Summary
Despite current treatment regimens, heart failure still remains one of the leading causes of morbidity and mortality in the world due to failure to adequately replace lost ventricular myocardium from ischemia-induced infarct. Although adult mammalian ventricular cardiomyocytes have a limited capacity to divide, this proliferation is insufficient to overcome the significant loss of myocardium from ventricular injury. However, lower vertebrates, such as the zebrafish and newt, have the remarkable capacity to fully regenerate their hearts after severe injury. Thus, there is great interest in studying these animal model systems to discover new regenerative approaches that might be applied to injured mammalian hearts. To this end, the zebrafish has been utilized more recently to gain additional mechanistic insight into cardiac regeneration because of its genetic tractability. Here, we describe two cardiac injury methods, a mechanical and a genetic injury model, for studying cardiac regeneration in the zebrafish.
Keywords: Heart regeneration, zebrafish, resection, genetic ablation, ischemia, nitroreductase
1. Introduction
Despite current treatment regimens, heart failure still remains one of the leading causes of morbidity and mortality in the world due to failure to adequately replace lost ventricular myocardium from ischemia-induced infarct. Although adult mammalian ventricular cardiomyocytes have a limited capacity to divide, this proliferation is insufficient to overcome the significant loss of myocardium from ventricular injury (1–3). As a result, the damaged human heart generates fibrous scar tissue and pathologically remodels in an attempt to unsuccessfully compensate for reduced cardiac function and altered cardiac architecture, subsequently leading to heart failure. Thus, developing innovative approaches to produce new healthy cardiomyocytes in order to replace damaged heart tissue may provide novel regenerative cellular therapies for treating heart failure.
Because of their robust capacity to regenerate a wide variety of tissues, including limb, brain, spinal cord, and retina, lower vertebrates, such as amphibians and fish, have been classically used to study organ regeneration (4). Many of these regeneration studies were initially performed in newts and Xenopus (5–12); however because of its genetic tractability, the zebrafish has been utilized more recently to gain additional mechanistic insight into vertebrate organ regeneration (13–19). As a consequence, researchers have taken advantage of these lower vertebrate models to further investigate cardiac regeneration with the hopes of translating these findings to the regeneration of mammalian hearts. Towards this end, similar cardiac injury and regeneration models used in lower vertebrate systems have recently been employed in mouse cardiac models (20). These studies reveal that early postnatal mouse hearts, like zebrafish hearts, possess the ability to regenerate from the proliferation of existing cardiomyocytes after ventricular apex resection. However, unlike zebrafish cardiac regeneration, this mouse cardiac regenerative potential appears to significantly diminish seven days after birth. Consequently, there is intense interest to investigate cardiac regeneration in these different animal models systems in order to identify new regenerative cellular strategies for repair of damaged adult human hearts.
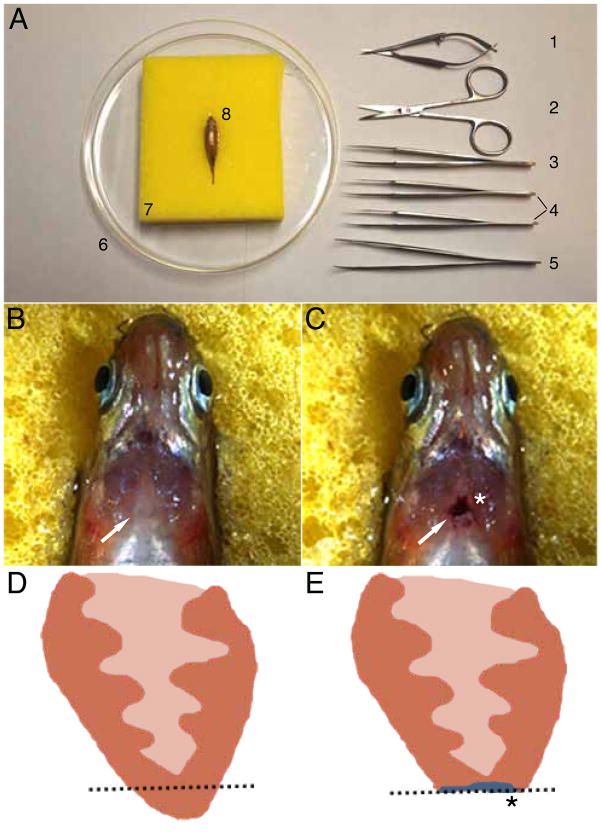
