Abstract
Dose-limiting side effects of centrally-acting opioid drugs have led to the use of topical opioids to reduce the pain associated with chronic cutaneous wounds. However, previous studies indicate that topical morphine application impairs wound healing. This study was designed to elucidate the mechanisms by which morphine delays wound closure. Rats were depleted of sensory neuropeptides by treatment with capsaicin, and full-thickness 4 mm diameter wounds were excised from the intrascapular region. Wounds were treated topically twice daily with 5 mM morphine sulfate, 1 mM substance P, 1 mM neurokinin A, or 5 mM morphine combined with 1 m M substance P or neurokinin A and wound areas assessed. During closure, wound tissue was taken 1, 3, 5, and 8 days post-wounding from control and morphine-treated rats and immunostained for neurokinin receptors and markers for macrophages, myofibroblasts, and vasculature. Results obtained from capsaicin-treated animals demonstrated a significant delay in the early stages of wound contraction that was reversed by neuropeptide application. Treatment of capsaicin-treated rats with topical morphine did not further delay wound closure, suggesting that topical opioids impair wound closure via the inhibition of peripheral neuropeptide release into the healing wound. Morphine application altered neurokinin-1 and neurokinin-2 receptor expression in inflammatory and parenchymal cells essential for wound healing in a cell-specific manner, demonstrating a direct effect of morphine on neurokinin receptor regulation within an array of cells involved in wound healing. These data provide evidence indicating a potentially detrimental effect of topical morphine application on the dynamic wound healing process.
Keywords: substance P, neurokinin A, primary afferent neuron, skin, macrophage, myofibroblast
1. Introduction
Chronic cutaneous wounds, such as burns and skin ulcers, often result in prolonged hospitalization and considerable morbidity and remain a significant burden on health care systems. Pain associated with chronic wounds can be particularly difficult to manage, and many patients experience pain despite the use of systemic analgesics. Systemic opioid drugs remain the standard course of care in providing analgesia to patients with cutaneous wounds. However, recent studies have focused on peripherally-located opioid receptors as alternative analgesic targets. Opioid analgesia was initially believed to originate exclusively from the activation of opioid receptors within the central nervous system. Accumulating evidence demonstrates the analgesic efficacy of peripherally-administered opioids. Analgesia obtained during peripheral opioid administration results from the activation of opioid receptors located on primary afferent sensory nerve terminals in peripheral tissues [1]. Peripheral opioid analgesia is particularly attractive since it lacks the typical central, dose-limiting side effects that can accompany systemic opioid administration. Furthermore, analgesics applied locally provide optimal drug concentrations at the site of the noxious stimulus, avoiding the need to titrate systemic doses into a therapeutic range and offering a new alternative in the treatment of chronic pain.
Several clinical studies have explored the peripheral application of opioids as a strategy for pain management. The most extensive studies evaluating the analgesic effect of peripheral opioid drugs involved intra-articular morphine for postoperative pain relief in patients undergoing arthroscopic knee surgery [2, 3]. Intra-articular injections of morphine have also been used to successfully alleviate the pain associated with rheumatoid and osteoarthritis [4, 5]. In addition, the topical application of morphine has been successfully explored as a strategy for reducing the pain associated with skin ulcers, burns, and oral mucositis [6-10].
Although topical opioid administration offers a promising new therapeutic strategy for alleviating pain, it may also adversely affect wound healing limiting the usefulness of this approach. Opioid receptor agonists exhibit anti-inflammatory effects [11, 12]. Activation of opioid receptors on primary afferent neurons reduces the excitability of these neurons suppressing the antidromic release of pro-inflammatory neuropeptides such as substance P (SP) and neurokinin-A (NKA) [1, 13, 14]. Sensory neuropeptides are essential for normal wound healing. Exogenous application of neuropeptides enhances wound repair [15-17] while their depletion impairs the process [18-21]. Neuropeptides facilitate wound healing by mediating early components of neurogenic inflammation, specifically enhancing inflammatory cell migration and function [22, 23], vasodilation [24], and plasma extravasation [25]. In addition, neuropeptides function as mitogens for smooth muscle cells, fibroblasts, keratinocytes, and endothelial cells in the closing wound [26-30].
The biological actions of SP and NKA are mediated via neurokinin receptors belonging to the G protein-coupled receptor superfamily. While SP and NKA can act as full agonists at all of the neurokinin receptors, the receptors are recognized with moderate selectivity showing preferential binding to the neurokinin-1 (NK-1) and neurokinin-2 (NK-2) receptor, respectively [31, 32]. NK-1 receptors are located on smooth muscle cells, endothelial cells, fibroblasts, keratinocytes, monocytes, macrophages, and lymphocytes, while NK-2 receptors have been identified on all of the previously mentioned with the exception of fibroblasts [33-40]. Pharmacologically antagonizing the biological responses of the neurokinin receptors has been shown to delay wound healing [41, 42]. Interestingly, a single neurokinin-1 receptor knockout mice study observed no significant variation in healing of wounds with morphine treatment [43]. However, tachykinins may act through multiple neurokinin receptors in the healing wound compensating for NK-1 deletion; alternatively, the larger wound size in that study may reflect the role of phases of wound healing that are less prominent in the smaller wound model used in the current study.
Most studies utilizing topical morphine application have focused on its analgesic properties. However, given the regulatory effects of morphine on inflammation, endothelial proliferation and angiogenesis, it is reasonable to infer that morphine may potentially alter the process of wound healing. Current observations in the literature demonstrate opposing effects of opioid receptor agonists on the inflammatory response and wound healing. At low concentrations, studies reveal proinflammatory actions of peripheral opioid agonists. Angiogenesis is enhanced in several contexts by morphine [44-47] or endogenous opioids [48]. Notably, closure of larger, open ischemic wounds in a rat model was accelerated by topical morphine treatment, but this occurred at a morphine dose several fold lower than in the current study [49]. Delayed wound closure in delta opioid receptor-deletion mutant mice has also been reported [50]. Conversely, morphine, as well as mu and delta opioid receptor-selective agonists inhibit plasma–induced exudation [51, 52]. Previous studies in this laboratory have consistently revealed that topical application of hydrogel infused with a clinically relevant concentration of morphine sulfate [8, 53] significantly delays closure rates of small, full-thickness cutaneous wounds in rats [54]. This delay occurs in a concentration-dependent manner (consistent with opioid-receptor mediated effects), is mimicked by NK-1 and NK-2 receptor antagonists, and can be reversed by the addition of the neuropeptides SP or NKA [41]. Taken together, these findings suggest that topical morphine impairs wound closure by inhibiting the peripheral release of SP and NKA into the healing wound. However, many non-neuronal cells important in wound healing that express neurokinin receptors and potentially respond to neuropeptides also express opioid receptors. Consequently, topical morphine application may alternatively delay wound closure as a result of direct inhibition of these cells and structures. Therefore, this study was designed to better characterize the mechanisms by which topical morphine inhibits wound closure by defining the expression patterns of neurokinin receptors and their regulation by morphine, and by evaluating the effects of depletion of sensory neuropeptides in the closing wound.
