Abstract
We have previously designed a conditionally replicative oncolytic adenovirus (CRAd) named Ad-F512 that can target both the stromal and the malignant melanoma cell compartments. The replication capacity of this CRAd is driven by a 0.5-Kb SPARC promoter fragment (named F512). To improve CRAd’s efficacy, we cloned into F512 motives responsive to hypoxia (hypoxia-responsive element (HRE)) and inflammation (nuclear factor kappa B) to obtain a chimeric promoter named κBF512HRE. Using luciferase as a reporter gene, we observed 10–15-fold increased activity under hypoxia and 10–80-fold induction upon tumor necrosis factor-α addition. We next constructed a CRAd (Ad-κBF512HRE) where E1A activity was under κBF512HRE regulation. Treatment of nude mice harboring established tumors made of a mix of SB2 melanoma cells and WI-38 fibroblasts with Ad-κBF512HRE led to the complete elimination of tumors in 100% of mice (8/8). Moreover, Ad-5/3-κBF512HRE, a viral variant pseudotyped with a chimeric 5/3 fiber, exerted a strong lytic effect on CAR-negative melanoma cells and was highly effective in vivo on established tumors made of melanoma cells and WI-38 fibroblasts, leading to the complete elimination of 4/5 tumors. These results indicate that this improved stroma-targeted oncolytic adenovirus can override the resistance of melanoma tumors and might become of significant importance for melanoma therapeutics.
INTRODUCTION
Although melanoma accounts for only 3–5% of all skin cancers, it is responsible for approximately 75% of all deaths from skin cancer worldwide and disproportionately targets young individuals, especially women (Wu et al., 2003; Lens and Dawes, 2004; Thompson et al., 2005). The standard therapy for the metastatic disease remained decarbazine with transient response rates of <10%, although interleukin-2 treatment seems to benefit a select group of patients, although with significant associated toxicity (Schadendorf et al., 2009). Two promising new agents, ipilimumab, a humanized mAb that blocks the T-cell surface protein CTLA-4, and the inhibitor of the BRAF gene, vermurafenib, seem to benefit<20% of patients and are limited by a relatively short duration that averages 6 months (Hodi et al., 2010; Chapman et al., 2011).
The use of oncolytic viruses for tumor targeting appears as a potential powerful approach for the treatment of advanced stages of cancer combined or not with radiotherapy or chemotherapy (Garber, 2006; Alemany, 2007; Choi et al., 2012). Direct treatment of accessible melanoma lesions with a replicative herpes simplex virus expressing granulocytemacrophage colony-stimulating factor (Oncovex-GMCSF) resulted in a 28% objective response in a phase II clinical trial encouraging the initiation of a phase III study (Senzer et al., 2009). Oncovex-GMCSF exhibits a strong antitumor lytic activity and a highly attenuated neurovirulence due to a mutation in the ICP34.5 gene (Senzer et al., 2009), although it was not specifically designed to attack cancer cells nor the supportive stroma. Few conditionally replicative oncolytic adenoviruses (CRAds) whose replication is driven by tumor-specific promoters and hence replicate mainly in the cancer niche (Hogg et al., 2010) have also entered clinical trials with promising therapeutic effects, although none have included melanoma patients yet (Dent et al., 2010). The lack of strong and specific responsive promoters and the difficulties to disseminate inside the tumor mass owing to the presence of stromal barriers hampered the therapeutic efficacy of oncolytic vectors (Cairns et al., 2006). We have recently designed a stroma-targeted CRAd, Ad-F512, whose replication is driven by a 0.5-kB SPARC promoter fragment named F512. The rationale underlying this was that SPARC is expressed in most stromal cells in close contact with the malignant compartment (Podhajcer et al., 2008). In human melanomas, SPARC is additionally expressed by the malignant cells themselves, indicating that the CRAd could eventually replicate in both cell compartments. Indeed, Ad-F512 exhibited therapeutic efficacy in vivo on human melanoma tumors xenografted in nude mice (Lopez et al., 2009). However, established melanomas made of a mix of human melanoma cells and human fibroblasts were highly resistant to viral attack (Lopez et al., 2009).
In the present study, we decided to make use of specific characteristics of the melanoma microenvironment and designed an improved version of Ad-F512. For this purpose, we incorporated in the 0.5-Kb SPARC promoter motives responsive to hypoxia and inflammation; this triple chimeric promoter enabled viral replication in the stromal and malignant cell compartment, and showed an enhanced response to hypoxia and inflammation. This improved adenovirus also showed enhanced in vivo capacity to eliminate resistant melanomas made of malignant cells mixed with human fibroblasts xenografted in nude mice.
