytes at day 7 in the draining LNs of IFNAR−/− mice (Supplemental Fig. 5B). Together, our studies with a panel of surface markers, different infection models, and cells derived from different locations confirmed an early and efficient neutrophil influx, which was accompanied by a limited recruitment of inflammatory monocytes, to the inflamed tissues in IFNAR−/− mice. This differential cellular recruitment is likely due to the differential production of cytokines and chemokines at early stages of parasite–host interaction.
FIGURE 4.
Recruitment of neutrophils and monocytes into infected ear tissues. WT and IFNAR−/− mice (n = 3–4 per group) were injected i.d. with 1 × 106 promastigotes in the ear. A, At 1, 2, and 7 d of infection, ear tissue cells were collected and stained with the indicated markers for FACS analysis. Shown are the percentages of neutrophils and monocytes. Data are presented as mean ± SD pooled from two independent repeats. The absolute numbers of tissue neutrophils (B) and inflammatory monocytes (C) were calculated at the indicated time points. Results shown are pooled from two independent repeats. *p < 0.05; **p < 0.01. D, The expression levels of indicated genes in infected ear tissues were analyzed by real-time PCR. Data are presented as relative changes in gene expression (fold) normalized to the 18S gene and are representative results from two independent repeats.
IFNAR−/− neutrophils promote parasite killing in vitro
Because neutrophils can influence L. major infection through direct interaction with Mϕs (27), we investigated the potential of parasite growth control in neutrophils and Mϕs in vitro. As shown in Fig. 5A, peritoneal Mϕs derived from WT and IFNAR−/− mice contained comparable parasite loads at 3 d postinfection with L. amazonensis parasites and efficiently killed parasites following treatment with LPS plus IFN-γ, suggesting no overt differences at the Mϕ level. When TG-elicited peritoneal neutrophils derived from WT and IFNAR−/− mice were infected with promastigotes (1:5 cell/parasite ratio), comparable infection rates (~50–60%) were reached at 4 h postinfection, suggesting no major differences in the uptake of parasites in two types of neutrophils. To mimic a natural infection setting (25), we cocultured preinfected neutrophils with Mϕs (10:1 neutrophil/Mϕ ratio) under different combinations for 3 d and examined parasite loads in the cultures by real-time PCR. We found that the coculture of infected IFNAR−/−, but not WT, neutrophils with either source of Mϕs resulted in a significant reduction in parasite loads (Fig. 5B; p < 0.01). As a control, we did similar studies with cells derived from STAT1−/− mice. As expected, STAT1−/− Mϕs failed to control parasite growth in response to LPS/IFN-γ treatment (Fig. 5A); however, STAT1−/− neutrophils were incompetent in promoting parasite killing in the coculture system (Fig. 5B). Therefore, IFNAR−/−, but not WT or STAT1−/−, neutrophils exhibited unique features in promoting parasite killing.
FIGURE 5.
IFNAR−/− neutrophils promote parasite killing. A, TG-elicited peritoneal Mϕs from WT, IFNAR−/−, and STAT1−/− mice were infected with promastigotes in the absence or presence of LPS (100 ng/ml) plus IFN-γ (100 ng/ml) at a cell/parasite ratio of 1:10 for 3 d. Parasite loads were determined by real-time PCR. B, TG-elicited peritoneal PMNs purified from WT, IFNAR−/− and STAT1−/− mice were preinfected with promastigotes (1:5 cell/parasite ratio) for 4 h. After washing, infected neutrophils were cocultured with peritoneal Mϕs (10:1 PMN/Mϕ ratio) for 3 d. Parasite loads were determined by real-time PCR. C and D, TG-elicited peritoneal neutrophils (1 × 107/ml) were infected with promastigotes in the absence or presence of LPS (20 ng/ml) at a cell/parasite ratio of 1:2 at 33°C for 6 h. NE activity (C) and MPO content (D) were measured in the supernatants. E and F, TG-elicited peritoneal neutrophils purified from WT, IFNAR−/− (E), and STAT1−/− (F) mice were infected with promastigotes in the absence or presence of IFN-α (200 U/ml) or IFN-γ (100 ng/ml) at the indicated cell/parasite ratios for 18 h. Neutrophil apoptosis in Gr-1–gated cells was assayed by staining with Annexin V and 7-AAD, respectively. The numbers indicate the percentages of positively stained cells. Representative results are shown from three independent repeats. *p < 0.05; **p < 0.01. PMN, polymorphonuclear neutrophil.
