Abstract
Modern, industrialized farming practices have lead to working conditions that include high levels of airborne dust. Agricultural workers inhale these complex organic dusts on a daily basis, leading to airway inflammation and higher risk for developing chronic obstructive pulmonary disease. The mechanisms regulating the organic dust-induced airway inflammatory response are not well-defined. We investigated whether overexpression of dimethylarginine dimethylaminohydrolase (DDAH) would lead to diminished pulmonary inflammation in an animal model of organic dust extract exposure. We instilled wild-type (WT) and DDAH overexpressing mice with an aqueous organic dust extract (ODE) collected from a swine confinement building. We found that inflammatory indices such as neutrophil influx and inflammatory cytokine production was lower in the DDAH overexpressing mice compared to WT after organic dust extract (ODE) instillation. We went on to determine how DDAH was mediating the decrease in inflammation induced by ODE. PKCα and PKCε play an essential role in the ODE inflammatory response. In a model of lung slices from WT and DDAH overexpressing mice, we demonstrated an increase in PKCα and PKCε in the WT mice exposed to ODE. This increase was diminished in the DDAH overexpressing mice exposed to ODE. We also tested an important component of the ODE, peptidoglycan (PGN). We noted a similar decrease in neutrophils and inflammatory cytokines in the DDAH overexpressing animals instilled with PGN compared to WT. In conclusion, our studies found a role for DDAH in regulating the ODE-triggered activation of epithelial PKCα and PKCε, a previously unrecognized mechanism of action. This ultimately results in diminished pulmonary inflammation.
Keywords: Pulmonary, lung, inflammation, swine confinement dust, peptidoglycan, Asymmetric dimethylarginine (ADMA), nitric oxide, CXCL-1, KC, CXCL-2, MIP1, IL-1β, precision cut lung slices, PKCα, PKCε, neutrophils, bronchoalveolar lavage
INTRODUCTION
Over the past several years, there has been a shift towards a more industrialized model of large animal farming. This has resulted in large concentrated animal feeding operations (CAFOs), where hundreds to thousands of animals are raised in a single facility. The large numbers of animals in a CAFO produce waste products that contribute to poor indoor air quality. Agricultural workers caring for these animals inhale these complex organic dusts on a daily basis, and in particular, swine confinement workers are at a high risk of developing chronic airway diseases. Exposed workers demonstrate decreased forced expiratory volume in 1 second (FEV1), which indicates obstructive lung disease. They also can have cross-shift variability in peak flows, indicating reactive airways disease.1 Bronchoalveolar lavage of exposed workers demonstrate airway neutrophil influx, as well as inflammatory cytokine production indicating increased airway inflammation.2, 3
The organic dust is complex and composed of numerous components including: a diversity of microbial products, particulate matter, feces, and animal feed. Recent studies demonstrate that gram-positive bacteria predominate in the microbial flora found in swine confinement facilities.4 The cell wall of the majority of gram-positive bacteria (>80%) contains peptidoglycan (PGN). PGN is also found to a lesser extent in the cell wall of gram-negative bacteria (<5%.). PGN appears to be driving the organic dust-induced inflammatory responses as evident by studies of the airway epithelium and phagocytes 5–8. It is also recognized that organic dust from swine confinement facilities induces the production of the inflammatory cytokines IL-6, IL-8, and TNF-α,9–11 and this response is mediated in part, by protein kinase C (PKC) activity in the airway epithelium.12 Animal models of airway inflammation triggered by organic dust exposure have also been developed and exposure to PGN resembles dust-induced airway inflammation.5, 6 Moreover, tracheal epithelial cell PKCα and PKCε activation parallels in vivo dust-induced inflammatory consequences.13 Despite these advances in the understanding of key organic dust components, the mechanisms regulating the organic dust-induced airway inflammatory response are not well-defined.