Various cardiac injury techniques have been utilized to study the process of cardiac regeneration in multiple animal models. The most common and well-established method has been the mechanical resection procedure (8, 13). In this cardiac injury model, an incision is created in the chest/ventral surface of the animal to expose the heart for mechanical ventricular apical transection with a pair of scissors (Figure 1) . Using this technique, several groups have reproducibly observed in zebrafish and newts that after resection of 15–20% of the ventricular apex, adult and juvenile hearts are able to generate sufficient new myocardium within several weeks to fully replace the transected apex (13, 14, 17, 18, 21). Because of its reliability, this cardiac injury model has been recently used in the mouse to investigate the cardiac regenerative potential in mammalian hearts (20). Moreover, in order to simulate myocardial infarction-like cardiac injuries, researchers have also used cryocauterization in both mouse and zebrafish hearts to study cardiac regeneration (19, 22). This technique involves contacting the cardiac ventricle with a copper filament cooled in liquid nitrogen, thereby causing necrotic death of the surrounding cells. Similar to myocardial infarctions, this injury results in fibrotic scar tissue formation, which is eventually resorbed to allow for integration of newly generated cardiomyocytes to the damaged myocardium. Although these invasive techniques can create a robust and fairly reproducible injury to the heart, they are unfortunately technically cumbersome, are difficult to employ in a high-throughput manner, structurally destroy the integrity of the overall cardiac architecture, remove other cardiac cell types beyond cardiomyocytes, such as endothelial, endocardial, and epicardial cells, and can potentially damage other organs and tissues besides the heart when exposing the heart for ventricular apical transection (i.e. skin, skeletal muscle, and bone).
Figure 1. Surgical transection of the zebrafish ventricular apex.
Six month old adult zebrafish is mounted in sponge ventral side up for incision of chest wall and transection of cardiac ventricle apex. (A) Materials required to surgically transect the ventricular apex include: (1) Vannas scissors, (2) Iris scissors, (3) #5 Dumont tweezers, (4) a pair of #55 Dumont tweezers, (5) WPI inc. Economy tweezers #7, (6) petri dish, and (7) sponge with slit cut into the top in order to hold (8) anesthetized zebrafish. Anesthetized six month old adult zebrafish mounted ventral side up (B) prior to surgical resection and (C) after resection. Cartoon schematic of a section of the ventricle (D) before and (E) after resection. Dashed line marks the plane of resection. White arrows point to the heart. Asterisks mark scarring after resection. Note blood clot that forms immediately after resection in C. Head is to the top and the tail is to the bottomin panels A–C.
To circumvent these mechanical cardiac injury issues, researchers have recently developed genetic ablation methods to noninvasively create organ/tissue-specific injuries, including cardiomyocyte specific ablations (23–32). For example, using a double transgenic system in zebrafish, which includes the Tg(cmlc2:CreER) line – cmlc2 cardiac specific promoter driving tamoxifen inducible CreER, and Tg(bactin2:loxp-mCherry-STOP-loxp-DTA) line– a ubiquitously expressing diphtheria toxin A chain (DTA) switch line, cardiac-specific expression of DTA, which blocks protein synthesis, can be induced with tamoxifen treatment in order to ablate cardiomyocytes (23). As a result, high dose tamoxifen injections into six month old zebrafish harboring both transgenes can specifically and reproducibly destroy over 60–80% of the ventricular myocardium, causing heart failure and in some cases death. In contrast to the ventricular apical transection model where these hearts cannot fully regenerate after more than a 20–25% cardiac resection (13), the DTA-genetically ablated ventricles were able to regenerate after ablation of over 60% of ventricular cardiomyocytes (23). Furthermore, these genetically ablated hearts also significantly regenerated faster than the mechanically resected hearts. These regenerative differences may be due to the mechanical resection model severely disrupting the cardiac architecture and removing other cardiac cell types, which may be necessary for more efficient myocardial regeneration (33).
