Abstract
Tunneling nanotubes (TnTs) are long, non-adherent, actin-based cellular extensions that act as conduits for transport of cellular cargo between connected cells. The mechanisms of nanotube formation and the effects of the tumor microenvironment and cellular signals on TnT formation are unknown. In the present study, we explored exosomes as potential mediators of TnT formation in mesothelioma and the potential relationship of lipid rafts to TnT formation. Mesothelioma cells co-cultured with exogenous mesothelioma-derived exosomes formed more TnTs than cells cultured without exosomes within 24-48 hours; and this effect was most prominent in media conditions (low-serum, hyperglycemic medium) that support TnT formation (1.3-1.9-fold difference). Fluorescence and electron microscopy confirmed the purity of isolated exosomes and revealed that they localized predominantly at the base of and within TnTs, in addition to the extracellular environment. Time-lapse microscopic imaging demonstrated uptake of tumor exosomes by TnTs, which facilitated intercellular transfer of these exosomes between connected cells. Mesothelioma cells connected via TnTs were also significantly enriched for lipid rafts at nearly a 2-fold higher number compared with cells not connected by TnTs. Our findings provide supportive evidence of exosomes as potential chemotactic stimuli for TnT formation, and also lipid raft formation as a potential biomarker for TnT-forming cells.
Keywords: Tunneling nanotubes, exosomes, lipid rafts, intercellular transfer, intercellular communication, mesothelioma
Introduction
Tunneling nanotubes (TnTs) are thin, non-adherent, actin-based cytoplasmic extensions that are formed by a wide variety of cell types when cultured in vitro.[1-3] There is a growing interest in examining TnTs as a novel mode of intercellular communication and functional networking among many cell types, including cancer.[3, 4] TnTs have the ability to directly mediate cell-to-cell communication by serving as long-range conduits for intercellular transfer of proteins, mitochondria, and other subcellular organelles.[1] Much of the work to date on these unique structures has focused on non-cancer cell types including dendritic cells and monocytes,[5] macrophages,[6, 7] T cells,[8, 9] B cells,[10] neutrophils,[11] neurons,[12] kidney cells,[13] endothelial progenitor cells,[14] and mesothelial cells.[9] More recently, we have shown that TnTs connect cells derived from malignant pleural mesothelioma and lung adenocarcinoma, both in vitro[2, 4] and in tumors resected from human patients.[2, 3] The functions of TnTs and the mechanisms by which they are selectively controlled during cancer growth remain poorly understood.
Exosomes and lipid rafts represent biological entities that may selectively facilitate TnT formation and cooperate with TnT-mediated intercellular communication. As small membrane-derived nanovesicles measuring 30-100 nm in diameter, exosomes are under investigation for their role in intercellular communication and as potential targets for the treatment of cancer. [15, 16] Additionally, exosome signaling is postulated to be mediated, in part, by lipid rafts, which are cholesterol-based microdomains located at focal points along the plasma membrane. Accumulation of lipid rafts along membrane domains (sometimes also designated as supramolecular activation clusters, or SMACs) has been associated with contact and exchange of information across immunological synapses.[17-20] In B cells specifically, such lipid raftenriched microdomains have been associated with TnT-like structures called cytonemes.[19] Cholesterol depletion has been shown to diminish signaling normally associated with lipid raft formation.[21] Furthermore, cholesterol accumulation has been proposed to have a direct effect on TnTs and similar nanotube structures.[9, 22, 23] Most notably, exosomes and lipid rafts appear to facilitate adherence and invasion of malignant cells in vitro[16].
Here, we explored exosomes as potential mediators of TnT formation and the potential relationship of lipid rafts to TnTs in mesothelioma cell models. Our results indicate that the addition of exogenous exosomes to mesothelioma cells in culture stimulates a higher rate of TnT formation within 24-48 hours, and that this stimulation is highest in a low-serum, hyperglycemic, acidic culture environment. Electron microscopic imaging confirmed the purity of the isolated exosomes and also co-localization at the base of and within TnTs. Time-lapse microscopic imaging provided visual evidence that TnTs can facilitate intercellular transport of these exosomes, further supporting the concept that mutually beneficial interactions take place between exosomes and TnTs. Further analysis demonstrated that lipid rafts were enriched in TnT-forming cells as compared to cells without TnTs, suggesting their role in the formation and/or maintenance of these cellular extensions.