A potential molecular pathway to target is asymmetric dimethylarginine (ADMA)/dimethylarginine dimethylaminohydrolase (DDAH). ADMA is an endogenous inhibitor of nitric oxide synthase (NOS), and DDAH is a naturally occurring inhibitor of ADMA. DDAH inactivates ADMA by hydrolyzing it into citrulline and dimethylamine. The DDAH/ADMA pathway can potentially play a role in lung inflammation. For instance, increases in ADMA have been shown to potentiate airway inflammation in a murine asthma model.14 However the role of DDAH/ADMA in organic dust-induced airway inflammation has not been described. And very little is known about pulmonary inflammation and the DDAH/ADMA pathway. Based on these collective observations, we hypothesized that increased DDAH would result in a diminished airway inflammatory response to agricultural organic dust and its component, PGN. To test this hypothesis, DDAH overexpressing mice were intranasally exposed with swine confinement organic dust extract (ODE) or PGN per established protocol,6, 13 and airway inflammatory consequences were investigated. These studies demonstrate a role for targeting the DDAH/ADMA pathway to reduce organic dust-induced airway inflammation.
METHODS
Organic dust collection and extract preparation
Settled dust was collected from horizontal surfaces in a swine confinement facility, housing approximately 500–700 head of hogs. An aqueous extract of the dust was prepared as previously published.12 Briefly, 1 gram of dust was placed in 10ml of Hank’s balanced salt solution and allowed to incubate at room temperature for 1 hour. The large particulate matter was removed by centrifugation for 20 minutes at 2000g, and the resulting supernatant was passed through a 0.22µm filter, a sterilization process that also removes coarse particles. The resulting supernatant was diluted to 12.5%, aliquoted and frozen at −80°C until used for instillation. The final diluted 12.5% ODE has been previously shown to elicit optimal lung inflammation in mice6 and contained 3–4 mg/ml of total protein as measured by spectrophotometry (NanoDrop Technologies, Wilmington, DE). Levels of muramic acid, a molecular component of peptidoglycan, were 424.0 pmol/mg (SEM ± 17.7 pmol/mg) as previously determined by mass spectrometry.15
Mouse model
All experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center.
The DDAH1 transgenic (Tg) mouse colony was established from a breeding pair of DDAH1 Tg mice on a C57BL/6 background. The specific strain was C57BL/6J-Tg(ACTB-DDAH1)1Jpck (Jackson laboratory, Bar Harbor, ME).14 For simplicity, these mice will be referred to as DDAH overexpressing mice in the rest of the manuscript. They were bred and maintained in micro-isolator cages and allowed rodent chow and water ad libitum.
The mice were used for experiments at the age of 6–12 weeks. They were genotyped to confirm their DDAH1 Tg status (data not shown), and wild-type (WT) C57BL/6 littermates were used as controls. We have previously confirmed that the DDAH overexpressing mice have high levels of DDAH1 in the lung, nearly 9 times as much as wild-type.14
Intranasal instillation of organic dust extract and peptidoglycan
The WT and DDAH overexpressing mice were intranasally instilled with organic dust extract (ODE) or peptidoglycan (PGN) (Staphylococcus aureus PGN: Sigma) or vehicle (sterile PBS) according to our established model.6, 13 Briefly, mice were anesthetized with isoflurane and held vertically while 50µl of ODE (12.5%), PGN (100 µg per 30µl) or sterile saline was inhaled through the nasal cavity and into the lungs. The mice were then monitored until awake and moving around normally after the treatment. No mice exhibited respiratory distress after instillation.
Bronchoalveolar lavage (BAL), indices of inflammation
At the end of the experiment, the mice were euthanized by pentobarbital injection (50mg/kg). Each trachea was cannulated, and 1ml of sterile phosphate buffered saline (PBS) was instilled into the lungs and ~800µl was recovered by aspiration. This process was repeated three times. The BAL fluid was centrifuged at 250g to collect cells. Cells from all 3 ml were resuspended, pooled, and spun onto slides with a Cytopro cytocentrifuge (Wescor, Logan, UT). Cytospun slides were stained with DiffQuik (DadeBehring, Newark, DE). Counts of the cells determined the differential ratio of cell types in 200 cells per slide per mouse.