Though the DTA-inducible genetic cardiac ablation shows great potential as a noninvasive cardiac injury model, this method can be technically and genetically difficult because intercrossing of two different transgenic lines is required to generate fish for this cardiac injury model. Thus, another promising genetic cell ablation model, which only requires one transgenic line and utilizes the bacterial nitroreductase (NTR) enzyme, has been used recently to specifically destroy tissues/organs in both mouse and zebrafish after treatment with the prodrug, metronidazole (MTZ) (30–32, 34). In this technique, tissue-specific NTR expression in vertebrate cells is innocuous; however, MTZ exposure to these NTR-expressing cells leads to the conversion of MTZ into a toxic compound, which then effectively ablates these cells through induction of apoptosis. Using the cmlc2, cardiac specific promoter, to drive expression of NTR in zebrafish hearts - Tg(cmlc2:NTR), cardiomyocytes can be reliably ablated after MTZ treatment (30). Moreover, to study the recovery and regeneration of the damaged heart, the MTZ can be effectively washed away to discontinue the ablations (35). Overall, these genetic ablation techniques are noninvasive, rapid, and easy to carry out, thus permitting researchers in the future to more easily execute high-throughput studies, such as chemical and forward genetic screens, of cardiac regeneration.
Overall, the surgical and genetic cardiac injury models are both useful and complementary tools to study different aspects of cardiac regeneration. Because of their genetic tractability (i.e. transgenics, mutants, morpholino knockdowns) and inherent regenerative capacity (36), the zebrafish has gained significant popularity for performing both cardiac injury models in order to dissect the cellular and molecular mechanisms of cardiac regeneration. In particular, zebrafish transgenic tools have been recently created to investigate critical signaling pathways that may be required for cardiac regeneration (14, 37) as well as to genetically fate map cells to identify the source of the cardiac regenerative tissue (17, 18). Thus, in this chapter, we describe protocols for performing both the surgical resection and the NTR-mediated genetic ablation cardiac injury models in the zebrafish heart. In the future, we anticipate that these protocols may be applicable in other animal models. Already, the mechanical cardiac injury model has been successfully used in mice, and recent mouse gene trap screens using NTR have been performed (31), which may yield cardiac specific NTR expressing lines that can be used for mouse NTR-mediated cardiac genetic ablation in the future.
2. Materials
2.1. Surgical resection of the zebrafish heart
Iris Scissors (Fine Science Tools 14060-09)
Vannas Scissors (World Precision Instruments 14003)
Dumont Tweezers #55 (World Precision Instruments 14099)
Dumont Tweezers #5 (World Precision Instruments 14098)
Economy tweezers #7 (World Precision Instruments 501981)
Petri Dish (BD Falcon 351058)
Double Sided Tape
Sponge
2.2. Transgenic fish lines
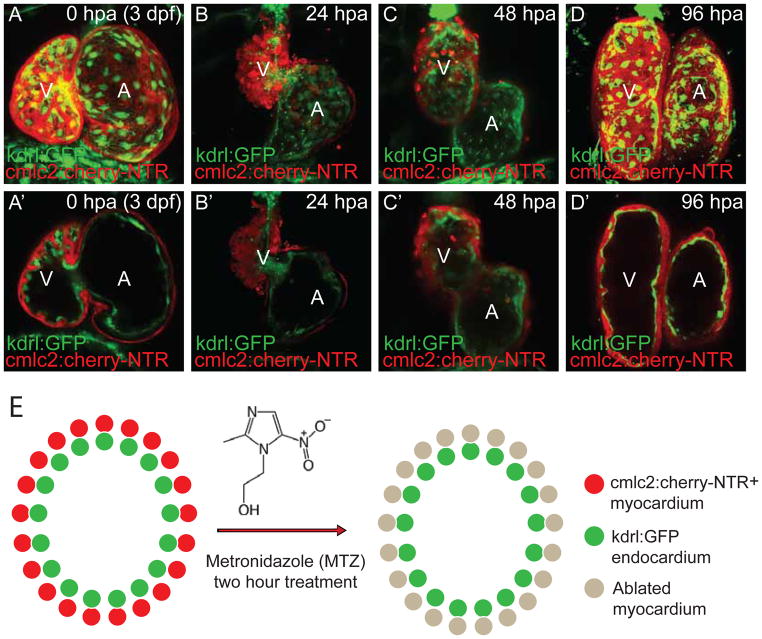
The Tg(cmlc2:mCherry-NTR) zebrafish line, which expresses the mCherry-nitroreductase fusion protein in the myocardium, was used to genetically ablate cardiomyocytes after MTZ treatment (Figure 2). In order to more easily observe the cardiac ablation, this line was crossed into the Tg(kdrl:GFP) reporter line (Figure 2), which expresses green fluorescent protein (GFP) in the endocardium and vasculature (38). During cardiac ablation, the cardiomyocytes are specifically destroyed and can be observed by the reduction of cherry red cardiomyocytes, whereas the endocardium marked in GFP remains intact (Figure 2, 3).