2. Experimental Procedures
2.1 Animals and Experimental Design
A cutaneous wound healing model was utilized to evaluate wound closure rates in rats. Sixty-six male Sprague-Dawley rats (Harlan, Indianapolis, IN) approximately 200-220 grams in body weight were randomly assigned to one of ten treatment groups. Rats were anesthetized by intraperitoneal administration of 65 mg/kg ketamine HCl and 5.5 mg/kg xylazine HCl (Webster Veterinary Supply, Sterling, MA) and the mid-periscapular region clipped and shaved. A 4-mm diameter full-thickness skin section was excised from the midline below the scapulae to a depth just above the panniculus carnosus muscle using a skin biopsy punch [55]. All rats were housed individually to prevent grooming or other disruption of healing. Animal facilities were temperature- and humidity-controlled with a 12-h dark–light cycle and food and water ad libitum. All surgical procedures and animal handling were performed in accordance with National Institutes of Health laboratory care standards and were approved by the University of Kansas Medical Center Animal Care and Use Committee.
2.2 Capsaicin Pretreatment
A 10 mg/ml capsaicin (Sigma-Aldrich, St. Louis, MO) solution containing 20% ethanol and 10% Tween 80 in physiological saline was prepared each day prior to treatment. Vehicle or capsaicin was injected subcutaneously under brief inhaled isoflurane anesthesia on three consecutive days (30 mg/kg on day 1, 50 mg/kg on day 2, 70 mg/kg on day 3) [56]. Animals were wounded 96 hours following the last injection. Solutions were prepared each day prior to treatment. The efficiency of the capsaicin pretreatment in depleting and/or destroying primary afferent neurons was determined on day 9 post-wounding utilizing immunohistochemistry to assess the number of calcitonin gene-related peptide-immunoreactive (CGRP-ir) intraepidermal nerve fibers in mid-periscapular skin.
2.3 Drug Preparation and Gel Administration
Morphine sulfate (25 mg/ml) (Abbott Laboratories, Inc., North Chicago, IL) was infused into IntraSite® Gel (amorphous hydrogel; Smith+Nephew, Hull, United Kingdom) at a concentration of 5 mM. This concentration of topical morphine is within the range of those previously investigated in clinical studies [7-10]. The peptides SP and NKA (Sigma-Aldrich) were solubilized in 0.9% saline to a concentration of 10 mM and then infused into IntraSite® Gel to a final concentration of 1 mM. Peptide concentrations were selected to provide similar ratios between drug concentration in the gel and drug affinity at the specific target receptor (roughly 1 × 106). Gel and drug solutions were mixed in 3 cc syringes by repeated passage through a Luer-lock stopcock. Drug solutions were made the day of use or one day prior. Control animals received IntraSite® Gel alone treatments. Saline was added to control gel to match the consistency of the drug-infused gels. Beginning one hour post-surgery, 150μL IntraSite® Gel alone or IntraSite® Gel infused with a drug was applied topically to the wound twice daily. Total numbers of rats treated were as follows: control, n=26; 5 mM morphine sulfate, n=19; 1 mM SP, 1 mM NKA, 5 mM morphine sulfate + 1 mM NKA, n=5 per group; 5 mM morphine sulfate + 1 mM SP, n=6. Of the control and 5 mM morphine sulfate-treated rats, 5-6 were used for analysis of wound closure over time, while the remainder were paired for tissue collection for immunohistochemical analyses at various times after wounding.
2.4 Wound Imaging and Data Analysis
Wound images were captured each morning prior to gel treatment using a hand-held digital camera. A bar attached to the camera provided a fixed focal distance target for wound imaging. A size standard with known surface area was affixed to the target bar and captured in each image. Wound surface area was determined using a computerized planimetric program (Scion Image, Fredrick, MD). The area occupied by the wound was defined by the boundary created by the granulation tissue or scab/intact tissue interface. Wound area data generated by Scion Image were converted from pixels to area units of mm2 by comparison to the known area of the fixed size standard.
2.5 Tissue Harvesting
Rats were decapitated and wound tissue including approximately 1.0 cm of surrounding intact skin was harvested. Skin was dissected from rats in each treatment group on post-wound day 0 (n=3), 1 (n=8), 3 (n=6), 5 (n=6), and 8 (n=6). Tissues were subsequently embedded in TBS tissue freezing medium (Electron Microscopy Sciences, Hatfield, PA), frozen on dry ice and serially cryosectioned throughout the wound site at 14 μm thickness. Individual sections were collected at ten-section intervals, placed on adjacent slides and stored at −80°C until staining.
2.6 Immunohistochemistry
Tissue sections utilized for CGRP staining (from vehicle and capsaicin pretreated rats on post-wound day 10) were post-fixed in Zamboni’s fixative for 5 minutes, blocked in 5% goat serum (Jackson Immunoresearch Labs, West Grove, PA) for 1 hour, and incubated overnight at room temperature with a polyclonal antibody (1:500 CGRP rabbit IgG, Chemicon, Temecula, CA). This was followed by incubation for 90 minutes at room temperature with a cy3-conjugated goat anti-rabbit IgG secondary antibody (1:200, Jackson Immunoresearch Labs, West Grove, PA). Sections from gel-only or morphine-treated rats on post-wound days 0, 1, 3, 5, and 8 were post-fixed in 4% paraformaldehyde for 5 minutes, blocked in 5% goat serum for 1 hour, and incubated overnight at room temperature with antibodies for the NK-1 receptor (rabbit polyclonal IgG, 1:200, Novus Biologicals, Littleton, CO) or the NK-2 receptor (rabbit polyclonal IgG, 1:200, Novus) followed by incubation for 90 minutes at room temperature with a cy3-conjugated goat anti-rabbit IgG secondary antibody (1:200, Jackson). No specific staining was observed in primary antiserum omission controls.
For double-labeling studies, sections were incubated with a polyclonal antibody, as listed above, in combination with either the macrophage marker macrosialin (rat polyclonal IgG, FITC conjugated, 1:200, clone FA-11, Serotec, Oxford, United Kingdom) or the myofibroblast marker α-smooth muscle actin (α-SMA, mouse monoclonal IgG, 1:500, clone 1A4, Sigma). Labeling of α-SMA was visualized by subsequent incubation (90 minutes at room temperature) with goat anti-mouse IgG secondary antibody conjugated to cy2 (1:500, Jackson). Primary antiserum omission controls for each nonconjugated antibody demonstrated no specific staining. Rat serum was added to the blocking serum of sections stained with macrosialin antisera to negate the impact of non-specific staining. In addition, a rat IgG antibody conjugated to FITC (1:100, Serotec, clone YTH71.3) was utilized as a negative control, and no specific staining was detected with this approach. In pilot studies, ED1 mouse monoclonal IgG (Chemicon), CD68 goat polyclonal (Santa Cruz, Santa Cruz, CA), ED1 mouse monoclonal IgG (Serotec), and CD68 mouse monoclonal IgG (Biomeda, Foster City, CA) antibodies were evaluated and demonstrated similar staining patterns. However, they exhibited reduced sensitivity compared to the antibody selected and were consequently not used for quantification.