The supernatant from the first BAL was stored at −80°C until the ELISA for IL-6, CXCL1, CXCL2, and TNF-α, IL-1β could be performed. The ELISA was performed on 50µl of BAL fluid according to the manufacturer’s instructions (R&D, Minneapolis, MN).
Precision-cut mouse lung slice model
Precision-cut mouse lung slices were prepared as previously reported16, 17 using naïve DDAH overexpressing and WT mice. Briefly, mice were euthanized with pentobarbital. The trachea was cannulated, and the chest cavity was opened. The lungs were slowly filled with a solution of 2% low melting point agarose (Invitrogen, Carlsbad, CA). The agarose was allowed to solidify at 4°C.
The lungs were then removed and placed in ice-cold HBSS for another 45 min. A vibrating microtome platform (model EMS-4000, Electron Microscope Sciences, Hatfield, PA) was used to cut 150 µm thick slices.
The slices were then placed in M-199 media and incubated at 37°C in a 5% CO2 incubator for 5 days with daily media changes. The slices were exposed to ODE, and ADMA (Sigma-Aldrich, St. Louis, MO.) by including these agents in their growth media at the final concentrations indicated.
PKCα/PKCε activity assay
Following experimental conditions, precision cut lung slices were sonicated and centrifuged for 30 min at 10,000g. The supernatant was removed (cytosolic fraction) and the pellet (particulate fraction) was resuspended in cell lysis buffer containing Triton X-100 (0.01%) and sonicated again. PKC isoform activity was determined in crude whole cell cytosolic and particulate fractions similar to methods described previously.18–20 A reaction mix was prepared with: phorbol myristate acetate (PMA; 24 µg/mL), dithiotreitol (30 mM), ATP (150µM), Mg-acetate (45mM), PKCα and ε isoform-specific substrate peptide (Calbiochem), and 10µCi/mL [γ-32P]-ATP were mixed in a Tris-HCl buffer (pH 7.5). The cytosolic or particulate samples (20µL) were added to 40 µL of the reaction mix and incubated for 15 min at 30°C. The reaction was halted by spotting 60µl onto a phosphocellulose paper (Whatman, Clinton, NJ). Then the papers were washed five times, dried, and counted in nonaqueous scintillant (National Diagnostics, Atlanta GA). PKCα and ε activity was expressed as picomoles of phosphate incorporated per minute per milligram of cellular protein.
Statistical Analysis
The data was analyzed using Prism 5 (GraphPad Software, La Jolla, CA) statistical software package. We used one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. A p-value of <0.05 was considered statistically significant. Data is graphed as mean ± SEM.
RESULTS
Inflammatory cell influx in response to organic dust extract (ODE) exposure is attenuated in the DDAH overexpressing mice
WT mice and DDAH overexpressing mice were intranasally instilled with either 12.5% ODE or saline. The animals were euthanized 5 hours later, and bronchoalveolar lavage (BAL) was performed. There was a marked increase in total cell influx following exposure to ODE in the WT animals as compared to saline. This effect was attenuated in the DDAH overexpressing animals (Figure 1A). The cell that contributed the most to this influx was neutrophils. Likewise, the number of neutrophils recruited was diminished in the ODE exposed DDAH overexpressing mice (Figure 1B). There was a much smaller, but statistically significant increase in lymphocytes following ODE exposure in WT and DDAH overexpressing mice (Figure 1D).
Figure 1. Inflammatory cell counts are diminished in the bronchoalveolar lavage (BAL) of mice following organic dust extract (ODE) exposure in the DDAH overexpressing mice.
Organic dust extract (ODE)-induced airway inflammatory cellular influx is decreased in DDAH transgenic overexpressing mice. C57BL/6 wild-type (WT) and DDAH overexpressing mice were treated with 12.5% ODE or saline and BAL fluid was collected 5 hours post-instillation (n = 9–11 mice/group). Asterisks denote statistical significance (*p<0.05, **p<0.01, ***p<0.001) vs. saline treated controls and hatch marks denote statistical significance (#p<0.05) vs. matched WT.
