Abstract
Alcoholism has a substantial heritability yet the detection of specific genetic influences has largely proved elusive. The strongest findings are with genes encoding alcohol metabolizing enzymes. A few candidate genes such as GABRA2 have shown robust associations with alcoholism. Moreover, it has become apparent that variants in stress-related genes such as CRHR1, may only confer risk in individuals exposed to trauma, particularly in early life. Over the past decade there have been tremendous advances in large scale SNP genotyping technologies allowing for genome-wide associations studies (GWAS). As a result, it is now recognized that genetic risk for alcoholism is likely to be due to common variants in very many genes, each of small effect, although rare variants with large effects might also play a role. This has resulted in a paradigm shift away from gene centric studies towards analyses of gene interactions and gene networks within biologically relevant pathways.
Keywords: Alcohol use disorders, ALDH2, ADH1B, GABRA2, GABRG1, AUTS2, SGIP1, 5-HTTLPR, HTR2B, HTR3B, HTR3A, CRHR1, MAOA, CHD13, Childhood trauma, Gene–environment interactions, Flushing response, Addictions array, GWAS, Exome sequencing, RNA-Seq, GSA, COGA, NESARC, Pharmacogenetics
INTRODUCTION
We published a comprehensive review of the genetics of alcoholism over a decade ago [1]. Since then, there have been significant advances in techniques available for mapping genes and as a result considerable changes in outlook have occurred. It is now generally accepted that genetic risk for alcoholism is likely to be due to common variants in numerous genes, each of small effect, however rare variants with large effects might also play a role. After years of family-based linkage studies and case-control candidate gene studies, attention has shifted to large scale genome-wide association studies (GWAS) for the detection of novel common variants (≥ 1%). Exome and whole genome sequencing studies for the detection of rare variants are beginning to emerge. Moreover, it is increasingly recognized that childhood trauma, particularly in the first few years of life, is a strong predictor for the development of later psychopathology, including alcoholism, and some of the genetic risk may only become apparent in the context of childhood trauma (i.e. gene x environment (G x E) interactions). However, it should be borne in mind that no matter how sophisticated genetic techniques might become, further advances in detecting genotype – phenotype associations are hampered by the fact that alcoholism is a heterogeneous phenotype. One way around this has been the use of intermediate phenotypes, including electrophysiological and imaging, that reflect mediating factors in behavior and are likely to be influenced by variation at fewer genes. Another approach is to refine the phenotype. In recent years there have been attempts at empirical classification of alcoholics into clinically relevant and potentially genetically distinct subgroups based on the large National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) [2] that will be discussed later. Finally, the diagnostic criteria for the alcoholism phenotype (now called alcohol use disorder (AUD)) have just been radically revised in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [3]. The aim of this review is to highlight some recent studies in human research that are of particular interest and not to provide exhaustive coverage of the literature.
ESSENTIAL FEATURES OF ALCOHOLISM
In most societies and throughout historical time, alcohol has been consumed to enhance well-being, social relationships and even health. However, a significant number of individuals eventually end up being unable to keep within safe limits of consumption and become addicted. The essential features of addiction are loss of control over consumption, obsessive thoughts about the drug and continuation of use despite knowledge of negative health and social consequences [4,5]. Therefore alcoholism has features of both impulsivity and compulsivity. Unhealthy drinking patterns frequently begin in adolescence [6] and early age of onset of alcohol use (< 15 years) predicts adult AUD [7,8]. By their early twenties, half of all alcoholics have developed their symptoms [9] however the transition from drinking onset to AUD varies with race/ethnicity [10].
PREVALENCE AND DIAGNOSTIC CRITERIA
Much has been learnt in recent years about the epidemiology of this disease across the USA from the NESARC, a study that was conducted by the National Institute on Alcohol Abuse and Alcoholism in a nationally representative sample of 43,093 adults in two waves, from 2001 to 2002 and 2004 to 2005 [2]. Studies using NESARC data have shown that the prevalence of lifetime DSM-IV [4] alcohol dependence and alcohol abuse (together called AUD) is 12.5% and 17.8% respectively [11]. AUD is twice as common in men as in women. However, the diagnostic criteria have recently been revised and it remains to be seen whether these changes will alter the proportions of individuals qualifying for a diagnosis. The DSM-5 [3] merges three of the four former alcohol abuse criteria (excluding alcohol-related legal problems that rarely occur in the community) with the former seven dependence criteria, adds craving as an additional criterion and creates one diagnosis: AUD. An individual experiencing at least 2 of these 11 criteria within 12 months qualifies for this diagnosis that is further coded by a severity scale.