2.7 Imaging and Quantification
Slides were mounted with Fluoromount G (Fisher) and viewed using a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY). Images of individual sections were captured with a Nikon Digital Sight Fi1 camera (Nikon). Immunoreactive morphological structures were quantified in 3 sections equally spaced throughout each wound. In each section, four regions, each equaling an area of 0.35 mm2, were selected randomly and analyzed by an observer blind to animal treatment. Intraepidermal nerve fibers positive for CGRP were assessed utilizing the European Federation of Neurological Societies guidelines for quantification [57]. Neurokinin receptor-ir area of keratinocytes was obtained by threshold discrimination and then divided by the total area analyzed and expressed as a percent of field area. For double-labeling studies, numbers of cells macrosialin- and NK-1 or NK-2 receptor-ir were averaged and expressed as percentage of neurokinin receptor-positive macrophages per unit area. Neurokinin receptor- and α-SMA-ir area of blood vessels and myofibroblasts were also obtained by threshold discrimination. Data are presented as percentage of neurokinin receptor-positive structures per unit area. For analysis of myofibroblasts, α-SMA-ir vasculature was excluded; only cells demonstrating a spindle-shaped morphology consistent with myofibroblast structure were included. All images were evaluated by an observer blinded to the treatment group using Metamorph (Molecular Devices, Downingtown, PA) analysis software.
2.8 Statistical Analyses
Statistical analyses were performed using SigmaStat (San Jose, CA). Data are reported as mean ± SEM. Main effects on wound closure were evaluated using two-way repeated measures analysis of variance with factors of drug treatment and time; effects of drug treatment and time on neurokinin receptor expressing macrophages and myofibroblasts were evaluated using separate two-way analyses of variance with factors of drug treatment and time. The effect of drug treatment on the area under the time course curve was assessed using one-way analysis of variance. Differences between treatment groups and within treatment groups over time were identified using Tukey post-hoc tests. Differences between means were considered significant when p < 0.05.
3. Results
3.1 Capsaicin pretreatment chemically ablates cutaneous sensory innervation
Pretreatment with the neurotoxin capsaicin was utilized to deplete sensory neuropeptides and selectively destroy small-diameter unmyelinated C-fibers and thinly myelinated Aδ fibers in adult rats. Functional cutaneous denervation of these fibers was assessed by immunohistochemistry using an anti-CGRP antibody. Intraepidermal nerve fibers positive for CGRP were quantified using European Federation of Neurological Societies guidelines [57]. Rats receiving three consecutive subcutaneous injections of capsaicin demonstrated an 80% reduction (p = 0.001) in intraepidermal CGRP-ir nerve fibers in the mid-periscapular region of the skin when compared to vehicle-treated controls (data not shown).
3.2 Neuropeptide replacement in morphine sulfate-infused gel restores cutaneous wound closure rates in denervated rats
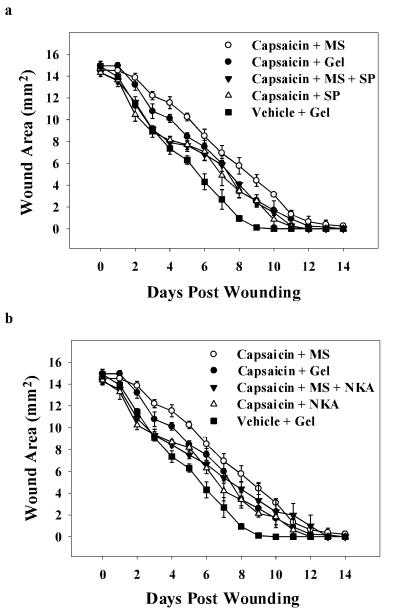
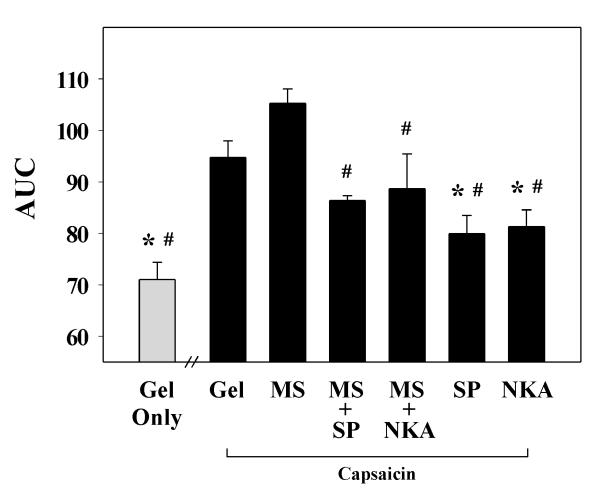
The impact of capsaicin pretreatment on cutaneous wound closure rates in rats was assessed using a standardized model of cutaneous wound healing. Repeated administration of capsaicin resulted in a significant delay in time required for full closure of cutaneous wounds; two-way ANOVA revealed a significant main effect of drug treatment on wound area (F(6, 279) = 14.0; p = 0.001). Vehicle-treated wounds closed 9.2 days post-wounding (Figure 1). Wounds of rats pretreated with capsaicin receiving applications of gel alone completely closed by post-wound days 11.75, correlating to a 28% increase in time to closure over controls (p = 0.02). One-way ANOVA revealed a significant main effect of drug treatment on the area under the time course curves (F(6, 26) = 12.0; p < 0.001). A 34% increase in the total wound area throughout the time course was also seen in capsaicin denervated wounds when compared to controls (p = 0.001; Figure 2).
Figure 1. Wound closure time course for capsaicin-treated rats receiving IntraSite™® gel treatments infused with morphine and/or neuropeptides.
IntraSite™® gel was applied to the wound twice daily through wound day 10. Wound size is presented as area (mm2) mean ± SEM and was determined by analysis of digital images. Note that the wounds of chemically denervated rats treated with gel-only fully closed significantly later than vehicle-only treated controls (n=5 or 6; p < 0.05; two-way repeated measures ANOVA, Tukey’s post hoc test).
Figure 2. Area under the wound closure time course curve for capsaicin-treated rats receiving IntraSite™® gel infused with morphine and/or neuropeptides.
Data are presented as area (mm2 × day) mean ± SEM. Rats received applications of IntraSite™® gel to the wound twice daily through wound day 10. Sensory denervation with capsaicin significantly delayed wound closure compared to vehicle-treated controls. Topical application of either 1 mM SP or NKA to chemically denervated wounds significantly accelerated wound closure compared to gel-only treated control. Addition of either 1 mM SP or NKA into gel infused with 5 mM morphine (MS) accelerated wound closure in capsaicin-treated rats (n = 5 or 6; *p < 0.05 when compared to capsaicin + gel-only treatment, # p < 0.05 compared to capsaicin + morphine treatment; one-way ANOVA, Tukey’s post hoc test).