RESULTS
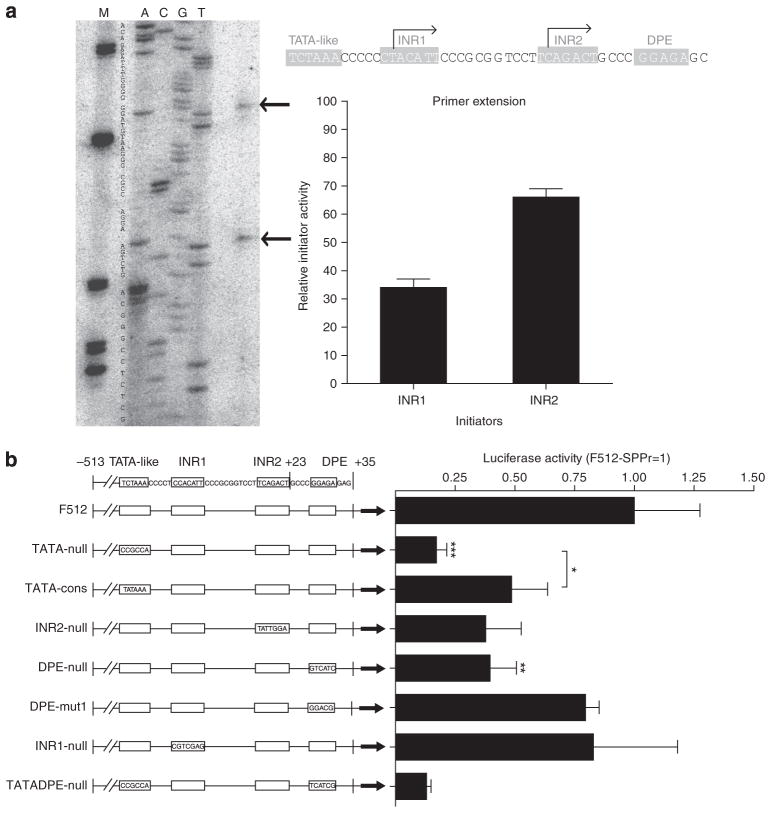
Analysis of the F512-SPARC promoter motifs
Before adding genetic motives responsive to hypoxia and inflammation, we performed a detailed study of the F512-SPARC promoter motifs to establish whether its potency or specificity can be improved. On the basis of in silico analysis and previous studies (Hafner et al., 1995), we observed the presence of two putative transcription initiator sequences (INRs) and a downstream promoter element (DPE) in addition to a TATA-like motif (TCTAAA). By using primer extension analysis on RNA extracted from A375N melanoma cells, we confirmed that SPARC mRNA transcription initiates from both the INR1 and the INR2 sites of the endogenous promoter at a ratio of 1: 2 (Figure 1a). Similar results were observed in cervical cancer HeLa cells, although F512 transcriptional activity was extremely low (data not shown). The existence of the DPE and the TATA-like elements, and the fact that both INRs were active, led us to consider that F512 could be a functional dual promoter where the DPE element could initiate transcription through INR1 and the TATA-like sequence might work with INR2 (Figure 1a). We generated seven mutated versions of F512 containing the consensus TATA box, DPE, or the null version of both (Figure 1b) placed upstream of luciferase. Replacing the TATA-box-like element with a nonsense sequence (TATA-null) reduced F512 activity to <20% of the activity of the native sequence (Figure 1b). Interestingly, replacing the native TATA-like box sequence by the consensus one also diminished F512 activity to 50% of the levels of the native sequence (Figure 1b). Mutational studies that rendered an INR2-null or DPE-null sequence led to 50% inhibition of F512 transcriptional activity (Figure 1b). Neither a DPE element that was mutagenized to obtain the other well-characterized DPE sequence (DPE-mut1) (Juven-Gershon and Kadonaga, 2010) nor an INR1-null sequence induced significant changes in F512 activity (Figure 1b). Thus, F512 works as a dual promoter using the TATA-like and DPE sequences to initiate transcription, although the TATA-like/INR2 motives seem to be the predominant one in terms of F512 transcriptional activity. As all the modifications that we performed resulted in lower levels of F512 activity, we decided to move forward with the wild-type version of F512.
Figure 1. Transcriptional activity of mutated versions of the SPARC promoter.
(a) Primer extension analysis of mRNA transcribed from the endogenous SPARC promoter region of A375N melanoma cells. The sequence and the main initiation sites are shown (see arrows). The location of each element (initiator sequence (INR), TATA-like, and downstream promoter element (DPE)) and the relative activity of each initiation site is shown (right panel). Note that there is a T in the INR1 of a (obtained from A375N cells) compared with a C in the INR1 sequence of b (obtained from human lymphocytes). (b) Luciferase activity of the different mutated versions of the F512-SPARC promoter. The bars represent mean±SD (n=3). Data were analyzed by one-way analysis of variance.
*P<0.05, **P<0.01, and ***P<0.001.
Incorporation to F512 of genetic elements responsive to melanoma microenvironment
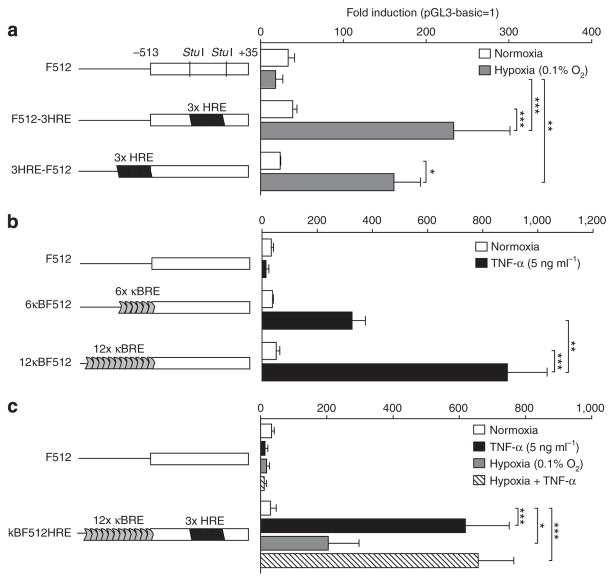
We constructed different plasmids that included six (101 bp) or twelve (202 bp) copies of the nuclear factor kappa B (NF-κB)-responsive element obtained from the HIV long terminal repeat sequence (Adriaansen et al. 2007; Khoury et al., 2007) combined or not with three copies of the hypoxia-responsive element (3 × HRE) obtained from the mouse VEGF promoter (from −889 to −939 bp) (Shima et al., 1996). Both elements were cloned at different positions with respect to F512, and the activity was assessed through luciferase expression assays.