GENETIC HETEROGENEITY IN ALCOHOLISM: AN EMPIRICAL CLASSIFICATION INTO HOMOGENEOUS SUBTYPES
There have been many attempts to classify alcoholics into more clinically homogeneous groups, for example in terms of age of onset, predisposing personalities, psychiatric comorbidity, severity of disease and withdrawal symptoms [1,12]. Etiological factors, including genetic influences, might be shared within each group. An empirical classification based on a factor analysis of NESARC data from nearly 1500 alcoholics identified five homogeneous subtypes: (a) young adult (32%), (b) functional (19%), (c) intermediate familial (19%), (d) young antisocial (21%) and (e) chronic severe (9%). These subtypes were distinguishable by family history of alcoholism, age of AUD onset, rates of antisocial personality disorder (ASPD), endorsement of specific DSM-IV AUD criteria, and comorbid mood, anxiety and substance use disorders [13]. The five subgroups had different clinical outcomes three years later [14]. This empirical classification of alcoholics in the general US population suggests that the groups might differ in genetic vulnerability to AUD which might have implications for pharmacogenetic approaches to treatment. Moreover, this empirical classification is relevant to the recruitment of alcoholics for research studies in order to reduce phenotype heterogeneity.
HERITABILITY
Analysis of data from a prospective New Zealand birth cohort of nearly 1000 individuals has shown that family history is a strong predictor of AUD and is associated with a more recurrent course and worse impairment [15]. It is well known that an important risk factor is having an alcoholic parent. It has now been firmly established, largely through numerous studies in thousands of twin pairs and some adoption studies, that the heritability (the genetic component of the variance) of all addictive substances lies between 40–70%; the heritability of alcoholism is around 50% [1,16]. Therefore genetic and environmental risk factors for the development of alcoholism are equally important. Interestingly, results from twin studies suggest a theoretical dissection of genetic risk in that there may be two pathways for genetic influence: an early onset pathway driven in part by genetic risk for externalizing disorders and a later, adult onset pathway driven by genetic risk factors that are specific for AUD [17,18].
THE GENETIC BASIS OF VULNERABILITY TO ALCOHOLISM
Genetic vulnerability to alcoholism may originate in personality traits (such as anxious, dysphoric temperament and impulsivity) that predispose to alcohol seeking behavior, differential response to the effects of alcohol or differential variation in the neurobiology underlying addiction and physiological response to stress. Although alcoholism is often comorbid with other psychiatric disorders the heritability is largely disease specific [1]. The exception is nicotine addiction with which there is a strong genetic correlation [1].
Genetic Influences on Alcohol Metabolism
The most robust finding for genetic influences on alcoholism remains with genes encoding ethanol metabolizing enzymes. These genetic variants have a high prevalence in East Asians and protect against the development of alcoholism.
Ethanol is metabolized largely in the liver by alcohol dehydrogenases (ADH) to the toxic acetaldehyde which is then converted to acetate by aldehyde dehydrogenases (ALDH), primarily by the mitochondrial enzyme ALDH2. The class I ADH enzymes encoded by the ADH1A, ADH1B and ADH1C genes contribute about 70% of the total ethanol oxidizing capacity, and the class II enzyme encoded by ADH4 contributes about 30% [19].
Approximately 45% of East Asians, particularly Japanese, are carriers of the ALDH2*2 allele (Glu504Lys, rs671) that encodes the inactive ALDH2 enzyme. After consumption of small quantities of alcohol by these individuals the toxin acetaldehyde rapidly accumulates, resulting in the very unpleasant flushing syndrome (facial flushing, tachycardia, sweating, headaches, nausea), colloquially called ‘Asian Glow’ or ‘Asian Blush’, that is protective against heavy drinking and therefore alcoholism [20]. The inactive ALDH2 variant is also associated with increased risk of esophageal cancer [21]. The ALDH2*2 allele is unique to East Asian populations.
The higher enzyme activity encoded by the polymorphisms ADH1B*2 (Arg48His, rs1229984), ADH1B*3 (Arg370Cys, rs2066702), and the ADH1C*1 haplotype (Arg272Ile350) enables more rapid conversion of ethanol to acetaldehyde, thereby also resulting in the flushing syndrome and also being protective against excessive alcohol consumption and alcoholism [22,23]. Carriers of both the ADH1B*2 and ALDH2*2 alleles have a particularly severe flushing response [22].
The ADH1B*2 allele, occurring at a frequency of 0.75 in East Asians, is uncommon in other populations with a frequency of ≤ 0.01 in Caucasians, African Americans and American Indians [24]. However, studies in large non-East Asian datasets of several thousand individuals have likewise demonstrated a strong protective effect of ADH1B*2 on alcoholism [25,26], and a study in 4500 largely Caucasian Australian twins found an association between ADH1B*2, flushing and alcohol consumption [27]. Other than in East Asians, the highest frequency for the protective ADH1B*2 allele is in Jewish populations, ranging from 0.20 to 0.31 [28–30]. Finally, the ADH1B*3 allele has been associated with a protective effect on risk for alcoholism in African-Americans and American Indians [31,32].