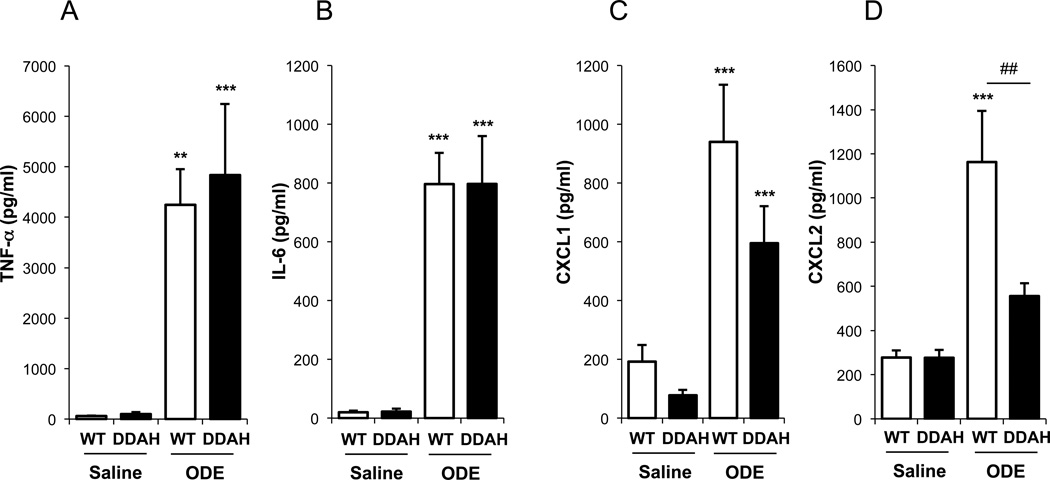
ODE-induced production of the chemokine CXCL2 is diminished in the DDAH overexpressing mice
To determine if the decrease in inflammatory cells in the BAL was related to a reduction in chemokine and/or cytokine production in the DDAH overexpressing animals, we measured TNFα, IL-6 and the mouse homologues of IL-8: CXCL1 (KC) and CXCL2 (MIP2α). In the WT animals, all of these cytokines/chemokines were increased following ODE exposure compared to saline (Figure 2). The increase in CXCL2 after exposure to ODE was diminished in the DDAH overexpressing mice compared to WT (Figure 2D). Compared to WT, there was also a decrease in CXCL1 in the DDAH overexpressing mice after ODE exposure, however it was not statistically significant (Figure 2C). There were no differences in the levels of TNFα or IL-6 between WT and DDAH overexpressing mice following ODE exposure (Figure 2A and B).
Figure 2. CXCL1 and CXCL2 are diminished in the BAL fluid of the DDAH overexpressing mice instilled with ODE.
C57BL/6 wild-type (WT) and DDAH overexpressing (DDAH) mice were instilled with 12.5% ODE or saline. BAL fluid was collected 5 hours after instillation of ODE, and cytokine/chemokine levels were quantitated by ELISA. The DDAH overexpressing mice had a significantly diminished production of CXCL2 (n = 9–11 mice/group). Asterisks denote statistical significance (**p<0.01, ***p<0.001) vs. saline treated controls and hatch marks denote statistical significance (##p<0.01) vs. matched WT.
PKCα and PKCε activation are dampened in the DDAH overexpressing mice in response to ODE exposure
We have previously shown that the sequential activation of PKCα and PKCε play a key role in the regulation of pulmonary inflammation in response to ODE exposure 11. To determine whether this pathway was altered in the DDAH overexpressing mice, we measured PKCα and PKCε activity in precision-cut lung slices. The lung slices from WT and DDAH overexpressing mice were stimulated ex vivo with 5% organic dust extract (ODE) with or without ADMA (100µM) for 1 hour for PKCα and 6 hours for PKCε. We have previously shown that these time points reflect the optimal stimulation for each isoform 11. We measured a significant increase in the activity of both PKCα (Figure 3A) and PKCε (Figure 3B) with ODE exposure. The activation of PKCα (Figure 3A) and PKCε (Figure 3B) was significantly diminished in the DDAH overexpressing mice. However, we were able to restore ODE-stimulated activation of PKCα (Figure 3A) and PKCε (Figure 3B) in the DDAH overexpressing mouse lung slices with ADMA.