The dual chimeric promoters that included 3 × HRE inside (F512-HRE) or upstream (HRE-F512) of F512 exhibited 6- and 7-fold increased activity in A375N SPARC-positive human melanoma cells compared with F512 under hypoxia, indicating that placement of 3 × HRE inside F512 did not affect its transcriptional activity (Figure 2a). The dual chimeric promoters containing 6 (6 κBF512) or 12 (12 κBF512) copies of the NF-κB-responsive element exhibited 9- and 17-fold induction, respectively, upon tumor necrosis factor-α (TNF-α) addition, indicating a superior activity of the latter (Figure 2b). No TNF-α effect on cell viability was observed under these conditions (data not shown).
Figure 2. Addition of nuclear factor kappa B (NF-κB) and hypoxia-responsive element (HRE) enhanced the transcriptional activity of the F512-SPARC promoter.
A375N melanoma cells were transfected with F512 or chimeric promoters containing (a) HRE, (b) NF-κB responsive elements (κBRE), or (c) both. Cells were placed under (a) hypoxia, (b) tumor necrosis factor-α (TNF-α), or (c) both. Values are expressed relative to the activity of the promoter-less vector pGL3-basic. Data expressed as the mean±SD (n=3) were analyzed by one-way analysis of variance followed by the Bonferroni test. *P<0.05, **P<0.01, and ***P<0.001.
Thus, we constructed a triple chimeric promoter where the 12κBRE motif was placed upstream of, and the HRE inside, F512. This triple chimeric promoter (kBF512HRE) exhibited 21-, 7-, and 22-fold increased activity in the presence of TNF-α, hypoxia, or TNF-α + hypoxia, respectively, compared with the activity of F512 in normoxia (Figure 2c). Although no additive effect was observed, the whole data indicate that the triple chimeric promoter would be able to respond to low oxygen tension and inflammatory conditions.
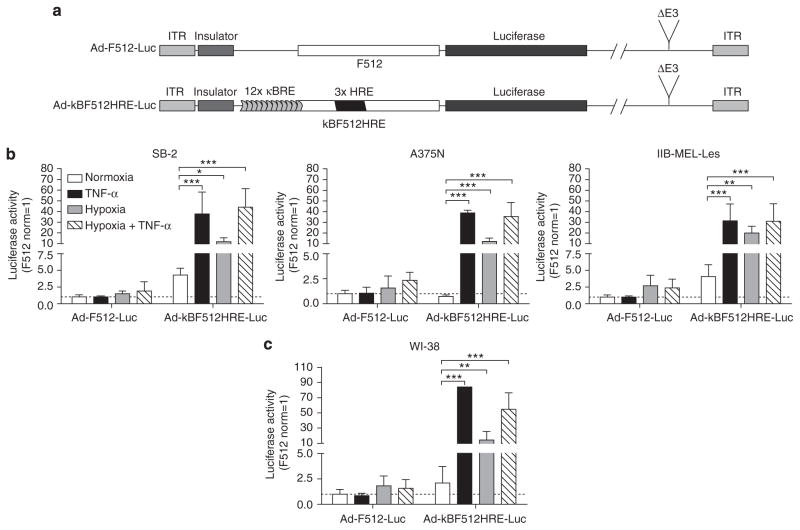
Activity of the chimeric promoter in an adenoviral context
The next step was to prepare a nonreplicative adenovirus where luciferase activity was driven by kBF512HRE (Ad-kBF512HRE-Luc) (Figure 3a). Ad-kBF512HRE-Luc exhibited 30–35, 10–20, and 30–35 increased fold activity in melanoma cells in the presence of TNF-α, hypoxia, or TNF-α + hypoxia, respectively, compared with Ad-F512 under the same conditions (Figure 3b). It is noteworthy that the chimeric promoter exhibited at least 60-fold increased activity in A375N and SB2 cells compared with the activity observed in IIB-MEL-LES cells. Ad-kBF512HRE-Luc was also highly active in WI-38 human fetal fibroblasts, reaching 84-, 14-, and 55-fold increased activity in the presence of TNF-α, hypoxia, or TNF-α+ hypoxia, respectively, compared with Ad-F512-Luc (Figure 3c). Overall, the data indicate that kBF512HRE responded to hypoxia and inflammatory conditions in an adenoviral context.
Figure 3. Transcriptional activity of the F512-SPARC promoter combined with NF-κB and hypoxia-responsive element (HRE) in an adenoviral context.