In contrast, topical application of either 1 mM SP or NKA to wounds of capsaicin-treated rats resulted in a significant decrease (17% and 15% respectively) in total wound area relative to denervated wounds treated with gel alone (p = 0.01 and p = 0.02; Figure 2). Treatment of denervated wounds with 5 mM morphine did not further delay wound closure compared to gel-only treated capsaicin controls. However, combining either 1mM SP or NKA with morphine-infused gel did significantly reduce the total wound area compared to morphine treatment of denervated wounds demonstrating acceleration in wound closure (p = 0.0002 and p = 0.04). Furthermore, addition of either neuropeptide into morphine-infused gel returned closure rates to those not significantly different from those in vehicle-treated rats.
3.3 Topical morphine sulfate treatment reduces the number of NK-1 and NK-2 receptor-positive macrophages in the healing wound
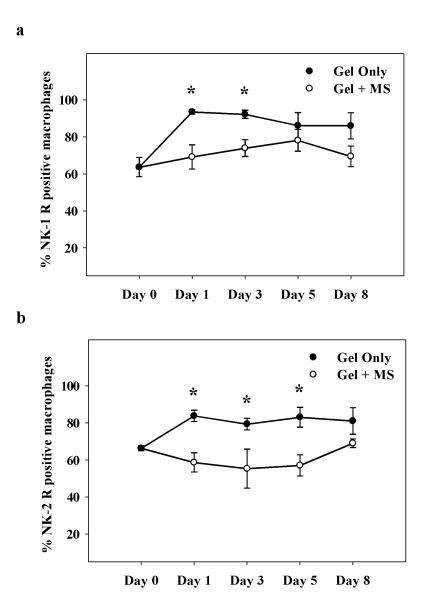
The effect of topical morphine on the number of NK-1 and NK-2 receptor-positive macrophages in healing wounds was assessed with immunohistochemistry using NK-1 and NK-2 receptor antiserum and the macrophage marker macrosialin (Figure 3). Numbers of macrophages expressing NK-1 and NK-2 receptor immunoreactivity increased significantly in gel-only treated controls following wounding with maximum numbers seen on wound day 1 (Figure 4a, b); two-way ANOVA indicated a significant main effect of drug treatment on percent NK-1 and NK-2 receptor-positive macrophages, respectively (NK-1: F(1, 38) = 21, p < 0.001; NK-2: F(1, 39) = 37; p = 0.001). Conversely, an increase in NK-1 or NK-2 receptor-immunoreactive (-ir) macrophages did not develop in wounds treated with topical morphine. Morphine administration significantly decreased macrosialin-ir cells positive for the NK-1 receptor (p = 0.02). Differences were significant on days 1 and 3 post-wounding (26% and 20%, respectively; Figure 4a). Macrophages positive for the NK-2 receptor decreased significantly in number (p = 0.01). Significant differences were observed on wound days 1 (30%), 3 (30%), and 5 (31%) with topical morphine treatment (Figure 4b).
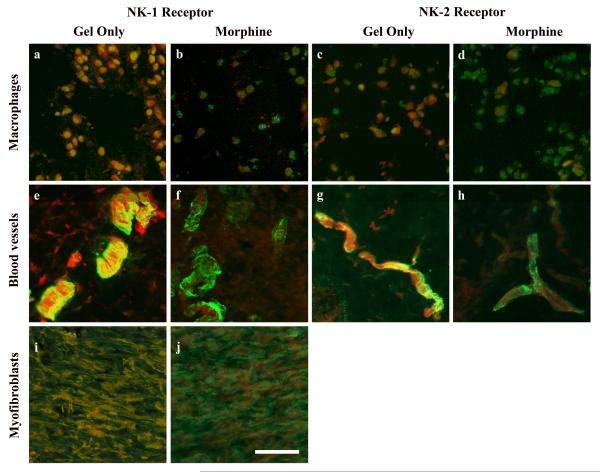
Figure 3. Photomicrographs of immunostaining in wound tissue during topical morphine treatment.
Cutaneous wounds were treated twice daily with IntraSite™® gel alone (a, c, e, g, i) or gel infused with 5 mM morphine sulfate (b, d, f, h, j). Images represent wound tissue dissected from rats on wound days 1 (a, b, c, d), 3 (e, f, g, h), and 8 (i, j). Neurokinin receptor positive cells were assessed in sections stained by direct immunofluorescence for macrosialin (green; a, b, c, d) or α-smooth muscle actin (red; e, f, g h, I j) in combination with indirect markers for the neurokinin-1 (a, b, e, f, i, j) or neurokinin-2 receptor (c, d, g, h). Scale bar = 100 μm.
Figure 4. Time course of NK-1 and NK-2 receptor-positive macrophages in morphine treated wounds.
Cutaneous wounds were treated twice daily with IntraSite™® gel alone or gel infused with 5 mM morphine sulfate (MS). Data are presented as percentage of neurokinin receptor-positive macrophages per unit area analyzed. Note that in gel-only treated controls, the number of NK-1 and NK-2 receptor-expressing macrophages increased following wounding. (a) Gel infused with 5 mM MS significantly reduced the number of NK-1 receptor positive macrophages within the healing wound. (b) Wounds treated with 5 mM MS had significantly fewer NK-2 receptor positive macrophages (n=3 or 4; *p < 0.05; two-way ANOVA, Tukey’s post hoc test).
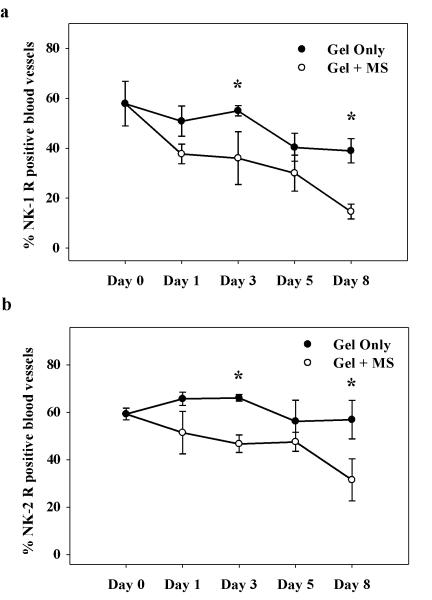
3.4 Topical morphine application decreases NK-1 and NK-2 receptor expression of vasculature in the healing wound
The density of blood vessels in the healing wounds was measured utilizing α-smooth muscle actin (α-SMA)-immunoreactivity. Two-way ANOVA revealed a significant main effect of drug treatment on percent NK-1 and NK-2 receptor-positive blood vessels, respectively (NK-1: F(1, 54) = 17.5, p = 0.001; NK-2: F(1, 35) = 14.3; p = 0.001). In control wounds, no significant differences in NK-1 or NK-2 receptor-positive blood vessels were observed across time (Figure 5a, b). However, topical morphine application significantly decreased NK-1 and NK-2 receptor expression in blood vessels when compared to gel-only treated controls (p = 0.04 and 0.03, respectively). A 34% and 62% reduction was observed in NK-1 receptor-ir blood vessels on wound days 3 and 8, respectively, while morphine decreased NK-2 expressing blood vessels by 30% and 45% on days 3 and 8.