The Neurobiology of Addiction: Dopamine Reward Circuitry and Interacting Stress Response Systems
The mesolimbic dopamine (DA) system is implicated in the development of all addictions and is also stimulated by stress [33]. This “reward” pathway originates in the ventral tegmental area of the midbrain and projects to the nucleus accumbens, the limbic system and the orbitofrontal cortex. The amygdala, hippocampus and medial prefrontal cortex send excitatory projections to the nucleus accumbens [34]. The feeling of euphoria experienced by humans subsequent to alcohol ingestion is associated with increased synaptic DA in the reward pathway that is entwined with complex changes in signal transduction pathways and numerous neurotransmitters including GABA, glutamate, serotonin (5-HT), opioid peptides and cannabinoids.
The transition to addiction involves multiple neuroadaptations and much of our understanding of these processes has so far been obtained from animal studies. However the use of microarrays and advances in next-generation RNA-sequencing (RNA-Seq) [35] have conferred the ability to quantify mRNA transcripts in postmortem brain and analyze expression differences between alcoholics and controls within gene networks [36–39].
Genetic variation in neurobiological pathways, including stress-response systems, may influence vulnerability to the development of permanent neurological changes in response to heavy alcohol use. Likewise, genetic variation may determine increased vulnerability to relapse in response to stressors.
STRATEGIES FOR IDENTIFYING GENETIC ASSOCIATIONS WITH ALCOHOLISM
Linkage Studies
Over the past few years numerous whole genome linkage studies have been performed in which the inheritance of phenotypes and genetic markers is followed in families [12,40]. Two influential linkage scans, one in a Southwestern American Indian tribe, a population isolate [41], and the other in the large, predominantly Caucasian Collaborative Study on the Genetics of Alcoholism (COGA) dataset [42] found evidence for linkage of AUD near the chromosome 4 GABAA receptor subunit gene cluster. A subsequent COGA scan found strong linkage of resting EEG beta power, an intermediate phenotype for alcoholism, to the same chromosome 4 region [43]. This finding led to the discovery of the association of GABRA2 with AUD, a robust, widely replicated finding that will be discussed below.
The ‘Educated Guess’ Approach – Candidate Genes on Arrays
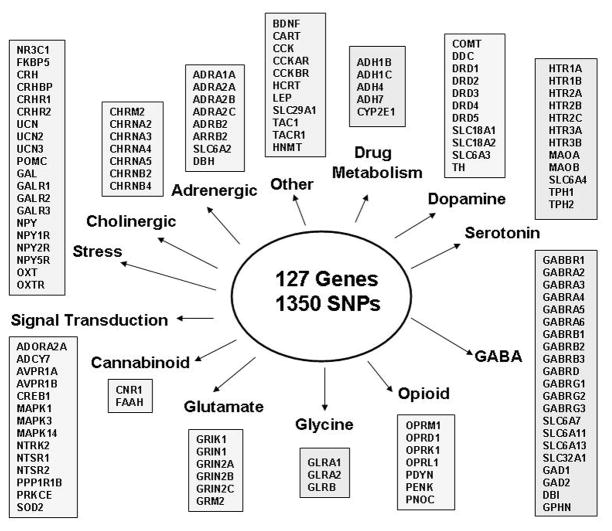
Because of the limitations of past technology, earlier studies were only able to analyze a few variants in a few candidate genes. With recent advances in genotyping technologies several laboratories have developed panels of markers in addiction related genes for mass genotyping together with ancestry informative markers (AIMS) to allow for the detection and correction of population stratification [44]. For example, Hodgkinson and colleagues [45] developed an Illumina GoldenGate ‘addiction array’ of 1465 haplotype tagging single nucleotide polymorphisms (SNPs) within 130 genes together with 186 AIMS. We have subsequently developed a stress-addictions array (Figure 1), largely based on neurobiological predictions. Many of the genes on this array have been associated with alcohol-related phenotypes in case-control studies but few results have been replicated [1,12,40].
FIGURE 1.
Stress-Addictions Array
This is an illustration of an Illumina GoldenGate array that was custom designed to include 1350 haplotype tagging single nucleotide polymorphisms (SNPs) within 127 stress- and addictions-related genes. This array was designed for Caucasian and African ancestry, hence the limited number of alcohol metabolism genes. The array also includes 186 ancestry informative markers.