(a) Scheme representing Ad-F512-Luc and Ad-kBF512HRE-Luc reporter adenoviruses (NF-κB responsive elements (κBRE)). Luciferase assay on (b) melanoma cells (SB2, A375N, and IIB-MEL-Les) and (c) human fetal fibroblasts (WI-38). Cells transduced with the different viruses were placed under normal oxygen levels (21% O2), hypoxia (0.1% O2), tumor necrosis factor-α (TNF-α) (5ngml−1), or hypoxia+ TNF-α. Error bars represent the mean±SD (n=3). Data are expressed relative to the activity of Ad-F512-Luc vector and were analyzed by one-way analysis of variance followed by the Bonferroni test. *P<0.05, **P<0.01, and ***P<0.001. ITR, inverted terminal repeat.
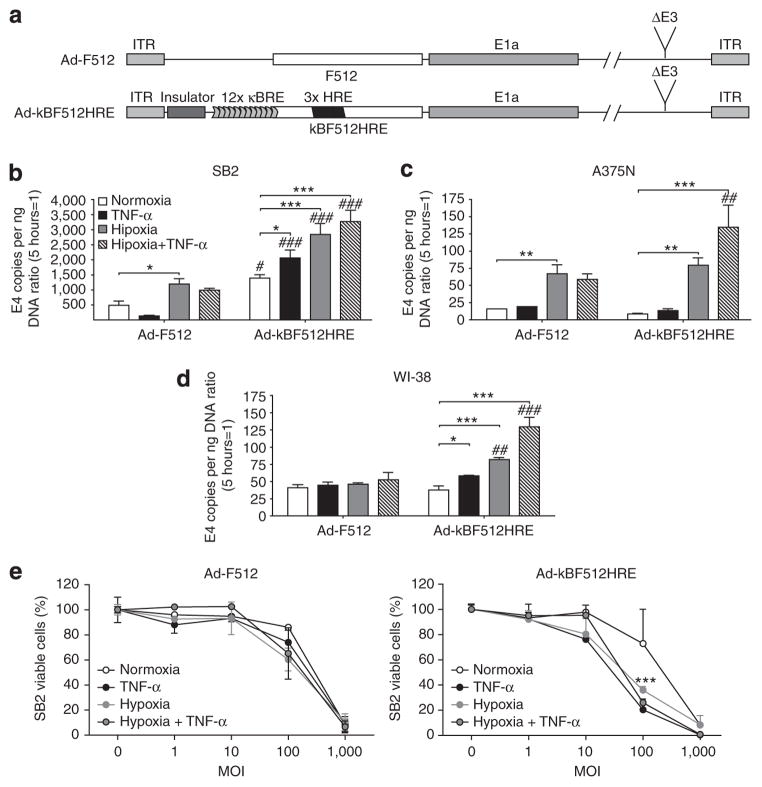
Construction of a CRAd driven by the kBF512HRE chimeric promoter
Thus, a CRAd named Ad-kBF512HRE, whose replication is driven by the kBF512HRE promoter, was constructed by inserting the insulator-kBF512HRE promoter cassette into the E1A region to regulate the expression of the E1a gene (Figure 4a). We evaluated its replication capacity by assessing the adenoviral genome copy number increase after 72 hours. Ad-kBF512HRE exhibited an enhanced replication capacity under TNF-α+ hypoxia in the two melanoma cell lines, although its overall activity was much higher in SB2 melanoma cells compared with A375N cells (Figure 4b, c, and e). The higher replication and lytic capacity of Ad-kBF512HRE in SB2 cells compared with A375N cells was probably related to the highest CAR levels observed in the former cells that allowed higher infectivity by the CRAd (see Supplementary Figure S1 online).
Figure 4. Replication capacity and in vitro lytic activity of Ad-kBF512HRE.
(a) Scheme representing the different conditionally replicative oncolytic adenoviruses (CRAds). (b–d) Cells were infected with 500 vp per cell and after 5 hours were placed under different conditions as depicted. Five and 72 hours later, cells were collected and their DNA was isolated to assess E4 gene copies. E4 data at 72 hours were normalized with the data at 5 hours. Error bars represent the mean±SD (n=3). (e) In vitro lytic activity of the CRAds on SB2 melanoma cells. Data were normalized to uninfected cells under the same experimental conditions and analyzed by one-way analysis of variance followed by the Bonferroni test. *P<0.05, **P<0.01, and ***P<0.001. *Relative to normoxia; #Relative to Ad-F512 under the same experimental conditions. ITR, inverted terminal repeat; MOI, multiplicity of infection.
Ad-kBF512HRE also showed 2.5-fold enhanced replication capacity in WI-38 fibroblast cells under TNF-α+ hypoxia, although the overall activity was lower than the one observed in SB2 cells (Figure 4d). Of note was the inhibitory effect exerted by TNF-α on Ad-F512 replication in SB2 cells that was not observed with the new CRAd carrying the chimeric promoter (Figure 4b). This effect was not related to TNF-α itself, as it showed no direct inhibitory activity on cell growth (data not shown).