Figure 5. Time course of NK-1 and NK-2 receptor-positive vasculature in morphine treated wounds.
Rats received twice-daily treatments of IntraSite™® gel. Data are presented as percentage of neurokinin receptor-positive blood vessels per unit area. (a & b) Application of 5 mM morphine sulfate (MS) to healing wounds significantly decreased NK-1 and NK-2 receptor levels in blood vessels within the wound (n=3 or 4; *p < 0.05; two-way ANOVA, Tukey’s post hoc test).
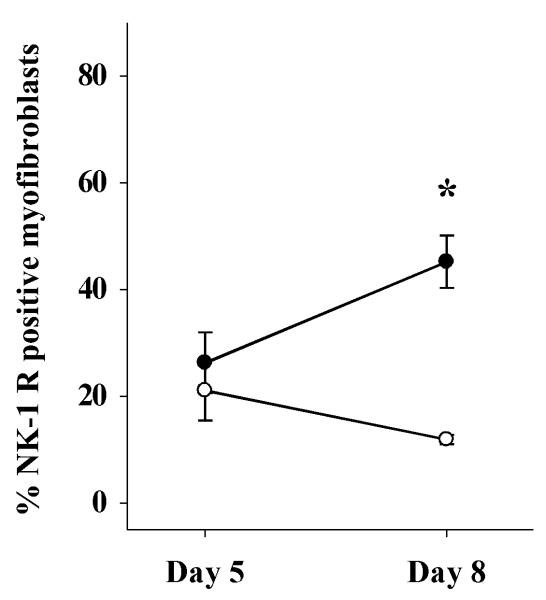
3.5 Fewer NK-1 receptor-ir myofibroblasts are present in morphine-treated wounds versus control
Myofibroblast density within the wound was measured using α-SMA-immunoreactivity. For the quantification of myofibroblasts, α-SMA-ir vasculature was excluded and only cells demonstrating a spindle-shaped morphology and organized in a sheath-like mass were quantified. NK-1 receptor-ir myofibroblasts levels were measurable beginning on day 5 post-wounding. Two-way ANOVA indicated a significant main effect of drug treatment on percent NK-1 receptor-positive myofibroblasts (NK-1: F(1, 11) = 12.4, p < 0.005). NK-1 receptor-expressing myofibroblasts increased in number from wound day 5 to 8 in gel-only treated controls (Figure 6). However, topical morphine treatment significantly inhibited this increase in expression (p = 0.002) resulting in a 74% decrease on day 8 post-wounding relative to gel-only treated controls. NK-2 receptor-ir myofibroblasts levels were not significantly altered by morphine treatment (data not shown).
Figure 6. NK-1 receptor-positive myofibroblasts in morphine treated wounds.
Wounds were treated twice daily with IntraSite™® gel alone or gel infused with 5 mM morphine sulfate (MS). Data are presented as percentage of neurokinin receptor-positive myofibroblasts per unit area. NK-1 receptor-positive myofibroblast levels were detectable on wound day 5. Gel infused with 5 mM MS significantly reduced the number of NK-1 receptor-positive myofibroblasts within the healing wound (n=3 or 4; *p < 0.05; two-way ANOVA, Tukey’s post hoc test).
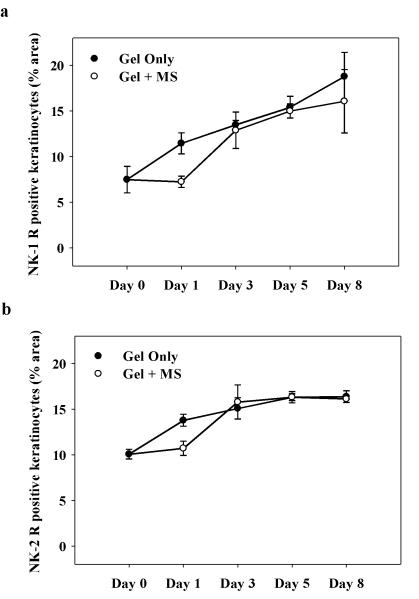
3.6 Numbers of NK-1 and NK-2 receptor-ir keratinocytes in control versus morphine-treated wounds are unchanged
NK1 and NK-2 receptor-immunoreactivity was assessed in keratinocytes of the healing wounds. Two-way ANOVA revealed a significant main effect of time on percent NK-1 and NK-2 receptor-positive keratinocytes, respectively (NK-1: F(1, 24) = 3.0, p < 0.001; NK-2: F(1, 42) = 18; p < 0.001). However, the main effect of drug treatment on percent NK-1 and NK-2 receptor-positive keratinocytes was not significant. NK-1 and NK-2 receptor-ir keratinocytes increased in number throughout the course of healing in both control and morphine-treated wounds, with the maximum percent area seen on day 8 post-wounding (Figure 7a, b). Morphine treatment did not significantly alter NK-1 or NK-2 receptor expression in keratinocytes in the healing wound.
Figure 7. Time course of NK-1 and NK-2 receptor-positive keratinocytes in wounds treated with topical morphine.
Rats were treated with IntraSite™® gel alone or gel infused with 5 mM morphine sulfate (MS) twice daily. Results are expressed as percent area of neurokinin receptor-ir keratinocytes in histological fields analyzed. The area of NK-1 and NK-2 receptor-ir keratinocytes increased with time course during wound closure. (a & b) No significant differences were detected between treatment groups (n=3 or 4; *p < 0.05; two-way ANOVA, Tukey’s post hoc test).
4. Discussion
The present study investigated the mechanisms underlying morphine-induced delays in wound closure. Topically applied morphine delays cutaneous wound closure in rats. Previous data suggest this delay occurs via inhibition of the peripheral release of neuropeptides from primary afferent neurons as morphine’s actions are concentration-dependent, emulated by neurokinin receptor antagonists, and reversed by exogenous application of the neuropeptides [41]. However, opioid receptors are not only located on peripheral nerve terminals but also on other neuropeptide-targeted cells essential in the healing process. Therefore, this study addressed whether morphine impairs healing directly through activation of opioid receptors on immune and/or parenchymal cells within the wound or by inhibiting the activation of primary afferent neurons thereby inhibiting the peripheral actions of neuropeptides.