IFNAR−/− neutrophils efficiently release enzymes and have a fast turnover rate
Upon phagocytosis or activation, neutrophils can release antimicrobial molecules and enzymes from the azurophilic granules, such as MPO and NE, which promote killing of bacteria (28) and Leishmania parasites (29). We tested whether parasite infection can trigger MPO and/or NE release from TG-elicited peritoneal neutrophils and whether such responses are favored in IFNAR−/− neutrophils. Infected IFNAR−/− neutrophil cultures showed significantly enhanced NE and MPO activities (Fig. 5C, 5D) compared with the cultures from WT controls. The enhanced release of MPO and NE in IFNAR−/− neutrophils was also observed after treatment with a low dose (20 ng/ml) of LPS (Fig. 5C, 5D). In addition to having microbicidal activities, neutrophils can produce immunoregulatory cytokines to trigger or shape the adaptive immune response (30). We next investigated cytokine production from peritoneal WT and IFNAR−/− neutrophils at 6 h after parasite injection via intracellular staining. We detected comparable percentages of IFN-γ+ and TNF-α+ neutrophils, as well as low percentages of IL-12+ and IL-10+ neutrophils, in the two groups of mice (Supplemental Fig. 6).
Given that apoptotic neutrophils can greatly modulate Mϕ function and influence the fate of parasites in Mϕs (27, 31), we tested whether enhanced parasite killing in IFNAR−/− mice is due to altered neutrophil apoptosis. TG-elicited peritoneal neutrophils were incubated with L. amazonensis parasites for 18 h. We found that IFNAR−/− neutrophils showed relatively greater spontaneous and parasite-induced apoptosis than did the WT controls, as judged by the percentages of Annexin V+ 7-AAD− cells gated on Gr-1+ cells (23% versus 18% without parasites, or 45% versus 33% at 1:1 infection ratio) (Fig. 5E). As expected, the addition of IFN-α decreased apoptosis in WT neutrophils (32); however, such treatment had no effect on IFNAR−/− cells (Fig. 5E). STAT1−/− neutrophils responded similarly to those in WT controls, with the exception of their defective responses to IFN-γ (Fig. 5F). Similarly, L. major-infected IFNAR−/− neutrophils showed significantly greater levels of apoptosis than did the WT controls (Supplemental Fig. 7). Collectively, these results indicate that an efficient release of granular enzymes and IFN-γ and a fast turnover rate in IFNAR−/− neutrophils may contribute to the enhanced parasite killing in these mice.