Ad-kBF512HRE exhibited improved antitumor activity in human melanoma xenografts
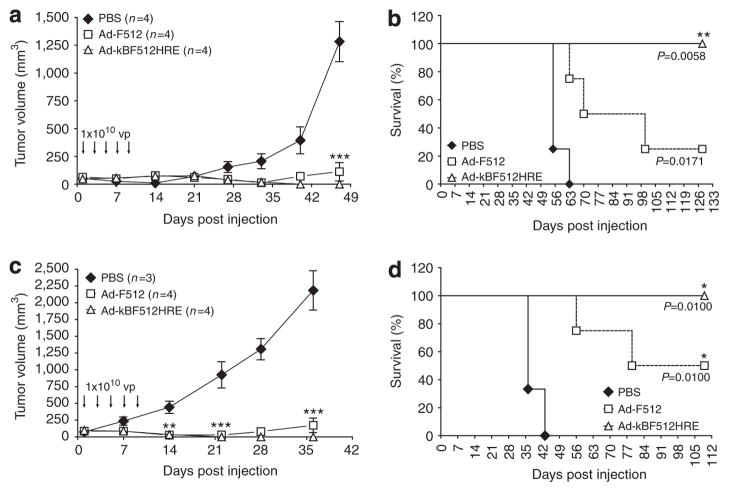
We next evaluated Ad-kBF512HRE antitumor activity in vivo in a mixed tumor model of SB2 melanoma cells and WI-38 fibroblasts that was described as partially resistant in vivo to Ad-F512 activity (Lopez et al., 2009). Established tumors were highly heterogenous in terms of hypoxic areas regardless of whether they were made of malignant cells alone or combined with WI-38 fibroblasts (Supplementary Figure S2a and b online). It was also noteworthy that, in mixed tumors, malignant cells accommodated mainly at the periphery of the tumor mass, whereas fibroblasts were more evenly distributed (Supplementary Figure S2c online). In initial experiments, we observed that administration of five intratumoral doses of 1 × 1010 vp of Ad-F512 to mice bearing mixed SB2/WI-38 tumors did not improve mice survival compared with three doses of the virus, and <40% of mice benefited from both treatment schemes (Supplementary Figure S2d–f online). In clear contrast, intratumoral administration of five doses of AdkBF512HRE to mice bearing mixed SB2/WI-38 tumors led to the complete elimination of tumors in 100% of mice in two independent experiments, compared with <40% tumor elimination in mice treated with Ad-F512 (Figure 5). Mostly important, tumors did not recur even after more than 100 days of follow-up. Hexon staining confirmed viral replication inside the tumor mass (Supplementary Figure S2g online).
Figure 5. In vivo therapeutic efficacy of Ad-kBF512HRE on established tumors.
Tumor growth (a, c) and Kaplan–Meier survival curves (b, d) corresponding to mice harboring tumors made of a mix of CAR-positive SB2 melanoma cells and WI-38 human fibroblasts. For further details see the Materials and Methods section. *P<0.05, **P<0.01, and ***P<0.001. PBS, phosphate-buffered saline.
Fiber exchange highlights the improved therapeutic efficacy of the CRAd based on the chimeric promoter
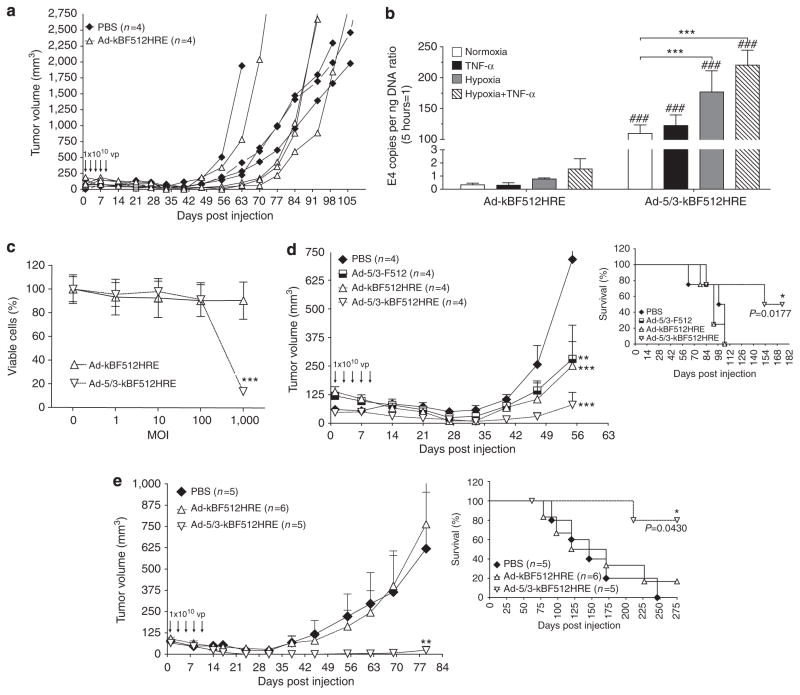
In clear contrast with the results obtained with SB2 tumors, administration of five doses of Ad-kBF512HRE to nude mice bearing IIB-MEL-LES tumors had no effect on tumor growth (Figure 6a). Flow-cytometer analysis demonstrated that IIB-MEL- LES cells expressed neglectable levels of CAR receptor while they expressed high levels of CD46, one of the receptors of adenovirus type 3 (Supplementary Figure S1 online).
Figure 6. In vivo therapeutic efficacy of Ad-5/3-kBF512HRE on CAR-negative tumors.
In vivo growth of subcutaneous tumors made of CAR-negative IIB-MEL-LES melanoma cells (a, d) or a mix of IIB-MEL-LES and WI-38 fibroblasts (e) treated intratumorally with five administrations of the viruses or phosphate-buffered saline (PBS). Kaplan–Meier curves are included (d, e). For further details, see the Materials and Methods section. (b) Conditionally replicative oncolytic adenovirus (CRAd) replication in IIB-MEL-LES cells assessed by E4 gene DNA copies. Error bars represent the mean±SD (n=3). *Relative to normoxia; #relative to Ad-kBF512HRE under the same experimental conditions. (c) In vitro lytic activity of the CRAds carrying native fiber 5 or the chimeric fiber 5/3 on IIB-MEL-LES cells. *P<0.05, **P<0.01, and ***P<0.001. MOI, multiplicity of infection; TNF-α, tumor necrosis factor-α.