Figure 2. In vivo cardiac genetic ablation and regeneration model.
3 dpf (days post-fertilization) Tg(cmlc2:mCherry-NTR; kdrl:GFP) larvae are treated with 5mM metronidazole (MTZ) for two hours. Live confocal imaging of ablated and regenerating heart at (A, A’) 0 hpa (hour post-ablation); (B, B’) 24 hpa; (C, C’) 48 hpa; and (D, D’) 96 hpa. A, B, C, D are full confocal image projections of the hearts and A’, B’, C’, D’ are representative confocal optical section of the A, B, C, D heart. (E) Schematic representation of myocardial genetic ablation strategy. cmlc2:mcherry-NTR (red) and kdrl:GFP (green) are expressed in the myocardium and endocardium, respectively. By 24 hpa (B, B’), much of the myocardium (red) is specifically ablated, whereas the endocardium (green) remains intact. Regeneration of cardiomyocytes begins ~48 hpf (C, C’) and continues to 96 hpa (D, D’) when the heart has fully regenerated. A, atrium; V, ventricle.
Figure 3. Cell death in Tg(cmlc2:mCherry-NTR) ablated hearts.
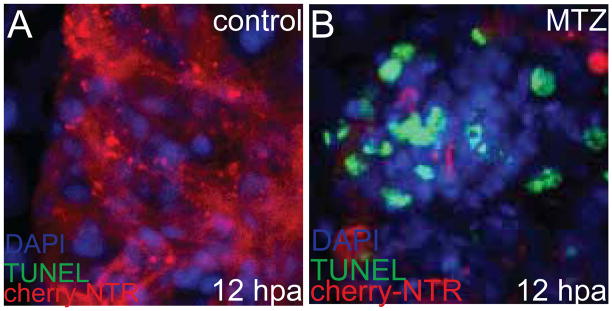
TUNEL staining (green) was performed in 3.5 dpf Tg(cmlc2:mCherry-NTR) larvae 12 hours after being treated with (A) DMSO or (B) 5mM metronidazole (MTZ) for two hours. TUNEL positive staining, which indicates apoptosis, has increased significantly in ablated cardiac ventricle (B) compared to DMSO-treated age-matched control (A, control). Red – cmlc2:mcherry-NTR; Green – TUNEL; Blue – DAPI/nuclei.
2.3. Reagents for cardiomyocyte ablation and cell death detection
Egg water for zebrafish embryos: 0.3 g Instant Ocean Sea Salt, 0.075 g CaSO4, 1 L distilled water.
20x Phenylthiourea (PTU) stock solution: 0.06% (w/v) 1-phenyl-2thiourea (Sigma P7629) dissolved in egg water.
Dimethyl sulfoxide (DMSO) control solution: 0.2% (v/v) DMSO (Sigma D8418) in egg water.
10 mM metronidazole (MTZ) solution: 1.712 g metronidazole (Sigma M1547), 2 mL DMSO, 1 L egg water (see Note 1).
-
10x Phosphate buffered saline (PBS) solution: 80 g NaCl, 2 g Kcl, 14.4 g
Na2HPO4, 2.4 g KH2PO4, 1 L distilled water, pH adjusted to 7.4 with 5 M NaOH.
Paraformaldehyde (PFA): 4% (w/v) paraformaldehyde (Sigma P6148) in 1x PBS.
PBST: 0.5% (v/v) Triton X-100 (Sigma T9284) in 1x PBS.
In situ cell death detection kit (Roche 11684795910).
2.4. Microscopy for regeneration analysis
20x Tricaine stock solution: 4 g Ethyl 3-aminobenzoate methanesulfate salt (Sigma A5040), 21 mL 1 M Tris-HCl (pH 9), 1 L distilled water, pH adjusted to 7.0 with 12 M HCl.
Low melting point agarose: 1% (w/v) SeaPlaque Agarose (Lonza 50101) dissolved in egg water. Microwave to dissolve, aliquot in 1.5 or 2 mL microcentrifuge tubes and keep at 37°C.