Coinjection and adoptive transfer of WT neutrophils promote parasite growth in vivo
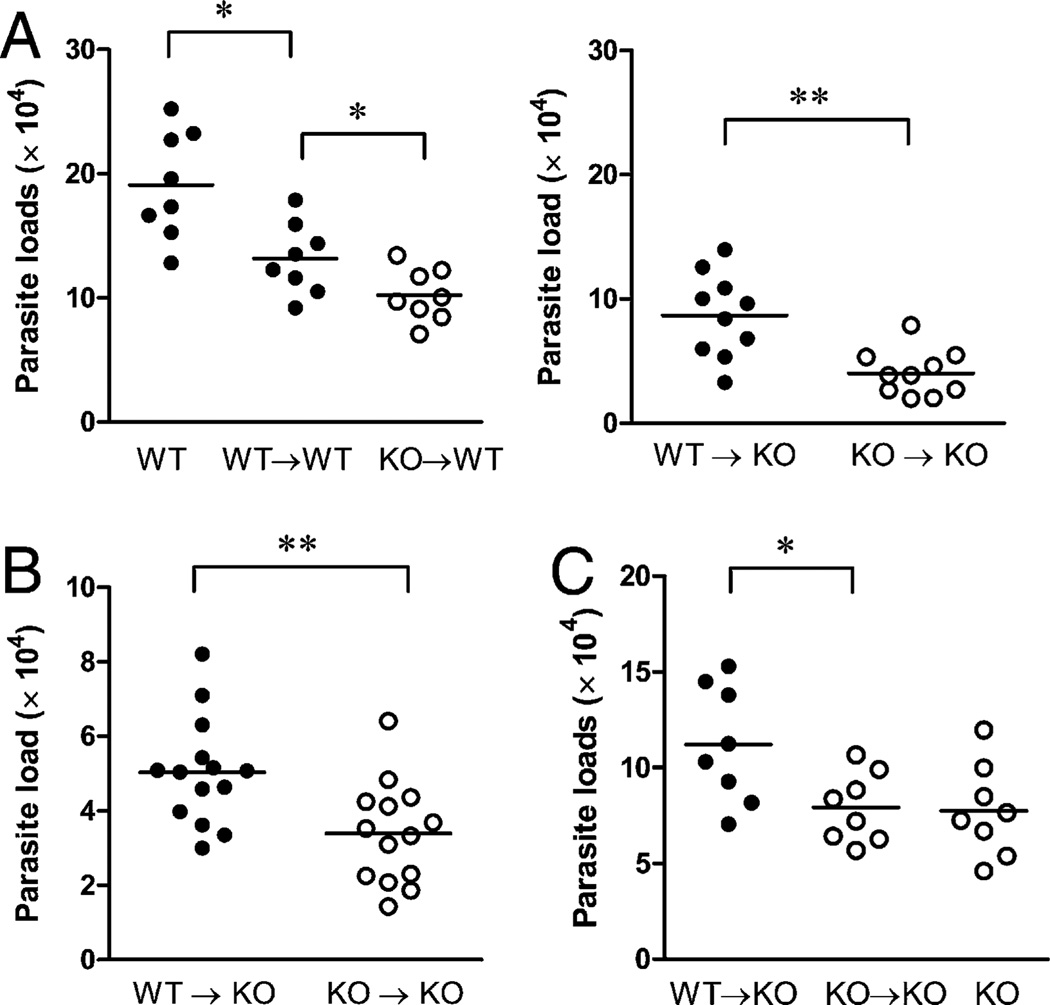
To examine the role of neutrophils in vivo, we took two complementary approaches: WT or IFNAR−/− neutrophils (purified from TG-elicited peritoneal cells) were coinjected with promastigotes or adoptively transferred into two types of recipients 1 d prior to the infection. First, we found that coinjection of WT neutrophils with parasites into WT mice or IFNAR−/− mice resulted in significantly greater tissue parasite loads than did the injection of IFNAR−/− counterparts, regardless of the type of recipient (Fig. 6A). Second, we adoptively transferred purified neutrophils derived from the bone marrow of WT or IFNAR−/− donors into IFNAR−/− recipients 1 d prior to parasite infection. It was evident that the adoptive transfer of WT neutrophils significantly promoted parasite growth at 1 wk (Fig. 6B) and 2 wk post infection (Fig. 6C). Together, these results suggested an infection-promoting effect of WT neutrophils and they indicated that the deficiency of IFNAR in neutrophils impaired such an effect.
FIGURE 6.
Coinjection or adoptive transfer of WT neutrophils promotes parasite growth in vivo. A, TG-elicited peritoneal neutrophils (2 × 106) purified from WT or IFNAR−/− mice were coinjected with 1 × 106 promastigotes in the ear of WT or IFNAR−/− recipients. Parasite numbers per ear at 2 wk postinfection were estimated by real-time PCR. B and C, Neutrophils were purified from bone marrow of WT or IFNAR−/− mice and adoptively transferred into IFNAR−/− recipients through the tail vein. One day later, recipient and control IFNAR−/− mice were injected with 1 × 106 promastigotes in the ear. Parasite numbers per ear at 1 wk (B, n = 14) and 2 wk (C, n = 8) of infection were estimated by real-time PCR. *p < 0.05; **p < 0.01.