Repeated subcutaneous administration of the neurotoxin capsaicin, the pungent vanilloid found in hot peppers, results in the depletion of sensory neuropeptides and permanent degeneration of small-diameter C-fibers, reducing inflammatory responses and ultimately diminishing wound healing [19, 21, 58, 59]. Capsaicin pretreatment was similarly utilized in this study to produce chemically “denervated” skin and thereby assess the impact opioid receptors located on primary afferent neurons have on morphine-induced delays in wound closure. The protocol used produces a 29% decrease in CGRP-ir neurons located in the dorsal root ganglion [56], a 74% reduction in inflammation-induced c-fos staining in the dorsal horn of the spinal cord [60], as well as a 90% reduction in CGRP-ir intraepidermal fibers in plantar skin [61]. Data from this study confirm significant functional sensory denervation, demonstrating an 80% reduction in cutaneous CGRP-positive nerver fibers following capsaicin pretreatment.
Neuropeptides peripherally released by primary afferent neurons following injury are essential for mediating the early components of neurogenic inflammation and initiating the healing process [62]. While other neuropeptides (e.g. CGRP) are undoubtedly relevant for normal wound healing, the focus of the studies included was SP and NKA. Consistent with previous reports, our data demonstrated that sensory denervation with capsaicin impairs wound healing, with wounds fully closing at times significantly later than those in rats treated with vehicle. However, exogenous application of either SP or NKA topically to the healing wounds attenuated the delay in capsaicin-denervated rats (to rates not significantly different than those in intact animals), emphasizing the importance of neuropeptides in the wound closure process. While opioid receptors are located on various cells and structures within the healing wound, topical morphine treatment of wounds in sensory denervated rats did not further delay wound closure. However, a trend of an increase in wound surface area with morphine treatment of capsaicin-denervated wounds was observed. Quantification of CGRP-ir neurons demonstrated an 80% reduction in small-diameter, peptide-containing fibers. Therefore, this delay could be attributed to incomplete sensory denervation followed by blockade of neuropeptide release by morphine application from remaining neurons. Furthermore, the addition of SP or NKA to morphine-infused gel accelerated wound closure to rates not significantly different from vehicle-treated controls. These results support an indirect action of morphine in delaying wound closure via primary afferent neurons, rather than a direct inhibitory effect of morphine on non-neuronal target cells activated by the tachykinins.
Activation of neurokinin receptors located in peripheral tissues initiates vasodilation and increased vascular permeability, stimulates the migration of immune cells and the production and release of inflammatory mediators from these cells, and induces proliferation of parenchymal cells within the healing wound [63].The results of this and previous studies implicate the actions of neuropeptides in the inhibition of wound closure by topical morphine application. Accordingly, the regulation of neurokinin receptor-expressing target cells in the closing wound was evaluated to explore the underlying effect morphine treatment has within the healing wound.
Macrophages are the predominant cell infiltrating the wound during the early, inflammatory phase of healing and are essential for the progression of normal wound healing [64]. Depletion of macrophages results in poor debridement, delayed fibroblast activation and inhibited fibroblast proliferation, and impaired wound closure [65]. We, and others, have previously demonstrated that topical morphine application both reduces and delays macrophage migration into the healing wound [54, 66]. Current results demonstrate a significant decrease in the number of NK-1 and NK-2 receptor-positive blood vessels in morphine-treated wounds throughout the healing process. Morphine can enhance endothelial proliferation and angiogenesis in several tissues and models, but at lower concentrations than that studied here [44-47]. It is possible that the relatively higher concentration of morphine used in this study produced local cytotoxicity rather than the promotion of healing reported in other studies [49]. However, evidence exists in the literature to suggest that high doses of morphine can directly impair angiogenesis. Recent findings by Lam et al. demonstrated that a high dose of intraperitoneal morphine impaired angiogenesis and mobilization of endothelial progenitor cells in an excisional wound model in mice [67], suggesting that our clinically-relevant dosing regimen may similarly directly effect macrophage recruitment. Combined, these findings may account for the reduction in macrophage numbers reported previously, and may result from decreased vasodilation and vascular permeability in the closing wound when treated topically with morphine.
In addition to mediating the inflammatory phase of wound healing, macrophages also initiate the second, proliferative phase [68-70]. During the proliferative phase the extracellular matrix is replaced by granulation tissue, which consists of macrophages, fibroblasts, collagen, and blood vessels [71]. Closure of full-thickness excision wounds occurs primarily via contraction of myofibroblasts. Macrophages release growth factors and cytokines responsible for the activation and proliferation of fibroblasts [69, 72]. Activated fibroblasts transdifferentiate into myofibroblasts expressing α-SMA, which provides their contractile potential [73, 74]. Additionally, tissue remodeling from granulation tissue to scar is dependent on continued synthesis and catabolism of collagen. The degradation of collagen is carried out by matrix metalloproteinases secreted by macrophages [75]. The results of the current study demonstrate an increase in the numbers of NK-1 and NK-2 receptor-expressing macrophages in the wound during closure. However, topical morphine application inhibits this increase in the proportion of neurokinin receptor-expressing macrophages. These data strongly suggest that the chemotactic and mitogenic effects on these cells in the healing wound, contributed in part by the actions of neuropeptides released from sensory nerve endings, would thereby be diminished by topical morphine application. Neuropeptides also directly initiate the activation, migration, and proliferation of fibroblasts [26, 28, 30]. Previous studies demonstrate wounds receiving topical morphine treatment contain considerably fewer myofibroblasts, which are essential for wound contraction [40]. Current observations show a significant decrease in numbers of NK-1 receptor-positive fibroblasts in morphine-treated wounds. These data demonstrate the dysregulation of neurokinin receptor-expressing inflammatory and parenchymal cells, and suggest that this dysregulation contributes to impaired wound closure.
In conclusion, the results of this study suggest that topical morphine application delays wound closure by inhibiting the peripheral release of SP and NKA and their subsequent actions at NK-1 and NK-2 receptors. These data strongly support a neuronal action of morphine in delaying wound closure, as opposed to direct inhibition of non-neuronal target cells within the wound. Dysregulation of neurokinin receptor-expressing vasculature, macrophages and fibroblasts (but not keratinocytes) secondary to blockade of the peripheral release of neuropeptides emerge as mechanisms that can significantly attenuate wound closure following topical morphine treatment.
Acknowledgments
This study was supported in part by DA12505 (KMcC) and a Basic Science Research Pilot Grant from the Lied foundation/KUMC Research Institute (KMcC) and an award from the KUMC Biomedical Research Training Program (JR). The authors would like to thank Michelle Winter and Donald Warn, Ph.D. for their expert technical assistance and the contributions of the KUMC IDDRC (NICHD HD 02528) core facilities.