The most robust candidate gene finding to date is for GABRA2 that was initially identified by linkage scans [41–43]. The GABAA gene cluster on chromosome 4 includes GABRA2, GABRG1 and GABRB1 that together encode the α1β1γ1 receptor that is almost exclusively found in the reward pathway. The initial finding came from COGA. This study showed a strong association between GABRA2 SNPs and alcoholism and resting EEG beta power [46]. Since then numerous studies, nearly all in Caucasians, have replicated this association although there have also been negative findings [47]. These studies have all identified the same two GABRA2 haplotype blocks, at least within Caucasians, American Indians and Asians. The significant association signals have been with the two, similar frequency yin yang haplotypes within the distal haplotype block. Many of the SNPs in allelic identity are conserved across species indicating the likelihood of selective pressure for this GABRA2 region distal to intron 3 [47]. Two subsequent studies in the COGA dataset have shown that the original evidence for association with AUD derived only from individuals with comorbid illicit drug dependence [48] and in adolescents, GABRA2 is associated with childhood conduct disorder symptoms, known to be a predictor of later-onset AUD [49]. Furthermore, GABRA2 variation appears to influence the subjective response to alcohol [50] and an fMRI study using a monetary incentive delay task has shown that GABRA2 variation is probably associated with impulsivity through variation of insula activity responses [51].
As yet, no GABRA2 functional variant has been detected to explain the yin yang haplotype (or tag SNP) associations with alcoholism-related phenotypes. HapMap data and other studies [52] reveal moderate long distance linkage disequilibrium across GABRA2 and the closely adjacent gene GABRG1 raising the possibility that the functional locus is in GABRG1. The results of several studies suggest that there are likely to be independent, complex contributions to alcoholism vulnerability from both linked genes [52–54].
Gene-Gene Effects within Biological Pathways
Biologically relevant combinations of functional variants within gene networks are likely to have additive effects on risk for addiction. Needless to say, even with high frequency variants, very large sample sizes are required to detect differences between carriers of different genotype combinations. Recent examples come from SLC6A4, the gene encoding the serotonin transporter, and HTR3A / HTR3B, the genes that encode the 5-HT3 receptor which mediates fast excitatory 5-HT transmission. We recently demonstrated additive effects between a functional SLC6A4 promoter polymorphism 5-HTTLPR and a common functional HTR3B coding polymorphism on risk for alcohol and drug dependence [55]. The explanation for these additive effects on addiction might be that the resulting increased synaptic 5-HT coupled with increased 5-HT3 receptor responsiveness might lead to enhanced DA transmission in the reward pathway. Likewise, another group was able to identify genotype combinations across SLC6A4, HTR3A and HTR3B that increased risk for AUD [56] and predicted the response of alcoholics to the 5-HT3 antagonist ondansetron [57]. Although exploratory at this stage and requiring very large sample sizes for validation, these kinds of studies could result in pharmacogenetic therapeutics [57]. At the present time, the only validated finding for pharmacogenetic underpinnings for response to therapeutics is for a functional polymorphism in the μ opioid receptor gene OPRM1, A118G (Asn40Asp) rs1799971 [58]. There have been mixed results from the numerous studies that have been performed, however a meta-analysis has shown that G allele carriers have lower relapse rates than AA homozygotes [59].
Gene x Environment Interactive Effects on Risk for Alcoholism
The experience of maltreatment and cumulative stressful life events prior to puberty and particularly in the first few years of life is associated with early onset of problem drinking in adolescence and alcohol and drug dependence in early adulthood [60,61]. The risk resilience balance for addiction may in part be due to the interaction between genetic variation and environment stressors (G x E) and this has been confirmed by twin studies of inferred genetic risk [62]. G x E interactive effects are likely for stress and anxiety related genes including HPA axis genes, GABAergic pathway genes and genes with a glucocorticoid response element located within their regulatory region such as MAOA, COMT and SLC6A4. Measured genotype studies to detect G x E effects have used a range of alcohol consumption and diagnostic phenotypes and stressors ranging from early life to adulthood past year life events [63]. To date, three studies have reported G x E effects on risk for AUD: CRHR1 variation and childhood sexual abuse in Australian participants [64]; CRHR1 variation and adulthood traumatic stress exposure in U.S. Caucasians [65] and MAOA variation and childhood sexual abuse in Southwestern American Indian women [66].
Genome-wide Association Studies (GWAS)
GWAS have no a priori hypothesis but ask whether a phenotype might be associated with any of a very large number of common SNPs distributed across the genome. The earliest GWAS chips included several hundreds of thousands of SNPs but more recent chips include up to 5 million. However, use of such large numbers of SNPs requires stringent corrections for false positive results with the current level set at around p values < 5×10−8. This has been a real challenge for GWAS of complex traits since few SNPs reach that degree of significance as can be seen from a catalogue prepared by the National Human Genome Research Institute (NHGRI). The results of all SNP-trait associations with p values < 1.0 × 10−5 in all published GWA studies (currently at 1659 publications, 10986 SNPs) have been catalogued by NHGRI and are freely available [67].