Materials and Methods (for additional details, see Supplementary Text)
Cell lines and culture medium
MSTO-211H cells were derived from a patient with biphasic mesothelioma (ATCC no. CRL-2081); VAMT is a sarcomatoid mesothelioma cell line; and H2052 is a mesothelioma cell line of epithelioid histology. Cell lines were kindly provided by Dr. Yuman Fong, Memorial Sloan-Kettering Cancer Center, and authenticated by the Core Fragment Analysis Facility at Johns Hopkins University using short tandem repeat profiling. Met5A (also known as MeT-5A) is an immortalized human mesothelial cell line derived from the pleural lining; and was purchased directly from the ATCC (CRL-9444). Cell lines were passaged using RPMI-1640 culture medium (Invitrogen, Carlsbad, CA) containing penicillin/streptomycin (Life Technologies, Carlsbad, CA) and plasmocin (Invivogen, San Diego, CA) antibiotics and tested negative for mycoplasma. All cells were passaged using 10% fetal bovine serum (FBS) in RPMI-1640 with 25 mM glucose, supplemented with 1% penicillin-streptomycin (P-S) and 2% L-glutamine, at normal pH (7.6). To stimulate nanotube formation, cells were grown in 2.5% FBS in RPMI-1640 containing 50 mM glucose, supplemented with 1% P-S, 2% L-glutamine with or without 10 mM ammonium lactate (Sigma Aldrich, St. Louis, Missouri) and acidification of medium to pH 6.6. All cultures were cultivated in 75cm2 tissue culture flasks (Falcon, Becton Dickson, Oxnard, CA) at 37°C in 5% CO2.
Isolation of exosomes
Exosomes were isolated from VAMT and Met5A cells using the Total Exosome Isolation Kit following the manufacturer's protocol (Invitrogen, Carlsbad, CA, Catl no. # 4478359). VAMT and Met5A cells were grown to confluence in T75 flasks using 5% FBS RPMI for 3 days. In order to minimize contamination and inclusion of endogenous exosomes from these cell lines, medium was removed on the third day of incubation and cells were washed x 3 with mTeSR1 basal (serum-free) medium (Stemcell Technologies, Vancouver, BC, Canada) and further grown in mTeSR1 basal medium for 2 days prior to exosome isolation. Briefly, supernatants from VAMT and Met5A cell cultures (18 ml each) were collected, and 9 ml was added to 15 ml Beckman centrifuge tubes (Cat no. #342082) and centrifuged at 2,000 x g for 30 minutes to remove cells and debris. Cell-free media were transferred (9 ml each) into fresh 15 ml centrifuge tubes, and 4.5 ml of exosome isolation reagent was added and mixed by inverting the tube x 3. Exosomes were precipitated by incubating the cell supernatant mixture at 4°C overnight and then collected the next morning by centrifugation at 10,000g for 1.0 hour, 4°C. Supernatant was carefully removed by aspiration. Exosome pellets were then resuspended in PBS, aliquoted in 100 μl fractions, and stored at -80°C until use. Purity of the preparations was assessed by SDS-PAGE, ELISA, and visual examination using electron microscopy as described below.
Addition of exogenous VAMT and Met5A exosomes to MSTO-211H mesothelioma cells and cell culture conditions
To determine the average numbers of nanotubes per cell (TnTs/cell) under various media conditions, with and without addition of exogenous VAMT- or Met5A-derived exosomes (5 × 106 exosomes added to a culture of 5×104 cells), MSTO-211H cells were first grown in 10% FBS RPMI-1640 medium for 72 hours. This medium was then removed 24 hours prior to experiments, and adherent cells in the flasks were washed x 3 with serum-free/vesicle-free mTeSR medium. To minimize exosome secretion by MSTO cells, fresh mTeSR medium was added for an additional 24 hours, and the next day flasks were placed at 4°C for four hours as previously reported [24]. Additionally, we elected to use exogenous exosomes derived from a different cell line (VAMT) to control for signaling from endogenous exosomes in culture. While several chemical compounds/agents have been shown to inhibit or suppress exosome secretion, we elected not to add this additional step due to unknown or non-specific effects of such compounds (such as amiloride, DMA, or inhibitors of Na+/H+ or Na+/Ca2+ transporters) on TnT formation and other cellular pathways [25, 26]. After four hours of incubation at 4°C, MSTO cells were trypsinized and co-cultured with or without exogenous VAMT or Met5A exosomes (5 × 105 exosomes added to 5 × 104 cells) in 6-well adherent tissue culture plates (Fisher Scientific, Pittsburgh, PA) under the following conditions: RPMI-1640 medium and 2.5% FBS at a normal pH of 7.6 with 25 mM or 50 mM glucose; RPMI-1640 medium with higher concentration of FBS (10%) with 25mM or 50 mM glucose at a normal pH of 7.6; or the same media used at low pH (6.6). Increased glycolysis is a well-established property of proliferating and metastatic cancer cells [27] and the low pH has been demonstrated to increase exosome release in mesothelioma [15].