Abbreviations
- (α-SMA)
α-smooth muscle actin
- (CGRP)
calcitonin gene-related peptide
- (-ir)
-immunoreactive
- (MS)
morphine sulfate
- (NKA)
neurokinin A
- (NK-1)
neurokinin-1
- (NK-2)
neurokinin-2
- (SP)
substance P
References
- [1].Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182–91. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- [2].Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–6. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- [3].Ho ST, Wang TJ, Tang JS, Liaw WJ, Ho CM. Pain relief after arthroscopic knee surgery: intravenous morphine, epidural morphine, and intra-articular morphine. Clin J Pain. 2000;16:105–9. doi: 10.1097/00002508-200006000-00003. [DOI] [PubMed] [Google Scholar]
- [4].Likar R, Schafer M, Paulak F, Sittl R, Pipam W, Schalk H, et al. Intraarticular morphine analgesia in chronic pain patients with osteoarthritis. Anesth Analg. 1997;84:1313–7. doi: 10.1097/00000539-199706000-00025. [DOI] [PubMed] [Google Scholar]
- [5].Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain. 1999;83:525–32. doi: 10.1016/S0304-3959(99)00156-6. [DOI] [PubMed] [Google Scholar]
- [6].Cerchietti LC, Navigante AH, Bonomi MR, Zaderajko MA, Menendez PR, Pogany CE, et al. Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer. 2002;95:2230–6. doi: 10.1002/cncr.10938. [DOI] [PubMed] [Google Scholar]
- [7].Flock P. Pilot study to determine the effectiveness of diamorphine gel to control pressure ulcer pain. J Pain Symptom Manage. 2003;25:547–54. doi: 10.1016/s0885-3924(03)00140-4. [DOI] [PubMed] [Google Scholar]
- [8].Long TD, Cathers TA, Twillman R, O’Donnell T, Garrigues N, Jones T. Morphine-Infused silver sulfadiazine (MISS) cream for burn analgesia: a pilot study. J Burn Care Rehabil. 2001;22:118–23. doi: 10.1097/00004630-200103000-00006. [DOI] [PubMed] [Google Scholar]
- [9].Watterson G, Howard R, Goldman A. Peripheral opioids in inflammatory pain. Arch Dis Child. 2004;89:679–81. doi: 10.1136/adc.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeppetella G, Paul J, Ribeiro MD. Analgesic efficacy of morphine applied topically to painful ulcers. J Pain Symptom Manage. 2003;25:555–8. doi: 10.1016/s0885-3924(03)00146-5. [DOI] [PubMed] [Google Scholar]
- [11].Jin S, Lei L, Wang Y, Da D, Zhao Z. Endomorphin-1 reduces carrageenan-induced fos expression in the rat spinal dorsal horn. Neuropeptides. 1999;33:281–4. doi: 10.1054/npep.1999.0040. [DOI] [PubMed] [Google Scholar]
- [12].Khalil Z, Sanderson K, Modig M, Nyberg F. Modulation of peripheral inflammation by locally administered endomorphin-1. Inflamm Res. 1999;48:550–6. doi: 10.1007/s000110050502. [DOI] [PubMed] [Google Scholar]
- [13].Schroeder JE, Fischbach PS, Zheng D, McCleskey EW. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- [14].Aimone LD, Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides. 1989;10:1127–31. doi: 10.1016/0196-9781(89)90003-x. [DOI] [PubMed] [Google Scholar]
- [15].Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005;230:271–80. doi: 10.1177/153537020523000407. [DOI] [PubMed] [Google Scholar]
- [16].Engin C. Effects of calcitonin gene-related peptide on wound contraction in denervated and normal rat skin: a preliminary report. Plast Reconstr Surg. 1998;101:1887–90. doi: 10.1097/00006534-199806000-00017. [DOI] [PubMed] [Google Scholar]
- [17].Kjartansson J, Dalsgaard CJ. Calcitonin gene-related peptide increases survival of a musculocutaneous critical flap in the rat. Eur J Pharmacol. 1987;142:355–8. doi: 10.1016/0014-2999(87)90073-2. [DOI] [PubMed] [Google Scholar]
- [18].Khalil Z, Helme R. Sensory peptides as neuromodulators of wound healing in aged rats. J Gerontol A Biol Sci Med Sci. 1996;51:B354–61. doi: 10.1093/gerona/51a.5.b354. [DOI] [PubMed] [Google Scholar]
- [19].Kjartansson J, Dalsgaard CJ, Jonsson CE. Decreased survival of experimental critical flaps in rats after sensory denervation with capsaicin. Plast Reconstr Surg. 1987;79:218–21. doi: 10.1097/00006534-198702000-00012. [DOI] [PubMed] [Google Scholar]
- [20].Peskar BM, Lambrecht N, Stroff T, Respondek M, Muller KM. Functional ablation of sensory neurons impairs healing of acute gastric mucosal damage in rats. Dig Dis Sci. 1995;40:2460–4. doi: 10.1007/BF02063255. [DOI] [PubMed] [Google Scholar]
- [21].Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–91. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]
- [22].Eglezos A, Andrews PV, Boyd RL, Helme RD. Modulation of the immune response by tachykinins. Immunol Cell Biol. 1991;69(Pt 4):285–94. doi: 10.1038/icb.1991.39. [DOI] [PubMed] [Google Scholar]
- [23].Eglezos A, Helme RD, Dandie GW, Andrews PV, Boyd RL. Substance P-mediated modulation of the primary antibody response. Adv Exp Med Biol. 1988;237:499–503. doi: 10.1007/978-1-4684-5535-9_76. [DOI] [PubMed] [Google Scholar]
- [24].Khalil Z, Helme RD. Serotonin modulates substance P-induced plasma extravasation and vasodilatation in rat skin by an action through capsaicin-sensitive primary afferent nerves. Brain Res. 1990;527:292–8. doi: 10.1016/0006-8993(90)91149-b. [DOI] [PubMed] [Google Scholar]
- [25].Khalil Z, Helme RD. Sequence of events in substance P-mediated plasma extravasation in rat skin. Brain Res. 1989;500:256–62. doi: 10.1016/0006-8993(89)90321-1. [DOI] [PubMed] [Google Scholar]
- [26].Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985;315:61–3. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- [27].Rameshwar P, Poddar A, Zhu G, Gascon P. Receptor induction regulates the synergistic effects of substance P with IL-1 and platelet-derived growth factor on the proliferation of bone marrow fibroblasts. J Immunol. 1997;158:3417–24. [PubMed] [Google Scholar]
- [28].Ziche M, Morbidelli L, Pacini M, Dolara P, Maggi CA. NK1-receptors mediate the proliferative response of human fibroblasts to tachykinins. Br J Pharmacol. 1990;100:11–4. doi: 10.1111/j.1476-5381.1990.tb12043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–78. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka T, Danno K, Ikai K, Imamura S. Effects of substance P and substance K on the growth of cultured keratinocytes. J Invest Dermatol. 1988;90:399–401. doi: 10.1111/1523-1747.ep12456487. [DOI] [PubMed] [Google Scholar]
- [31].Mussap CJ, Geraghty DP, Burcher E. Tachykinin receptors: a radioligand binding perspective. J Neurochem. 1993;60:1987–2009. doi: 10.1111/j.1471-4159.1993.tb03484.x. [DOI] [PubMed] [Google Scholar]
- [32].Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev. 1994;46:551–99. [PubMed] [Google Scholar]
- [33].Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnett N, Payan D. Skin-nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- [34].Bowden JJ, Baluk P, Lefevre PM, Vigna SR, McDonald DM. Substance P (NK1) receptor immunoreactivity on endothelial cells of the rat tracheal mucosa. Am J Physiol. 1996;270:L404–14. doi: 10.1152/ajplung.1996.270.3.L404. [DOI] [PubMed] [Google Scholar]