The strongest and most consistent findings for GWAS for AUD are for alcohol metabolizing genes, as in a recent study in an East Asian (Korean) sample of alcoholics in which ALDH2 and ADH1B showed up as GWAS signals with genome-wide significance [68]. One of the few other GWAS with a significant result was a meta-analysis of an alcohol consumption phenotype in 26,316 individuals from 12 European ancestry population based samples with replication genotyping in another 21,185 individuals that found a genome-wide significant result for one SNP in the autism susceptibility candidate gene 2 (AUTS2) [69]. Subsequent analysis showed that AUTS2 was implicated in alcohol consumption in mice and alcohol sensitivity in drosophila [69]. This gene plays a role in neurodevelopment, at least in zebrafish and mice [70].
Other major findings from GWAS for AUD, alcohol-related phenotypes and comorbid diseases are listed and discussed in Rietschel and Treutlein [12]. A number of new chromosomal regions and candidate genes have been identified, however other than for the alcohol metabolizing genes there are few if any commonalities across studies. The initial expectation was that robust candidate gene associations with alcoholism, for example the widely replicated GABRA2 finding would be replicated in GWAS but this has not been the case. In a GWAS of nearly 2000 alcoholics and 2000 controls, five GABRA2 SNPs had only nominal association (p < 0.05) with AUD, with odds ratios of 1.1 – 1.2 [71]. Other commonly reported candidate loci for AUD (e.g. in DRD2, COMT, ADH1C, OPRM1) have not been replicated in GWAS of AUD [72]. It should also be noted that genetic variants that confer risk only in trauma-exposed individuals (G x E interactions) will not be picked up by GWAS.
One of the earlier GWAS of AUD illustrates the kinds of manipulations that are being undertaken in the absence of genome-wide significant results to make sense of the wealth of data. Treutlein and colleagues [73] performed GWAS in 487 male inpatient alcoholics and 1358 controls and identified 121 SNPs with p values < 10−4. These SNPs were genotyped in a follow-up sample of 1024 male inpatient alcoholics and 996 controls. A total of 15 SNPs showed significant association with the same allele as in the GWAS; nine of these were located in genes including CDH13 and ADH1C, previously associated with AUD. In the combined analysis, two closely linked intergenic SNPs met criteria for genome-wide significance. Using max drinks as an excessive consumption phenotype, Kapoor and colleagues [74] performed one GWAS in over 2300 individuals from the COGA dataset and another GWAS in 2600 individuals derived from the Study of Addiction: Genes and Environment (SAGE) dataset. Neither study had genome-wide significant results but the investigators noted that among the top SNPs in each dataset, far more showed the same direction of effect in the other dataset than would be expected by chance. Other investigators have looked for commonalities of sub-threshold SNPs across several GWAS. It is interesting that several psychiatric disorder GWAS have identified genes encoding cadherins that mediate cell-cell adhesion, are involved in intracellular signaling pathways and may alter functional connectivity [75]. For example, CDH13 has shown up at sub-threshold levels in several studies of addiction including alcohol, nicotine and methamphetamine dependence [73,75,76].
Innovative statistical approaches are being pioneered to make biological sense out of GWAS data. Rather than analyzing each individual SNP, gene-set analysis (GSA) methods have been proposed that evaluate global evidence of association with a set of related genes enabling the identification of cellular or molecular pathways or biological pathways that are implicated in alcoholism, for example by using all 200 pathways listed in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [77]. Another approach that has been proposed is to use stratified False Discovery Rate methods to uncover new loci likely to replicate in independent samples. One recent study has demonstrated enrichment of polygenic effects, particularly for SNPs tagging regulatory and coding genic elements [78]. For example, a study in 33,332 patients and 27,888 controls used a combination of polygenic risk score analyses and pathway analyses to support a role for calcium channel signaling genes across five psychiatric disorders [79].
As discussed above, few robust associations between specific gene variants and complex behaviors have been identified. It may well be that very large sample sizes on the order of hundreds of thousands are required to provide sufficient statistical power. Even then, the gains may not be that great. A recent GWAS was able to identify three independent SNPs at genome-wide significance (p = 1 × 10−9) influencing educational attainment, a moderately heritable trait, in a discovery sample of 101,069 individuals and this finding was replicated in a sample of 25,490. However, the effect sizes for the SNPs were small (0.02%) and these three SNPs together accounted for only 2% of the variance in both educational attainment and cognitive function [80]. A GWAS in a community sample of Australian alcoholics also estimated that the effect size for SNPs was very small, each SNP contributing at most to 0.25% of the variance in AD risk [81].
Awareness of the need for large sample sizes for GWAS has resulted in the formation of large scale collaborations for sharing data, such as the Psychiatric Genomics Consortium [82]. However, one risk of this approach is to potentially increase phenotypic heterogeneity. Qualified investigators can access freely available GWAS datasets via the database of Genotypes and Phenotypes (dbGaP) [83] and several studies have used this resource for replication samples.