Glass bottom culture dish (MatTek P35G-0-14-C)
Stereomicroscope
Confocal microscope
3. Methods
3.1 Surgical resection of the zebrafish heart
Affix sponge to a petri dish using double-sided tape (Figure 1A).
Using a razor blade, cut a slot into the sponge big enough to hold an adult zebrafish (Figure 1A).
Anesthetize zebrafish (>3 months old) with tricaine solution and place ventral side up into the sponge (Figure 1A–C).
Determine location of the heart by observing motion under the skin.
Observing under a stereomicroscope, make an incision in the skin above the heart using iris scissors. This will expose the silver-colored pericardial sac.
Open the pericardial sac using #55 tweezers (Figure 1A3).
Using #5 tweezers (Figure 1A3), which are not too sharp, gently pull up the apex of the ventricle to expose it.
Using Vannas scissors (Figure 1A1), remove approximately 20% of the ventricular apex.
Once the wound has clotted and stopped bleeding, the fish can be returned to a tank (Figure 1C).
Use a plastic transfer pipette to gently push water across the gills of the fish until it is able to swim freely on its own (generally 3–5 minutes).
Allow the fish to recover for several days (full recovery is usually between 30–60 days). The chest incision normally heals without problems (within 24–48 hours, with more than 80% of resected fish surviving following a procedure.
3.2 MTZ –dependent ablation and cell death detection
Collect eggs from a pair mating of Tg(cmlc2:mCherry-NTR) and Tg(kdrl:GFP) fish. Incubate the eggs at 28°C in egg water until they reach the desired stage for ablation (Figure 2). To maintain optical clarity of the zebrafish embryo or larvae, add PTU after gastrulation to prevent pigmentation of the fish. Cardiac ablation can be performed as soon as cmlc2 is expressed (16–18 hpf). However, in order to investigate that cardiac recovery is due to cardiac regeneration rather than normal cardiac development, we have specifically ablated at 3–4 days post-fertilization (dpf), when cardiogenesis has completed and the proliferation rate of the cardiomyocytes has dramatically slowed (39).
Before ablation, sort the larvae into two groups: 1) cmlc2:mCherry-NTR+ siblings; +0.2% DMSO (negative control), and 2) cmlc2:mCherry-NTR+ siblings; +MTZ (experimental) (Figure 2). Use a minimum of 30 larvae per group in order to account for any variability.
Remove as much egg water as possible and add 5 mM MTZ solution or 0.2% DMSO control solution at 28°C. Incubate at 28°C in the dark for 4 hours (see Note 2).
Remove the solution at the end of the incubation. Wash 4–5 times with egg water and replace with fresh egg water + PTU. Check under the microscope over the next few hours for any indication of cell death, such as decreased levels of mCherry fluorescence, decreased ventricular function, or blood pooling in the heart (Figure 2B, B’ and C).
In addition to these indicators of myocardial dysfunction and cardiomyocyte ablation as outlined in point 4, TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) can be used to assess and confirm cell death (Figure 3). Thus, following cardiac ablation, larvae can be briefly fixed with 4% PFA solution, washed three times with 1x PBS, and permeabilized with PBST for 2 hours or longer at room temperature. Next, 50 μL of staining solution is added, and the larvae are incubated at 37°C for 1 hour. The samples are then examined under the microscope (see Note 3).
3.3 Post-ablation regeneration analysis
The optical clarity of the zebrafish embryo/larvae is particularly useful to monitor heart regeneration in vivo in zebrafish (Figure 2C, C’, D, D’) and is maintained in the zebrafish larvae by preventing the pigmentation of the zebrafish using PTU (see 3.2.1). In order to image the zebrafish cardiac regeneration in vivo, transfer fish to a 1.5 mL Eppendorf tube that contains 37°C pre-warmed liquid 1% low melting point agarose with 3–4 drops of tricaine. Transfer the anesthetize larvae with low melt agarose using a pipette onto a Mat-Tek glass bottom dish (see 2.4.3). Use a long flat Eppendorf gel loading pipette tip to position the larvae so that the entire heart can be observed. This must be done quickly before the agarose solidifies (see Note 4). Once the fish is positioned and agarose solidifies, the larvae can then be imaged under the microscope. Afterwards, the agarose can be broken apart with forceps to free the larvae for transfer back to egg water. Regeneration normally occurs by 3 to 4 days post-ablation.