Discussion
Contrary to a previous report showing a protective effect of exogenous IFN-β against L. major infection in susceptible mice (18), our study, for the first time, indicates a detrimental role for endogenous type I IFN signaling in host defense against nonhealing cutaneous leishmaniasis caused by L. amazonensis parasites. Consistent with previous studies, which demonstrated that the lack of type I IFN signaling impaired the production of inflammatory cytokines/chemokines (e.g., IFN-γ, TNF-α, IP-10, and inducible NO synthase) in different infection models (3, 4, 6), we also found that the attenuated cutaneous leishmaniasis in IFNAR−/− mice was due to a reduced, rather than an enhanced, Th1 response (Fig. 1). Because Leishmania parasites preferentially infect and replicate within poorly activated or alternatively activated Mϕs, the local cytokine/chemokine milieu that favors monocyte/Mϕ recruitment and/or parasite replication could promote disease progression rather than infection control. Our previous studies indicated that treatment with IFN-γ enhanced the replication of L. amazonensis amastigotes in Mϕs in vitro (33) and that the administration of IL-1β exacerbated lesion development in mice (34). Therefore, the IFNAR deficiency may reduce the inflammatory and immune responses by altering leukocyte recruitment and cytokine/chemokine production. This study reveals an important role for type I IFNs in regulating neutrophil/monocyte recruitment, neutrophil turnover, and Leishmania infection.
CD11b+ Gr-1+ (Ly-6C+) inflammatory monocytes play an essential role in host–pathogen interactions because they can differentiate in situ into tissue Mϕs and dendritic cells (26, 35); regulate adaptive immune responses, such as Th1 response development (36); and participate in host defenses against microbial infection (37). Yet, the factors that regulate the immigration of these inflammatory monocytes are a matter of debate. CCR2 expression is known to be a key player in monocyte migration, especially in terms of its emigration from the bone marrow (38), whereas the involvement of CX3CR1, CCR6, and P-selectin glycoprotein ligand-1 are also reported (39–41). A recent study also suggests a role for a TLR7-mediated innate signaling pathway in the recruitment of inflammatory monocytes (42). In this study, we found that IFNAR deficiency caused a defective recruitment of inflammatory monocytes into the peritoneal cavity and ear skin and skin-draining LNs (Figs. 2–4). Considering the similar levels of CD11b+Ly-6C+ monocytes in the blood of naive and infected WT and IFNAR−/− mice (Supplemental Fig. 5), we speculate that IFNAR deficiency does not affect the emigration of monocytes from bone marrow but rather impairs the recruitment of these cells into inflamed tissues, probably because of the reduced production of inflammatory mediators, such as CCL2 and CCL5 (Fig. 4D, Supplemental Fig. 2). Further studies are ongoing to define the regulation of monocyte recruitment-related chemokines by type I IFN signaling. Regardless of the regulation mechanisms, we believe that the defective/delayed recruitment of monocytes (safe targets for Leishmania replication) partially contributes to the limited parasite replication at the inoculation site, and that monocytes/Mϕs, by themselves, are insufficient to control parasite growth in vitro (Fig. 5A).
In the current study, it is unclear which cell type(s) produce type I IFNs during initial responses to Leishmania parasites. Some studies indicated that plasmacytoid dendritic cells, rather than myeloid dendritic cells, can produce appreciable levels of IFN-α/β following infection with different species of Leishmania via a TLR9-dependent mechanism (16), whereas other studies suggested that inflammatory monocytes are the predominant source of type I IFNs (43). Given that inflammatory monocytes are the rapidly recruited population in our peritoneal injection and cutaneous leishmaniasis models, it is possible that inflammatory monocytes contribute to initiate type I IFN production; we are investigating this possibility.