One way of bypassing the sample size issue is to study intermediate phenotypes in population isolates that are more genetically and environmentally homogeneous. The first published GWAS on resting EEG power, an intermediate phenotype for alcoholism, was performed in a sample of 322 Plains American Indians [76]. Although the diagnostic phenotype of alcoholism did not generate genome-wide significant statistical signals, the EEG GWAS identified three genes, one of which (SGIP1) was associated with alcoholism, an effect that might be mediated via the same brain mechanisms accessed by EEG power. Convergence of findings at the sub-threshold level with previous findings in genetic studies of addictions suggested that the intermediate phenotype approach can potentially identify genes that have a general effect on addiction even in datasets of modest size, notably of population isolates [76].
Direct Sequencing of Rare Variants
Some researchers have hypothesized that there may be large panels of rare functional variants, each of large effect, that predict risk for alcoholism with different variants occurring in different people. It is becoming increasingly easy, and the costs are rapidly decreasing, to detect rare variants using next-generation sequencing. Sequencing is rapidly becoming the key tool for characterization of the genetic basis of human diseases [84]. Clearly very large sample sizes are required to detect large panels of rare variants and there are significant bioinformatic requirements to deal with vast quantities of data. One such successful study performed exon-focused sequencing of impulsive individuals derived from a Finnish population isolate and identified a stop codon in HTR2B (1% frequency) that was unique to Finns. The stop codon carriers performed violently impulsive acts, but only whilst intoxicated with alcohol [85].
CONCLUSIONS
Alcoholism is known to be moderately heritable yet the search for genetic vulnerability factors has proven to be more difficult than originally thought and to date only a small proportion of the genetic variance has been accounted for. Over the past decade there have been tremendous advances in large scale SNP genotyping technologies and next generation sequencing and these technologies, including GWAS arrays and whole genome sequencing, are now widely available. Results of GWAS suggest that numerous common variants with very small effect and potentially rare variants with large effects are likely to encode proteins within, or regulate, numerous biological pathways. The current hope is that with very large sample sizes, GWAS will provide novel information about genetic underpinnings of alcoholism, including gene pathways that are altered in disease.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Conflict of Interest
Mary-Anne Enoch declares that she has no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
References
Recently published papers of particular interest have been highlighted as”
*Of importance
**Of major importance
- 1.Enoch M-A, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3:144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed August 2013];National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Available at ( http://pubs.niaaa.nih.gov/publications/arh29-2/74-78.htm)
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: 1994. [Google Scholar]
- 5.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 6.Donovan JE, Molina BS. Types of Alcohol Use Experience From Childhood Through Adolescence. J Adolesc Health. 2013 May 15; doi: 10.1016/j.jadohealth.2013.03.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund MM, Egeland B, Oliva EM, Collins WA. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103 (Suppl 1):23–35. doi: 10.1111/j.1360-0443.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse 2001. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 10.Alvanzo AA, Storr CL, La Flair L, et al. Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend. 2011;118(2–3):375–82. doi: 10.1016/j.drugalcdep.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 12.Rietschel M, Treutlein J. The genetics of alcohol dependence. Ann N Y Acad Sci. 2013;1282:39–70. doi: 10.1111/j.1749-6632.2012.06794.x. [DOI] [PubMed] [Google Scholar]
- 13.Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91(2–3):149–58. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss HB, Chen CM, Yi HY. Prospective follow-up of empirically derived Alcohol Dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol And Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior, health status, and treatment seeking. Alcohol Clin Exp Res. 2010;34(6):1073–83. doi: 10.1111/j.1530-0277.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 15.Milne BJ, Caspi A, Harrington H, et al. Predictive value of family history on severity of illness: the case for depression, anxiety, alcohol dependence, and drug dependence. Arch Gen Psychiatry. 2009;66:738–47. doi: 10.1001/archgenpsychiatry.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol Med. 2011;41:1507–16. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley TD, Edenberg HJ. Genes encoding enzymes involved in ethanol metabolism. Alcohol Res. 2012;34:339–44. [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi S, Matsushita S, Masaki T, et al. Influence of genetic variations of ethanol metabolizing enzymes on phenotypes of alcohol-related disorders. Ann N Y Acad Sci. 2004;1025:472–80. doi: 10.1196/annals.1316.058. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PJ, Enoch M-A, Goldman D, et al. The Alcohol Flushing Response: An Unrecognized Risk Factor for Esophageal Cancer from Alcohol Consumption. PLoS Med. 2009 Mar 24;6(3):e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CC, Lu RB, Chen YC, et al. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Zhifeng Zhou, Hodgkinson CA, et al. Haplotype-Based Study of the Association of Alcohol Metabolizing Genes with Alcohol Dependence in Four Ethnically Diverse Populations. Alcohol Clin Exp Res. 2011;35:304–316. doi: 10.1111/j.1530-0277.2010.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bierut LJ, Goate AM, Breslau N, et al. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17:445–50. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherva R, Rice JP, Neuman RJ, et al. Associations and interactions between SNPs in the alcohol metabolizing genes and alcoholism phenotypes in European Americans. Alcohol Clin Exp Res. 2009;33:848–57. doi: 10.1111/j.1530-0277.2009.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macgregor S, Lind PA, Bucholz KK, et al. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Hum Mol Genet. 2009;18:580–93. doi: 10.1093/hmg/ddn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr LG, Foroud T, Stewart T, et al. Influence of ADH1B polymorphism on alcohol use and its subjective effects in a Jewish population. Am J Med Genet. 2002;112:138–43. doi: 10.1002/ajmg.10674. [DOI] [PubMed] [Google Scholar]
- 29.Neumark YD, Friedlander Y, Durst R, et al. Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res. 2004;28:10–14. doi: 10.1097/01.ALC.0000108667.79219.4D. [DOI] [PubMed] [Google Scholar]
- 30.Shea SH, Wall TL, Carr LG, Li TK. ADH2 and alcohol-related phenotypes in Ashkenazic Jewish American college students. Behav Genet. 2001;31:231–9. doi: 10.1023/a:1010261713092. [DOI] [PubMed] [Google Scholar]
- 31.Edenberg HJ, Xuei X, Chen HJ, et al. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006;15:1539–49. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- 32.Wall TL, Carr LG, Ehlers CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am J Psychiatry. 2003;160:41–6. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- 33.Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–83. [PMC free article] [PubMed] [Google Scholar]
- 34.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hitzemann R, Bottomly D, Darakjian P, et al. Genes, behavior and next-generation RNA sequencing. Genes Brain Behav. 2013;12(1):1–12. doi: 10.1111/gbb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flatscher-Bader T, Harrison E, Matsumoto I, Wilce PA. Genes associated with alcohol abuse and tobacco smoking in the human nucleus accumbens and ventral tegmental area. Alcohol Clin Exp Res. 2010;34(7):1291–302. doi: 10.1111/j.1530-0277.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Lewohl JM, Harris RA, et al. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin Exp Res. 2007;31(9):1460–6. doi: 10.1111/j.1530-0277.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z, Yuan Q, Mash DC, Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc Natl Acad Sci U S A. 2011;108(16):6626–31. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farris SP, Miles MF. Ethanol modulation of gene networks: implications for alcoholism. Neurobiol Dis. 2012;45(1):115–21. doi: 10.1016/j.nbd.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126(1):91–9. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81(3):216–21. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Reich T, Edenberg HJ, Goate A, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81(3):207–15. [PubMed] [Google Scholar]
- 43.Porjesz B, Almasy L, Edenberg HJ, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99(6):3729–33. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducci F, Roy A, Shen PH, et al. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166(9):1031–40. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgkinson CA, Yuan Q, Xu K, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–15. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enoch M-A. The role of GABAA Receptors in the Development of Alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal A, Edenberg HJ, Foroud T, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36(5):640–50. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 49.Dick DM, Bierut L, Hinrichs A, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 50.Roh S, Matsushita S, Hara S, et al. Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res. 2011;35(3):400–7. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villafuerte S, Heitzeg MM, Foley S, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17(5):511–9. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enoch M-A, Hodgkinson CA, Yuan Q, et al. GABRG1 and GABRA2 haplotypes as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–54. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Covault J, Gelernter J, Jensen K, et al. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33(4):837–48. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ittiwut C, Yang BZ, Kranzler HR, et al. GABRG1 and GABRA2 variation associated with alcohol dependence in African Americans. Alcohol Clin Exp Res. 2012;36(4):588–93. doi: 10.1111/j.1530-0277.2011.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enoch M-A, Gorodetsky E, Hodgkinson CA, et al. Functional Genetic Variants that Increase Synaptic Serotonin and 5-HT3 Receptor Sensitivity Predict Alcohol and Drug Dependence. Mol Psychiatry. 2011;16:1139–46. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seneviratne C, Franklin J, Beckett K, et al. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum Genet. 2013 Jun 12; doi: 10.1007/s00439-013-1319-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Johnson BA, Seneviratne C, Wang XQ, et al. Determination of Genotype Combinations That Can Predict the Outcome of the Treatment of Alcohol Dependence Using the 5-HT3 Antagonist Ondansetron. Am J Psychiatry. 2013 Jul 30; doi: 10.1176/appi.ajp.2013.12091163. Epub ahead of print. Although the results are preliminary, this is one of the first studies to show the way forward for developing individualized treatment for alcoholism, based on genotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorsell A. The μ-opioid receptor and treatment response to naltrexone. Alcohol Alcohol. 2013;48(4):402–8. doi: 10.1093/alcalc/agt030. [DOI] [PubMed] [Google Scholar]