Figure 3. PKCε and PKCα activity is dampened in response to stimulation with organic dust in the DDAH overexpressing mice; and this response can be rescued by ADMA.
Lung slices prepared from WT and DDAH overexpressing mice were exposed to 5% ODE in their media. A) PKCα activity in the slices was measured after 1 hour, and B) PKCε activity was measured after 6 hours of exposure. There was a significant increase in PKCα and PKCε after exposure to ODE. This response was dampened in the DDAH overexpressing mice. The ODE-stimulated activation of PKC was restored in the DDAH overexpressing mouse lung slices by the addition of 100 µM ADMA (n = 9–11 mice/group). Asterisks denote statistical significance (**p<0.01) vs. saline treated controls and hatch marks denote statistical significance (##p<0.01, ###p<0.001) vs. matched WT.
DDAH overexpression dampens inflammatory cell influx triggered by peptidoglycan, an important inflammatory component of the organic dust
Peptidoglycan (PGN) is an important inflammatory component of the organic dust found in swine confinement buildings.5 Because of this, and to remain consistent with our previous published studies showing a role for PGN,5–7 we investigated whether overexpression of DDAH would lead to diminished inflammation in response to peptidoglycan. WT and DDAH overexpressing mice were intranasally instilled with 100µg of peptidoglycan or sterile saline. After 24 hours, the mice were euthanized and bronchoalveolar lavage (BAL) was performed. We noted an increase in total cells (p<0.01) and neutrophils (p<0.05) in the WT animals instilled with peptidoglycan as compared to saline (Figure 4). Peptidoglycan did not stimulate a significant increase in cellular influx in the DDAH overexpressing mice (Figure 4A). There were no differences in numbers of macrophages or lymphocytes between groups (Figure 4C and 4D).
Figure 4. Inflammatory cell counts in the bronchoalveolar lavage (BAL) of mice instilled with peptidoglycan (PGN), a component of the ODE, is diminished in the DDAH overexpressing mice.
WT and DDAH overexpressing mice were instilled with saline or PGN (100µg), and BAL was performed 24 hours after instillation. PGN triggered an increase in total cells (**p<0.01) and neutrophils (*p<0.05) in the WT animals as compared to saline treatment. This response was reduced in the DDAH overexpressing mice. There were no differences in macrophages or lymphocytes under any condition tested (n=8–10/group).
DDAH overexpression diminishes the inflammatory cytokine response produced by peptidoglycan
Cytokine/chemokine levels were also measured in the BAL fluid from the wild-type and DDAH overexpressing animals instilled with peptidoglycan or saline. As compared to saline, PGN induced a significant increase in CXCL1 (KC) (Figure 5A), IL-6 (Figure 5B) and IL-1β (Figure 5C) in the wild-type mice. DDAH overexpressing mice demonstrated significantly diminished CXCL1 (p< 0.01) and IL-6 (p<0.01) production triggered by peptidoglycan exposure as compared to exposed WT mice (Figure 5A and B).
Figure 5. CXCL1 and IL-6 are diminished in the BAL fluid of the DDAH overexpressing mice instilled with PGN.
Wild-type and DDAH overexpressing mice were instilled with saline or PGN (100µg), and BAL was performed 24 hours after instillation. Cytokine/chemokine levels were measured by ELISA. Peptidoglycan triggered an increase in CXCL1 compared to saline control (*p<0.05), IL-6 (**p<0.01) and IL-1β (*p<0.05) in the WT mice. As compared to ODE exposed WT animals, ODE-induced CXCL1 and IL-6 were significantly reduced in DDAH overexpressing mice (##p<0.01) (n=8–10/group).