We have provided solid evidence that a rapid and sustained recruitment of neutrophils is a unique feature in the early stages of L. amazonensis infection in IFNAR−/− mice (Figs. 3, 4). The efficient neutrophil recruitment in these mice is most likely due to the production of soluble factors, because the soluble factors produced in the IFNAR−/− mice directly promoted the recruitment of neutrophils but not inflammatory monocytes (Fig. 3D). Although the nature of the soluble factors in these supernatants was not examined in this study, we speculate that there may be an involvement of Spp1, IL-1β, and CXCL2 because of their early induction and high expression levels in the ear tissues in infected IFNAR−/− mice (Fig. 4D, Supplemental Fig. 2). It was reported that the activation of the IL-1R/MyD88 signaling pathway in resident skin cells is critical for neutrophil recruitment in bacterial infection (44). Of relevance to this study, Shahangian et al. (7) recently demonstrated that the induction of type I IFNs by primary influenza virus infection inhibited the production of keratinocyte-derived chemokine (KC/CXCL1) and CXCL2, resulting in impaired neutrophil responses against a secondary challenge with S. pneumoniae. Therefore, results from our Leishmania infection study support the view that endogenous or viral-induced type I IFN signaling can negatively regulate the production of neutrophil-recruiting cytokines/chemokines and that the lack of IFNAR promotes the production of such molecules and rapid influx of neutrophils to the infection site.
The function of recruited neutrophils in the fate of engulfed Leishmania parasites and the outcome of infection are subjects of active research. It was proposed that neutrophils can act like “Trojan horses” for harboring and transporting parasite into Mϕs (45). Peters et al. (25) revealed via in vivo imaging that neutrophils can efficiently capture L. major parasites delivered by sand flies or needles and that neutrophils can release live parasites and undergo apoptosis. Therefore, neutrophils may promote Leishmania infection by rescuing/protecting promastigotes from death in the extracellular space or by facilitating parasite infection in monocytes/Mϕs. Using neutralizing Abs, some studies showed that neutrophil depletion reduced lesion development in L. major-infected susceptible mice (46), but it increased parasite loads in L. major or L. braziliensis infection in genetically resistant strains of mice (47, 48). However, the opposite results were observed by Peters et al. (25). A recent study showed that although most Leishmania donovani parasites were rapidly killed by phagocytosis, only a small number of parasites targeted to the nonlytic compartments of the cells survived in neutrophils (49). Collectively, these studies highlight a critical, but complex, role for neutrophils in Leishmania infection. We propose that the net (detrimental versus protective) effects of neutrophils are influenced by the intrinsic sensitivity of Leishmania spp. to neutrophil-based killing mechanisms and the activation status of neutrophils.
The formation of neutrophil extracellular traps (NETs) is one possible mechanism underlying microbial killing by neutrophils. NETs are composed of decondensed chromatin mixed with deiminated histones and a panel of granular proteins/peptides, including MPO and NE (50, 51). It was shown that infection with L. amazonensis promastigotes can trigger NET formation, parasite entrapment, and parasite killing (52) and that pretreatment of L. amazonensis promastigotes with histone proteins H2A and H2B markedly reduced parasite survival and infectivity in Mϕs (Y. Wang, L. Xin, V. Popov, S.M. Beverley, K-P. Chang, M. Wang, and L. Soong, manuscript in preparation). However, it remains unclear whether and how NET and histone proteins contribute to the outcome of infection in vivo. The most interesting and important findings in this study are the detrimental role of WT neutrophils in promoting parasite infection in vivo and the protective role of IFNAR−/− neutrophils in limiting parasite infection in vitro and in vivo (Figs. 1, 5, 6). It is evident from this study that, in the absence of IFNAR, there was an increased and sustained neutrophil influx at the infection site, along with an increased release of antimicrobial molecules from neutrophils and fast turnover of neutrophils. At least under in vitro conditions, the efficient killing of parasites was determined by IFNAR−/− neutrophils, not IFNAR−/− Mϕs or WT neutrophils (Fig. 5B). Although the molecular details about how the IFNAR−/− neutrophils interact with parasites in vivo require further investigation, we speculate that activating the IFNAR signaling pathway via exogenous IFN-α (Figs. 1, 2) or concurrent viral infection may have a detrimental effect on Leishmania infection.