- 59.Chamorro AJ, Marcos M, Mirón-Canelo JA, et al. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17(3):505–12. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- 60.Enoch M-A. The Role of Early Life Stress as a Predictor for Alcohol and Drug Dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenton MC, Geier T, Keyes K, et al. Combined role of childhood maltreatment, family history, and gender in the risk for alcohol dependence. Psychol Med. 2013;43(5):1045–57. doi: 10.1017/S0033291712001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young-Wolff KC, Enoch M-A, Prescott CA. The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clin Psychol Rev. 2011;31:800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Enoch M-A. The Influence of Gene x Environment Interactions on the Development of Alcoholism and Drug Dependence. Curr Psychiatry Rep. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. A comprehensive review of an emerging field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson EC, Agrawal A, Pergadia ML, et al. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15(1):1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ray LA, Sehl M, Bujarski S, et al. The CRHR1 gene, trauma exposure, and alcoholism risk: a test of G × E effects. Genes Brain Behav. 2013;12(4):361–9. doi: 10.1111/gbb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ducci F, Enoch M-A, Hodgkinson C, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–7. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 67*. [Accessed August 2013];National Human Genome Research Institute (NHGRI) catalog of GWA studies. Available at: http://www.genome.gov/gwastudies/ A very useful website, continually updated, to access the results of all published GWAS.
- 68.Park BL, Kim JW, Cheong HS, et al. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet. 2013;132(6):657–68. doi: 10.1007/s00439-013-1281-8. [DOI] [PubMed] [Google Scholar]
- 69**.Schumann G, Coin LJ, Lourdusamy A, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A. 2011;108(17):7119–24. doi: 10.1073/pnas.1017288108. This GWAS illustrates the value of collaborative studies resulting in large datasets that have sufficient power to identify a SNP with genome-wide significance. It also demonstrates the importance of using animal models to identify the biological relevance of novel genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oksenberg N, Stevison L, Wall JD, Ahituv N. Function and regulation of AUTS2, a gene implicated in autism and human evolution. PLoS Genet. 2013;9(1):e1003221. doi: 10.1371/journal.pgen.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bierut LJ, Agrawal A, Bucholz KK, et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107(11):5082–7. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olfson E, Bierut LJ. Convergence of genome-wide association and candidate gene studies for alcoholism. Alcohol Clin Exp Res. 2012;36(12):2086–94. doi: 10.1111/j.1530-0277.2012.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Treutlein J, Cichon S, Ridinger M, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66(7):773–84. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kapoor M, Wang JC, Wetherill L, et al. A meta-analysis of two genome-wide association studies to identify novel loci for maximum number of alcoholic drinks. Hum Genet. 2013 Jun 7; doi: 10.1007/s00439-013-1318-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redies C, Hertel N, Hübner CA. Cadherins and neuropsychiatric disorders. Brain Res. 2012;1470:130–44. doi: 10.1016/j.brainres.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 76.Hodgkinson CA, Enoch M-A, Srivastava V, et al. Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proc Natl Acad Sci U S A. 2010;107:8695–8700. doi: 10.1073/pnas.0908134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biernacka JM, Geske J, Jenkins GD, et al. Genome-wide gene-set analysis for identification of pathways associated with alcohol dependence. Int J Neuropsychopharmacol. 2013;16(2):271–8. doi: 10.1017/S1461145712000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schork AJ, Thompson WK, Pham P, et al. All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013;9(4):e1003449. doi: 10.1371/journal.pgen.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cross-Disorder Group of the Psychiatric Genomics Consortium. Smoller JW, Craddock N, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rietveld CA, Medland SE, Derringer J, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340(6139):1467–71. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heath AC, Whitfield JB, Martin NG, et al. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. 2011;70(6):513–8. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Psychiatric GWAS Consortium. A framework for interpreting genomewide association studies of psychiatric disorders. Mol Psychiatry. 2009;14:10–17. doi: 10.1038/mp.2008.126. [DOI] [PubMed] [Google Scholar]
- 83. [Accessed August 2013];The database of Genotypes and Phenotypes (dbGaP) Available at http://www.ncbi.nlm.nih.gov/gap.
- 84.Goldstein DB, Allen A, Keebler J, et al. Sequencing studies in human genetics: design and interpretation. Nat Rev Genet. 2013;14(7):460–70. doi: 10.1038/nrg3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85**.Bevilacqua L, Doly S, Kaprio J, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–6. doi: 10.1038/nature09629. One of the first sequencing studies to successfully identify and trace the effects of a novel functional allele in complex behavioral phenotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]