Footnotes
Shake vigorously until MTZ is dissolved. The MTZ may be more difficult to completely dissolve in a higher stock solution concentration. MTZ may be toxic at high concentrations or after prolonged exposure, so use gloves when preparing MTZ solution.
The concentration and incubation times with MTZ solution should be experimentally determined based on the age of the fish and the degree of damage desired. For example, while two hour 5 mM MTZ treatments at 3 dpf is sufficient to cause substantial damage to the heart, it may require 6–8 hours at 5 dpf to achieve the same degree of damage.
For better penetration, open the pericardial sac with forceps before permeabilization or proteinase K digest followed by a 4% PFA re-fixation.
Do not transfer too many fish at the same time otherwise the agarose will solidify before all the fish are positioned. If working with an inverted microscope, position 3–4 dpf larvae with the heads down close to the glass bottom slide and tilt the tail up 45 degrees. For 5 dpf or later, rotate the body to the side as well.
For time-lapse imaging, use 0.5% low melt agarose and as little agarose as possible to allow for better diffusion of egg water to the fish. Fill the dish with egg water and change it every day to keep the fish alive.
References
- 1.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 4.Major RJ, Poss KD. Zebrafish Heart Regeneration as a Model for Cardiac Tissue Repair. Drug Discov Today Dis Models. 2007;4:219–225. doi: 10.1016/j.ddmod.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mochii M, Taniguchi Y, Shikata I. Tail regeneration in the Xenopus tadpole. Dev Growth Differ. 2007;49:155–161. doi: 10.1111/j.1440-169X.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin G, Chen Y, Slack JM. Regeneration of neural crest derivatives in the Xenopus tadpole tail. BMC Dev Biol. 2007;7:56. doi: 10.1186/1471-213X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberpriller JO, Oberpriller JC, Aafedt BC. Changes in binucleation and cellular dimensions of rat left atrial myocytes after induced left ventricular infarction. Am J Anat. 1987;179:285–290. doi: 10.1002/aja.1001790310. [DOI] [PubMed] [Google Scholar]
- 8.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 9.Flink IL. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat Embryol (Berl) 2002;205:235–244. doi: 10.1007/s00429-002-0249-6. [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Yoshino J, Kado K, Tochinai S. Brain regeneration in anuran amphibians. Dev Growth Differ. 2007;49:121–129. doi: 10.1111/j.1440-169X.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 11.Mescher AL, Neff AW. Limb regeneration in amphibians: immunological considerations. ScientificWorldJournal. 2006;6(Suppl 1):1–11. doi: 10.1100/tsw.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 13.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 14.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 15.Poss KD. Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol. 2007;18:36–45. doi: 10.1016/j.semcdb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating MT. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- 17.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 20.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lien CL, Schebesta M, Makino S, Weber GJ, Keating MT. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006;4:e260. doi: 10.1371/journal.pbio.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovasc Pathol. 2008;17:6–13. doi: 10.1016/j.carpath.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akazawa H, Komazaki S, Shimomura H, Terasaki F, Zou Y, Takano H, Nagai T, Komuro I. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem. 2004;279:41095–41103. doi: 10.1074/jbc.M313084200. [DOI] [PubMed] [Google Scholar]
- 25.Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 26.Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave KA, Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–7642. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee P, Morley G, Huang Q, Fischer A, Seiler S, Horner JW, Factor S, Vaidya D, Jalife J, Fishman GI. Conditional lineage ablation to model human diseases. Proc Natl Acad Sci U S A. 1998;95:11371–11376. doi: 10.1073/pnas.95.19.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 29.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 30.Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 31.Medico E, Gambarotta G, Gentile A, Comoglio PM, Soriano P. A gene trap vector system for identifying transcriptionally responsive genes. Nat Biotechnol. 2001;19:579–582. doi: 10.1038/89343. [DOI] [PubMed] [Google Scholar]
- 32.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PD, Shin D, Chi NC, Shin CH, Schlegel A, Halpern M, Stainier DY. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol. 2009;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beis D, Stainier DY. In vivo cell biology: following the zebrafish trend. Trends Cell Biol. 2006;16:105–112. doi: 10.1016/j.tcb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 38.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 39.de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]