- [35].Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–60. [PubMed] [Google Scholar]
- [36].Krause JE, Takeda Y, Hershey AD. Structure, functions, and mechanisms of substance P receptor action. J Invest Dermatol. 1992;98:2S–7S. doi: 10.1111/1523-1747.ep12462082. [DOI] [PubMed] [Google Scholar]
- [37].Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–6. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- [38].Qian BF, Zhou GQ, Hammarstrom ML, Danielsson A. Both substance P and its receptor are expressed in mouse intestinal T lymphocytes. Neuroendocrinology. 2001;73:358–68. doi: 10.1159/000054653. [DOI] [PubMed] [Google Scholar]
- [39].Haley KJ, Sunday ME, Osathanondh R, Du J, Vathanaprida C, Karpitsky VV, et al. Developmental expression of neurokinin A and functional neurokinin-2 receptors in lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1348–58. doi: 10.1152/ajplung.2001.280.6.L1348. [DOI] [PubMed] [Google Scholar]
- [40].Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, et al. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R) Exp Dermatol. 2000;9:42–52. doi: 10.1034/j.1600-0625.2000.009001042.x. [DOI] [PubMed] [Google Scholar]
- [41].Rook JM, McCarson KE. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol. 2007;74:752–7. doi: 10.1016/j.bcp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schmassmann A, Waser B, Flogerzi B, Reubi JC. Expression of functional neurokinin-1 receptors in regenerative glands during gastric wound healing in rodents. Gastroenterology. 2004;126:784–95. doi: 10.1053/j.gastro.2003.11.052. [DOI] [PubMed] [Google Scholar]
- [43].Cao T, Grant AD, Gerard NP, Brain SD. Lack of a significant effect of deletion of the tachykinin neurokinin-1 receptor on wound healing in mouse skin. Neuroscience. 2001;108:695–700. doi: 10.1016/s0306-4522(01)00435-3. [DOI] [PubMed] [Google Scholar]
- [44].Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res. 2006;3:171–80. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- [45].Farooqui M, Li Y, Rogers T, Poonawala T, Griffin RJ, Song CW, et al. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–31. doi: 10.1038/sj.bjc.6604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- [47].Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [48].Dai X, Cui SG, Wang T, Liu Q, Song HJ, Wang R. Endogenous opioid peptides, endomorphin-1 and -2 and deltorphin I, stimulate angiogenesis in the CAM assay. Eur J Pharmacol. 2008;579:269–75. doi: 10.1016/j.ejphar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [49].Poonawala T, Levay-Young BK, Hebbel RP, Gupta K. Opioids heal ischemic wounds in the rat. Wound Repair Regen. 2005;13:165–74. doi: 10.1111/j.1067-1927.2005.130207.x. [DOI] [PubMed] [Google Scholar]
- [50].Bigliardi-Qi M, Gaveriaux-Ruff C, Zhou H, Hell C, Bady P, Rufli T, et al. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation. 2006;74:174–85. doi: 10.1111/j.1432-0436.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- [51].Lei YH, Rogers DF. Effects and interactions of opioids on plasma exudation induced by cigarette smoke in guinea pig bronchi. Am J Physiol. 1999;276:391–7. doi: 10.1152/ajplung.1999.276.3.L391. [DOI] [PubMed] [Google Scholar]
- [52].Romero A, Planas E. Anti-exudative effects of opioid receptor agonists in a rat model of carrageenan-induced acute inflammation of the paw. Eur J Pharmacol. 2005;511:207–17. doi: 10.1016/j.ejphar.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [53].Twillman RK, Long TD, Cathers TA, Mueller DW. Treatment of painful skin ulcers with topical opioids. J Pain Symptom Manage. 1999;17:288–92. doi: 10.1016/s0885-3924(98)00140-7. [DOI] [PubMed] [Google Scholar]
- [54].Rook JM, Hasan W, McCarson KE. Temporal effects of topical morphine application on cutaneous wound healing. Anesthesiology. 2008;109:130–6. doi: 10.1097/ALN.0b013e31817b5ac3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu M, Warn JD, Fan Q, Smith PG. Relationships between nerves and myofibroblasts during cutaneous wound healing in the developing rat. Cell Tissue Res. 1999;297:423–33. doi: 10.1007/s004410051369. [DOI] [PubMed] [Google Scholar]
- [56].Zhou L, Zhang Q, Stein C, Schafer M. Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther. 1998;286:1000–6. [PubMed] [Google Scholar]
- [57].Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–58. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- [58].Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–9. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- [59].Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- [60].Zhang Q, Schaffer M, Elde R, Stein C. Effects of neurotoxins and hindpaw inflammation on opioid receptor immunoreactivities in dorsal root ganglia. Neuroscience. 1998;85:281–91. doi: 10.1016/s0306-4522(97)00647-7. [DOI] [PubMed] [Google Scholar]
- [61].Schicho R, Skofitsch G, Donnerer J. Regenerative effect of human recombinant NGF on capsaicin-lesioned sensory neurons in the adult rat. Brain Res. 1999;815:60–9. doi: 10.1016/s0006-8993(98)01094-4. [DOI] [PubMed] [Google Scholar]
- [62].Schaffer M, Beiter T, Becker HD, Hunt TK. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg. 1998;133:1107–16. doi: 10.1001/archsurg.133.10.1107. [DOI] [PubMed] [Google Scholar]
- [63].Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–86. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–40. [PubMed] [Google Scholar]
- [65].Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- [66].Dinda A, Gitman M, Singhal PC. Immunomodulatory effect of morphine: therapeutic implications. Expert Opin Drug Saf. 2005;4:669–75. doi: 10.1517/14740338.4.4.669. [DOI] [PubMed] [Google Scholar]
- [67].Lam CF, Chang PJ, Huang YS, Sung YH, Huang CC, Lin MW, et al. Prolonged use of high-dose morphine impairs angiogenesis and mobilization of endothelial progenitor cells in mice. Anesth Analg. 2008;107:686–92. doi: 10.1213/ane.0b013e31817e6719. [DOI] [PubMed] [Google Scholar]
- [68].Diegelmann RF, Schuller-Levis G, Cohen IK, Kaplan AM. Identification of a low molecular weight, macrophage-derived chemotactic factor for fibroblasts. Clin Immunol Immunopathol. 1986;41:331–41. doi: 10.1016/0090-1229(86)90004-8. [DOI] [PubMed] [Google Scholar]
- [69].Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–84. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- [70].Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174–9. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- [71].Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–86. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- [72].Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- [73].Gabbiani G. The biology of the myofibroblast. Kidney Int. 1992;41:530–2. doi: 10.1038/ki.1992.75. [DOI] [PubMed] [Google Scholar]
- [74].Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- [75].Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]