DISCUSSION
We have shown that overexpression of DDAH leads to diminished pulmonary inflammation in response to challenge with ODE or PGN, an important component of the dust. We have shown decreased influx of neutrophils in response to ODE and PGN in the DDAH overexpressing mice compared to wild-type (Figure 1 and 4). This corresponds to decreases in the chemokines CXCL1 (KC) and CXCL2 (MIP2α)(Figure 2), which are important in the recruitment of neutrophils to the lung.21 The diminished production of inflammatory chemokines/cytokines in the DDAH overexpressing mice was even more prominent when we used peptidoglycan alone, an important inflammatory component of the dust (Figure 5).
The ADMA/DDAH pathway has been recognized for its role in cardiovascular and cerebrovascular disease. Increased ADMA contributes to vascular injury and cardiac oxidative stress whereas overexpression of DDAH (resulting in decreased ADMA) attenuates these abnormalities and has been associated with improved endothelial function. However, less is known about the role of ADMA/DDAH in lung injury. In a murine model of acute lung injury triggered by intraperitoneal lipopolysaccharide (LPS), LPS increased ADMA and decreased DDAH activity, in whole lung homogenate.22 It has also been found that in murine asthma, ADMA infusions potentiate allergic airway inflammatory consequences.14 In the current study, we found a functional consequence of over-expressing DDAH. Namely, DDAH overexpression diminishes the inflammatory effects of a direct insult to the lung, whether by organic dust or peptidoglycan. These findings of diminished pulmonary inflammation in DDAH overexpressing mice complement findings in other organ systems such as the vascular endothelium. Tanaka et al. showed a decrease in vascular inflammatory cytokines MCP-1, TNFα, interferon γ, and transforming growth factor-β23 after heart transplant in DDAH overexpressing mice. We saw a similar decrease in inflammatory chemokines and cytokines in the BAL fluid of DDAH overexpressing mice after stimulation with peptidoglycan.
Our studies found a role for DDAH in regulating the activation of epithelial PKCα and PKCε, a previously unrecognized mechanism of action. Lung slices from DDAH overexpressing animals were unable to appropriately activate PKCα and PKCε in response to stimulation with organic dust (Figure 3). In airway epithelial cells, the sequential activation of PKCα and PKCε are essential signaling events in the inflammatory cascade triggered by organic dust.11 In the DDAH overexpressing animals, inactivation of PKCα and PKCε was fully restored by adding back ADMA (Figure 3). This finding supports that the inflammatory process is being regulated at the level of the DDAH/ADMA pathway, rather than from off target effects that may be present in the DDAH overexpressing animals.
Our study has several limitations. The first is that the mice are exposed to an extract of organic dust, rather than inhaling organic dust itself. The process of making the aqueous extract could potentially change the inflammatory response by concentrating or diluting certain components of the dust. In addition, we have filtered out the coarse particles which may also change the inflammatory response to the dust. However, a model where rats were directly exposed to swine confinement air has shown similar changes in pulmonary inflammation.24, 25 Likewise, swine confinement workers show similar changes in inflammatory cytokines in their BAL fluid.9, 26. Another limitation of this study is that we did not directly determine the role of each of the many components of the ODE. We focused on peptidoglycan because previous studies have shown that it was one of the most active components. However, the ODE is a very complex mixture and other components such as LPS also likely play a role. It is also likely that there is synergy between the multiple components of the ODE.
In summary, our experiments demonstrate that DDAH plays a role in the regulation of pulmonary inflammation induced by organic dust, and that this observation can also be extended to peptidoglycan. Peptidoglycan has been increasingly shown to be a major inflammatory mediator of large animal agricultural organic dust exposures. Agricultural workers in these environments are exposed to large amounts of the dust that can lead to pulmonary inflammation and progress to COPD. As current therapeutic options are limited for exposed and symptomatic workers, these current studies provide evidence to support future research into novel approaches of targeting the ADMA/DDAH pathway to prevent and/or reduce environmental inhalant-induced lung disease. Specifically, HMG-CoA reductase inhibitors or statins are one potential class of therapeutic agents recognized to increase DDAH, which would be available for translational approaches. Finally, understanding the underlying mechanisms of agricultural organic dust triggered inflammation, and how that inflammation can be modulated is an important step towards improved therapies for this condition.