Neutrophil apoptosis is an important step in Leishmania infection. It was reported that L. major infection can delay the apoptosis of human blood-derived neutrophils (53) and that engulfing infected/apoptotic neutrophils promotes silent invasion of parasites into Mϕs (45). We used elicited peritoneal neutrophils that mimic the infiltrated neutrophils in tissues and found that L. amazonensis infection actually promoted apoptosis of murine neutrophils (Fig. 5). At this stage, it is unclear whether the discrepancy observed in these studies is due to different host cell sources, parasite species, or experimental settings. Nevertheless, our neutrophil infection rates (~50–60% at 4 h postinfection) were consistent with reports on phagocytosis-induced neutrophil apoptosis through CD11b/CD18 (54). Given that type I IFNs can inhibit neutrophil apoptosis through the PI3K signaling pathway (32), it is not surprising that the deficiency of IFNAR promoted spontaneous or infection-induced neutrophil apoptosis (Fig. 5). Because the uptake of apoptotic neutrophils (not B cells) or neutrophil granules increased the antimicrobial activity of Mϕs against intracellular mycobacteria and parasites (27, 31), it is possible that the increased apoptosis in L. amazonensis-infected IFNAR−/− neutrophils indirectly contributes to parasite killing in Mϕs.
In summary, the highlight in this study is that type I IFN signaling regulates the innate immunity to Leishmania infection through several mechanisms, including the differential recruitment and activation of neutrophils and inflammatory monocytes. The sustained recruitment of neutrophils, but limited recruitment of monocytes, at an early stage of infection would regulate the availability of monocytes (safe targets) for parasites. The efficient influx and fast turnover of neutrophils and the enhanced release of granular enzymes can promote neutrophil-mediated parasite killing. Thus, regulating type I IFN signaling is an important means for regulating innate immunity to intracellular protozoan parasites and the outcome of the infection.
Supplementary Material
Acknowledgments
We thank Mark Griffin for technical assistance with cell sorting, Dr. T. Wang for helpful discussion, and Mardelle Susman for assisting in manuscript preparation.
This work was supported by National Institutes of Health Grants AI043003 (to L.S.).
Abbreviations used in this paper
- 7-AAD
7-aminoactinomycin D
- i.d.
intradermally
- IFNAR
type I IFNR
- LN
lymph node
- Mϕ
macrophage
- MPO
myeloperoxidase
- NE
neutrophil elastase
- NET
neutrophil extracellular trap
- PMN
polymorphonuclear neutrophil
- Spp1
secreted phosphoprotein 1
- TG
thioglycollate
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 2.Decker T, Müller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso G, Midiri A, Biondo C, Beninati C, Zummo S, Galbo R, Tomasello F, Gambuzza M, Macrì G, Ruggeri A, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J. Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 4.Weighardt H, Kaiser-Moore S, Schlautkötter S, Rossmann-Bloeck T, Schleicher U, Bogdan C, Holzmann B. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J. Immunol. 2006;177:5623–5630. doi: 10.4049/jimmunol.177.8.5623. [DOI] [PubMed] [Google Scholar]
- 5.Meissner NN, Swain S, Tighe M, Harmsen A, Harmsen A. Role of type I IFNs in pulmonary complications of Pneumocystis murina infection. J. Immunol. 2005;174:5462–5471. doi: 10.4049/jimmunol.174.9.5462. [DOI] [PubMed] [Google Scholar]
- 6.Biondo C, Midiri A, Gambuzza M, Gerace E, Falduto M, Galbo R, Bellantoni A, Beninati C, Teti G, Leanderson T, Mancuso G. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J. Immunol. 2008;181:566–573. doi: 10.4049/jimmunol.181.1.566. [DOI] [PubMed] [Google Scholar]
- 7.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagarajan UM, Prantner D, Sikes JD, Andrews CW, Jr, Goodwin AM, Nagarajan S, Darville T. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 2008;76:4642–4648. doi: 10.1128/IAI.00629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu H, Fan Y, Joyee AG, Wang S, Han X, Bai H, Jiao L, Van Rooijen N, Yang X. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 2008;181:2092–2102. doi: 10.4049/jimmunol.181.3.2092. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis M. Recombinant murine beta interferon enhances resistance of mice to systemic Mycobacterium avium infection. Infect. Immun. 1991;59:1857–1859. doi: 10.1128/iai.59.5.1857-1859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, III, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner J, Orme IM. The expression of early resistance to an infection with Mycobacterium tuberculosis by old mice is dependent on IFN type II (IFN-gamma) but not IFN type I. Mech. Ageing Dev. 2004;125:1–9. doi: 10.1016/j.mad.