Therefore, we engineered Ad-kBF512HRE with a chimeric 5/3 fiber to redirect viral entry. This pseudotyped CRAd, Ad-5/3-kBF512HRE, exhibited 405-, 230-, and 145-fold increased replication capacity on IIB-MEL-LES cells in the presence of TNF-α, hypoxia, and TNF-α+ hypoxia, respectively, compared with Ad-kBF512HRE under the same conditions (Figure 6b). This increased replication was accompanied by an enhanced lytic activity (Figure 6c). Fiber pseudotyping also improved viral lytic capacity on WI-38 fibroblast, as these cells expressed higher levels of CD46 than CAR (Supplementary Figure S1 online and data not shown).
On the basis of the in vitro data, we further assessed the in vivo efficacy of Ad-5/3-kBF512HRE on CAR-negative, CD46-positive, IIB-MEL-LES-established melanoma xenografts. Administration of five doses of 1 × 1010 vp of Ad-5/3-kBF512HRE on mice bearing established IIB-MEL-LES melanomas led to the complete elimination of tumors in 2/4 mice and a strong delay in tumor growth in one additional mouse (Figure 6d), with a statistically significant increase in survival rate (Figure 6d, insert).
Next, we established whether Ad-kBF512HRE retargeting through pseudotyping with the 5/3 chimeric fiber can also render mixed tumors made of IIB-MEL-LES cells and WI-38 fibroblasts sensitive to oncolytic attack. Treatment of mice bearing established tumors made of IIB-MEL-LES and WI-38 cells with five doses of 1 × 1010 vp of Ad-5/3-kBF512HRE led to the complete tumor elimination in 4/5 mice, a strong delay in tumor growth in 1 additional mouse, and a significant increase in the overall survival (Figure 6e). The effect of the non-pseudotyped CRAd did not differ significantly from phosphate-buffered saline (PBS) (Figure 6d and e).
DISCUSSION
Here we show that incorporation of motives responsive to hypoxia and inflammation to a stroma-targeted conditionally replicative adenovirus greatly improved the therapeutic efficacy of the virus, leading to the elimination of human melanomas established in nude mice. Virus retargeting following engineering of a chimeric 5/3 adenovirus serotype fiber to target melanoma tumors devoid of CAR expression confirmed the increased potency of this improved oncolytic adenovirus.
To enhance viral replication, we first accomplished a deep study of the F512-SPARC promoter fragment. Mutational analysis demonstrated that both the TATA-like and the DPE sequences were active and that the native sequences of both motifs exhibited better activity than the consensus one. Thus, the F512-SPARC promoter is, to our knowledge, the first native promoter in which both the TATA and the DPE sequences are transcriptionally active, suggesting that F512 is a mixed-mode promoter (Juven-Gershon and Kadonaga, 2010). Further efforts to establish whether these alternate initiation sites could be associated with specific cell types did not succeed. Thus, the possibility that F512 will be active in different cell types depending on the availability of the TATA-like or the DPE-related transcription factors warrants further investigation.
Further to the molecular analysis of the F512 promoter, we incorporated motives responsive to hypoxia and inflammation that are hallmarks of more aggressive melanomas and have been shown to correlate with bad prognosis (Bedogni and Powell, 2009). Few reports described the incorporation of genetic responsive elements to promoters for enhanced transcriptional regulation of therapeutic genes (Yew et al., 2001; Greco et al., 2002; Lee et al., 2002; Liu et al., 2004; Lee et al., 2006; Guo et al., 2007; Poulsen et al., 2008), but only one study attempted to combine NF-κB and HRE elements in a plasmid that conferred only a slight enhanced activity to the KDR promoter when assessed in primary murine endothelial cells (Modlich et al., 2000). Here we observed that both in a plasmid and in an adenoviral context the triple chimeric promoter increased its transcriptional activity greatly, leading to enhanced viral efficacy under hypoxia and upon TNF-α addition in human melanoma cells and fetal fibroblasts. Moreover, the oncolytic virus carrying this triple chimeric promoter showed enhanced in vivo therapeutic efficacy both on tumors made of melanoma cells alone and on tumors made of a mix of melanoma cells and stromal cells. Although we were unable to observe an in vitro synergistic effect when the replication/lytic capacity of Ad-κBF512HRE was assessed in the presence of hypoxia+ TNF-α, we can envisage an in vivo positive loop because hypoxia has been shown to enhance transient changes in NF-κB levels (Vaupel, 2010). Pseudotyping this virus with a chimeric fiber 5/3 overrides the natural resistance of a melanoma cell line devoid of CAR receptors such as IIB-MEL-LES. This is consistent with previous studies showing that some human fresh melanoma might not express CAR (Rivera et al., 2004). Thus, assessment of viral receptor expression in fresh melanoma samples before treatment can help predict viral success in a potential clinical trial.