Acknowledgments
Declaration of all sources of funding for the research reported in the manuscript: Study supported by grants from the National Institute of Environmental Health Sciences (2R01: ES019325 to JAP), National Institute of Occupational Safety Health (2R01OH008539-01 to DJR and (K08AA019503 to KLB). This project was also supported by CDC-NIOSH award (U54 OH010162) to the Central States Center for Agricultural Safety and Health (CS-CASH).
REFERENCES
- 1.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, et al. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. American Journal of Respiratory and Critical Care Medicine. 1995;151:47–53. doi: 10.1164/ajrccm.151.1.7812571. [DOI] [PubMed] [Google Scholar]
- 2.Nathell L, Malmberg P, Lundbäck B, Nygren A. Impact of occupation on respiratory disease. Scand J Work Environ Health. 2000;26:382–389. doi: 10.5271/sjweh.558. [DOI] [PubMed] [Google Scholar]
- 3.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Current Opinion in Allergy and Clinical Immunology. 2012;12:126. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehme B, Létourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol. 2008;10:665–675. doi: 10.1111/j.1462-2920.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 5.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol. 2011;45:711–719. doi: 10.1165/rcmb.2010-0427OC. PMID: 21278324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1049–L1054. doi: 10.1152/ajplung.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008;122:375–382. doi: 10.1016/j.jaci.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, et al. Repeat organic dust exposure–induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol. 2007;120:366–373. doi: 10.1016/j.jaci.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Larsson BM, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax. 1997;52:638–642. doi: 10.1136/thx.52.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, et al. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase cepsilon in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1163–L1170. doi: 10.1152/ajplung.00103.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt TA, Slager RE, Heires AJ, DeVasure JM, VonEssen SG, Poole JA, et al. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. American Journal of Respiratory Cell and Molecular Biology. 2010;42:706. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93:289–296. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 13.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, et al. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein E, Weigel J, Buford MC, Holian A, Wells SM. Asymmetric dimethylarginine potentiates lung inflammation in a mouse model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2010;299:L816–L825. doi: 10.1152/ajplung.00188.2010. PMID: 20889675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. Journal of Toxicology and Environmental Health, Part A. 2010;73:684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyatt TA, Kharbanda KK, McCaskill ML, Tuma DJ, Yanov D, DeVasure J, et al. Malondialdehyde–acetaldehyde-adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey KL, LeVan TD, Yanov DA, Pavlik JA, DeVasure JM, Sisson JH, et al. Non-typeable haemophilus influenzae decreases cilia beating via protein kinase C epsilon. Respiratory Research. 2012;13:49. doi: 10.1186/1465-9921-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt TA, Sisson JH, Allen-Gipson DS, McCaskill ML, Boten JA, Devasure JM, et al. Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase C epsilon-dependent manner. 2012;181:431–440. doi: 10.1016/j.ajpath.2012.04.022. PMID: 22677421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyatt TA, Kharbanda KK, McCaskill ML, Tuma DJ, Yanov D, DeVasure J, et al. Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. PMID: 21958604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Smith A, Kumar S, Aggarwal S, Rehmani I, Snead C, et al. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: Role of asymmetric dimethylarginine. Vascul Pharmacol. 2009 doi: 10.1016/j.vph.2009.11.010. PMID: 19962451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Sydow K, Gunawan F, Jacobi J, Tsao PS, Robbins RC, et al. Dimethylarginine dimethylaminohydrolase overexpression suppresses graft coronary artery disease. Circulation. 2005;112:1549–1556. doi: 10.1161/CIRCULATIONAHA.105.537670. [DOI] [PubMed] [Google Scholar]
- 24.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respiratory Research. 2005;6:50. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charavaryamath C, Singh B. Pulmonary effects of exposure to pig barn air. J Occup Med Toxicol. 2006;1 doi: 10.1186/1745-6673-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med. 1994;150:973–977. doi: 10.1164/ajrccm.150.4.7921472. [DOI] [PubMed] [Google Scholar]