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Röllinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 18.Mattner J, Wandersee-Steinhäuser A, Pahl A, Röllinghoff M, Majeau GR, Hochman PS, Bogdan C. Protection against progressive leishmaniasis by IFN-beta. J. Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 19.Une C, Andersson J, Orn A. Role of IFN-alpha/beta and IL-12 in the activation of natural killer cells and interferon-gamma production during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 2003;134:195–201. doi: 10.1046/j.1365-2249.2003.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa VM, Torres KC, Mendonça RZ, Gresser I, Gollob KJ, Abrahamsohn IA. Type I IFNs stimulate nitric oxide production and resistance to Trypanosoma cruzi infection. J. Immunol. 2006;177:3193–3200. doi: 10.4049/jimmunol.177.5.3193. [DOI] [PubMed] [Google Scholar]
- 21.Koga R, Hamano S, Kuwata H, Atarashi K, Ogawa M, Hisaeda H, Yamamoto M, Akira S, Himeno K, Matsumoto M, Takeda K. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J. Immunol. 2006;177:7059–7066. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- 22.Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am. J. Trop. Med. Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 23.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanabria MX, Vargas-Inchaustegui DA, Xin L, Soong L. Role of natural killer cells in modulating dendritic cell responses to Leishmania amazonensis infection. Infect. Immun. 2008;76:5100–5109. doi: 10.1128/IAI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, Calich VL, Coutinho SG, Lopes MF, DosReis GA. Macrophage interactions with neutrophils regulate Leishmania major infection. J. Immunol. 2004;172:4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 28.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J. Immunol. 2005;174:1557–1565. doi: 10.4049/jimmunol.174.3.1557. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, Lungarella G, DosReis GA. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 31.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 32.Wang K, Scheel-Toellner D, Wong SH, Craddock R, Caamano J, Akbar AN, Salmon M, Lord JM. Inhibition of neutrophil apoptosis by type 1 IFN depends on cross-talk between phosphoinositol 3-kinase, protein kinase C-delta, and NF-kappa B signaling pathways. J. Immunol. 2003;171:1035–1041. doi: 10.4049/jimmunol.171.2.1035. [DOI] [PubMed] [Google Scholar]
- 33.Qi H, Ji J, Wanasen N, Soong L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect. Immun. 2004;72:988–995. doi: 10.1128/IAI.72.2.988-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin L, Li Y, Soong L. Role of interleukin-1beta in activating the CD11c(high) CD45RB− dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect. Immun. 2007;75:5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 36.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 39.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, Kellner ES, Nacionales D, Barker T, Kelly-Scumpia K, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, van Rooijen N, Moldawer L, Satoh M, Reeves WH. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003;11:210–214. doi: 10.1016/s0966-842x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 46.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis JA. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 47.Lima GM, Vallochi AL, Silva UR, Bevilacqua EM, Kiffer MM, Abrahamsohn IA. The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunol. Lett. 1998;64:145–151. doi: 10.1016/s0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 48.Novais FO, Santiago RC, Báfica A, Khouri R, Afonso L, Borges VM, Brodskyn C, Barral-Netto M, Barral A, de Oliveira CI. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J. Immunol. 2009;183:8088–8098. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 49.Gueirard P, Laplante A, Rondeau C, Milon G, Desjardins M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell. Microbiol. 2008;10:100–111. doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 50.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 51.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 52.Guimarães-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceição-Silva F, Saraiva EM. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Müller K, Solbach W, Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 54.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.