The tumor microenvironment involves the malignant cells themselves, fibroblasts, endothelial cells, and inflammatory cells. This microenvironment is characterized by low levels of oxygen tension and increased proinflammatory conditions that might enhance NF-κB responsiveness (Melnikova and Bar-Eli, 2009). New blood vessel formation by endothelial cells and enhanced remodeling of the extracellular matrix by neighboring fibroblasts to create either new tracks or barriers help tumors develop beyond an initial avascular growth and protect malignant cells from the attack of therapeutic drugs. Moreover, the dynamic cross-talk between malignant melanoma cells and surrounding stromal cells is a key event for allowing dissemination (van Kempen et al., 2008). Melanoma and inflammatory cells induce fibroblast proliferation through the release of platelet-derived growth factor, basic fibroblast growth factor, and transforming growth factor-β, whereas fibroblasts produce a series of growth factors such as IGF1, hepatocyte growth factor, basic fibroblast growth factor, and transforming growth factor-β that can support melanoma cell proliferation (Hsu et al., 2002; Hendrix et al., 2003).
Recent studies have shown encouraging studies looking at combining the oncolytic adenovirus H101 with an small interfering RNA against Bcl2 in different human uveal melanoma cells lines (Huang et al., 2012). A couple of studies have also shown that a CRAd carrying IL-24 helped in inducing apoptosis of melanoma cells following radiotherapy (Chai et al., 2012; Jiang et al., 2012). Thus, the possibility of acting on human melanoma tumors with an oncolytic virus that can target both the stromal and the malignant compartment, respond to melanoma microenvironment, and whose infectivity can be tailored according to cell surface expression of viral receptors can be of significant importance for melanoma therapeutics.
MATERIALS AND METHODS
Cell lines
See Supplementary Material online.
Plasmids and recombinant adenoviruses
See Supplementary Material online.
Primer extension
A measure of 1 μg of poly(A) RNA extracted from A375N melanoma cell line using PolyATtract mRNA Isolation System (Promega, Madison, WI, USA) was annealed with 1 pmol of [γ-32P] end-labeled reverse primer (5′-TCTCCAGCGGTTCCATCTTCCAGC-3′). The reverse transcripts were generated according to the protocol of Primer Extension System-AMV Reverse Transcriptase (Promega), using 55 °C as annealing temperature for primer and RNA binding. The same primer was used for sequencing with fmol DNA Cycle Sequencing System (Promega) according to the supplier’s protocol using 55 °C as melting temperature. The extension products from primer extension and sequencing were analyzed by 8M urea–6% polyacrylamide gel electrophoresis. The products were detected by gel drying and exposure to phosphorimaging screens (Molecular Dynamics, Madison, WI) for quantitative analysis using a Storm scanner and ImageQuant software (Storm 860 by Molecular Dynamics, now GE Healthcare, Madison, WI).
Luciferase assays and E4 real-time PCR
See Supplementary Material online.
Flow cytometry
Cells at 80% confluence were washed with PBS, detached with 5mM EDTA, resuspended in DMEM/F12 containing 10% fetal bovine serum (FBS), washed again with PBS, and resuspended at a concentration of 5 × 106 per ml in cold PBS containing 10% FBS and 1% sodium azide (PBS/FBS/NaAz). For further details see Supplementary Material online.
In vitro and in vivo assays and ethics statement
All experiments were approved by the Institutional Animal Care and Use Committee of the Fundación Instituto Leloir (Protocol no. 30OP). The Fundación Instituto Leloir has an approved Animal Welfare Assurance as a foreign institution with the Office of Laboratory Animal Welfare, Number A5168-01.
Six- to eight-week old male athymic N:NIH (S)-nu mice (obtained from the animal facility of the Faculty of Veterinary, University of La Plata) were subcutaneously injected on the left flank with 5 × 106 melanoma cells mixed or not with 2 × 106 WI-38 fibroblasts. When the average tumor volume reached 100mm3, mice received 1 × 1010 vp/mouse of CRAd or vehicle (PBS) administered intratumorally on days 1, 3, and 7 (three doses) or 1, 3, 5, 7, and 9 (five doses). Sequential doses were administered in different areas of the tumor, trying to avoid leakage. Tumor size was estimated once a week with caliper measurements (volume=0.52 × (width)2 × length). Mice were followed up and then killed when tumors reached approximately 2cm3; in several cases, data are presented as tumor growth of individual mice, whereas in other cases, data are presented as average tumor volume. All animals under study received food and water ad libitum. Survival rates were calculated with the Kaplan– Meier method, and multiple comparisons of survival were evaluated by the Bonferroni-corrected method. A P-value 0.05/K (where K= number of comparisons) was considered statistically significant. Data analysis was performed with the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA).
Acknowledgments
This work was supported by grants from the National Agency for Promotion of Science and Technology (Anpcyt) Argentina, CONICET, Fundación Bunge y Born, Argentina, and 2P50CA101955 UAB/UMN Spore in Pancreatic Cancer. We are indebted to the continuous support of Amigos de la Fundación Instituto Leloir para la Investigación en Cancer (AFULIC), Argentina. We acknowledge the technical support of Florencia Straminsky.
Abbreviations
- CRAd
conditionally replicative oncolytic adenovirus
- DPE
downstream promoter element
- FBS
fetal bovine serum
- HRE
hypoxia-responsive element
- INR
initiator sequence
- NF-κB
nuclear factor kappa B
- PBS
phosphate-buffered saline
- TNF-α
tumor necrosis factor-α
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary Material is linked to the online version of the paper at http://www.nature.com/jid
References
- Adriaansen J, Khoury M, de Cortie CJ, et al. Reduction of arthritis following intra-articular administration of an adeno-associated virus serotype 5 expressing a disease-inducible TNF-blocking agent. Ann Rheum Dis. 2007;66:1143–50. doi: 10.1136/ard.2006.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Bedogni B, Powell MB. Hypoxia, melanocytes and melanoma - survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 2009;22:166–74. doi: 10.1111/j.1755-148X.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- Chai L, Liu S, Mao Q, et al. A novel conditionally replicating adenoviral vector with dual expression of IL-24 and arresten inserted in E1 and the region between E4 and fiber for improved melanoma therapy. Cancer Gene Ther. 2012;19:247–54. doi: 10.1038/cgt.2011.84. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-W, Lee J-S, Kim SW, et al. Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev. 2012;64:720–9. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Hamed HA, et al. The development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther. 2010;128:375–84. doi: 10.1016/j.pharmthera.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Greco O, Marples B, Dachs GU, et al. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 2002;9:1403–11. doi: 10.1038/sj.gt.3301823. [DOI] [PubMed] [Google Scholar]
- Guo X, Evans TR, Somanath S, et al. In vitro evaluation of cancer-specific NF-kappaB-CEA enhancer-promoter system for 5-fluorouracil prodrug gene therapy in colon cancer cell lines. Br J Cancer. 2007;97:745–54. doi: 10.1038/sj.bjc.6603930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Zimmermann K, Pottgiesser J, et al. A purine-rich sequence in the human BM-40 gene promoter region is a prerequisite for maximum transcription. Matrix Biol. 1995;14:733–41. doi: 10.1016/s0945-053x(05)80016-2. [DOI] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Hess AR, et al. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RT, Garcia JA, Gerard RD. Adenoviral targeting of gene expression to tumors. Cancer Gene Ther. 2010;17:375–86. doi: 10.1038/cgt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M-Y, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–36. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Jia R, Zhao X, et al. Recombinant oncolytic adenovirus H101 combined with siBCL2: cytotoxic effect on uveal melanoma cell lines. Br J Ophthalmol. 2012;96:1331–8. doi: 10.1136/bjophthalmol-2011-301470. [DOI] [PubMed] [Google Scholar]
- Jiang G, Zhang K, Jiang AJ, et al. A conditionally replicating adenovirus carrying interleukin-24 sensitizes melanoma cells to radiotherapy via apoptosis. Mol Oncol. 2012;6:383–91. doi: 10.1016/j.molonc.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juven-Gershon T, Kadonaga JT. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol. 2010;339:225–9. doi: 10.1016/j.ydbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M, Adriaansen J, Vervoordeldonk MJ, et al. Inflammation-inducible anti-TNF gene expression mediated by intra-articular injection of serotype 5 adeno-associated virus reduces arthritis. J Gene Med. 2007;9:596–604. doi: 10.1002/jgm.1053. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee YS, Kim JM, et al. A novel chimeric promoter that is highly responsive to hypoxia and metals. Gene Ther. 2006;13:857–68. doi: 10.1038/sj.gt.3302728. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim HS, Yu R, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002;6:415–21. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wang X, Ma YX, et al. CMV enhancer/human PDGF-beta promoter for neuron-specific transgene expression. Gene Ther. 2004;11:52–60. doi: 10.1038/sj.gt.3302126. [DOI] [PubMed] [Google Scholar]
- Lopez MV, Viale DL, Cafferata EG, et al. Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses. PLoS One. 2009;4:e5119. doi: 10.1371/journal.pone.0005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova VO, Bar-Eli M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009;22:257–67. doi: 10.1111/j.1755-148X.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- Modlich U, Pugh CW, Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- Podhajcer OL, Benedetti L, Girotti MR, et al. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:691–705. doi: 10.1007/s10555-008-9146-7. [DOI] [PubMed] [Google Scholar]
- Poulsen TT, Pedersen N, Juel H, et al. A chimeric fusion of the hASH1 and EZH2 promoters mediates high and specific reporter and suicide gene expression and cytotoxicity in small cell lung cancer cells. Cancer Gene Ther. 2008;15:563–75. doi: 10.1038/cgt.2008.24. [DOI] [PubMed] [Google Scholar]
- Rivera AA, Davydova J, Schierer S, et al. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Algarra SM, Bastholt L, et al. Immunotherapy of distant metastatic disease. Ann Oncol. 2009;20(Suppl 6):vi41–50. doi: 10.1093/annonc/mdp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor–encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Shima DT, Kuroki M, Deutsch U, et al. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–83. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- van Kempen LCLT, Rijntjes J, Mamor-Cornelissen I, et al. Type I collagen expression contributes to angiogenesis and the development of deeply invasive cutaneous melanoma. Int J Cancer. 2008;122:1019–29. doi: 10.1002/ijc.23147. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol. 2010;32:125–7. [PubMed] [Google Scholar]
- Wu XC, Chen VW, Steele B, et al. Cancer incidence in adolescents and young adults in the United States, 1992–1997. J Adolesc Health. 2003;32:405–15. doi: 10.1016/s1054-139x(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Yew NS, Przybylska M, Ziegler RJ, et al. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther. 2001;4:75–82. doi: 10.1006/mthe.2001.0415. [DOI] [PubMed] [Google Scholar]