Quantification of TnTs after addition of exogenous VAMT and Met5A exosomes to MSTO-211H mesothelioma cells
A 20x objective lens on a Olympus IX70 inverted microscope (Olympus Corporation) was used to count the number of cells and TnTs in 10 fields per medium condition at regular time intervals (0, 24, 48, and 72 hours). To visually identify TnTs via microscopic imaging of live cell cultures, we used parameters described previously [2, 3] These included (i) lack of adherence to the substratum of tissue culture plates, including visualization of TnTs passing over adherent cells; (ii) TnTs connecting two cells or extending from one cell were counted if the width of the extension was appropriately narrow and estimated to be < 1000 nm in width; and (iii) a narrow base at the site of extrusion from the plasma membrane. Two co-authors (E.L. and V.T.) performed visual identification and counting of TnTs. Cellular extensions that were not clearly within the above-described parameters were not included in the final count. The average number of TnTs per cell (TnTs/cell) was counted to control for continued cell proliferation in cell culture and exclude the possibility that increases in TnTs were due to an increased cell numbers. Experiments were performed in duplicate, and the results were averaged.
Electron-Microscopic (EM) Imaging of Nanotubes
To perform scanning and transmission EM, 1-3 × 106 MSTO-211H cells were cultured on Thermanox plastic tissue culture 25 mm cover slips (Lux Scientific Corporation). The fixative consisted of 2.5% glutaraldehyde/2% paraformaldehyde in 0.075 M sodium cacodylate buffer (pH 7.5; 10 ml, Electron Microscopy Sciences, Hatfield, PA) and was added directly to the overlying medium.
Lipid raft staining
Lipid raft (cholera toxin B) staining was done using freshly prepared Biotinylated-Cholera Toxin B (Molecular Probes, cat#C34779, 1 μg/mL), which was incubated for 15 minutes at 4°C and then washed with chilled phosphate-buffered saline (PBS). Slides were fixed with 4% paraformaldehyde for 10 minutes at RT and then washed in PBS for 5 minutes. Aliquots of 100 μl of Reagent A (Avidin DH solution) and Reagent B (biotinylated enzyme from ABC Vector Kit (PK4001)) were added to 5 ml 2% bovine serum albumin (BSA)/PBS for 30 min at RT. Slides were washed in PBS three times for 5 min each at RT. Tyramide Alexa was prepared in amplification buffer with 0.0015% H2O2; and the final concentration was 1:100. One hundred microliters of this solution (Invitrogen TSA kit) was applied to each cover slide for 15 minutes at RT, and the slides were again washed in PBS 3 times for 5 minutes each at RT.
Statistical Analysis
To assess quantitative differences in TnTs as well as relative lipid raft fluorescence, we performed Student's t-test using Excel 2013 (Microsoft, Redmond, WA). Error bars in the graphs represent means ± standard deviation. P values of <0.05 were considered statistically significant.
Results
Isolation of exogenous exosomes
We previously demonstrated that mesothelioma cells from different histologies (sarcomatoid VAMT and biphasic MSTO-211H) form TnTs in co-culture.[2] Having demonstrated that these two different cell lines establish TnT connections, we elected to collect exosomes from VAMT cells and add them to MSTO cells to control for the likelihood that MSTO cells produce their own exosomes in culture. We also elected to isolate exosomes from Met5A, a non-malignant mesothelial cell line. Exosome isolation from both cell lines were analysed by 10% SDS-PAGE analysis (Figure 1A), as well as a Western blot using standard exosome-verified protein markers such as HPS70, CD63, CD81, and CD9 (Figure 1B). We further confirmed and quantified exosome isolation using ELISA protocols (Figure 1C). VAMT and Met5A exosomes contained 1022.09 and 1067.84 pg of CD9 protein/ml, respectively.
Figure 1. Confirmation and characterization of exosomes.
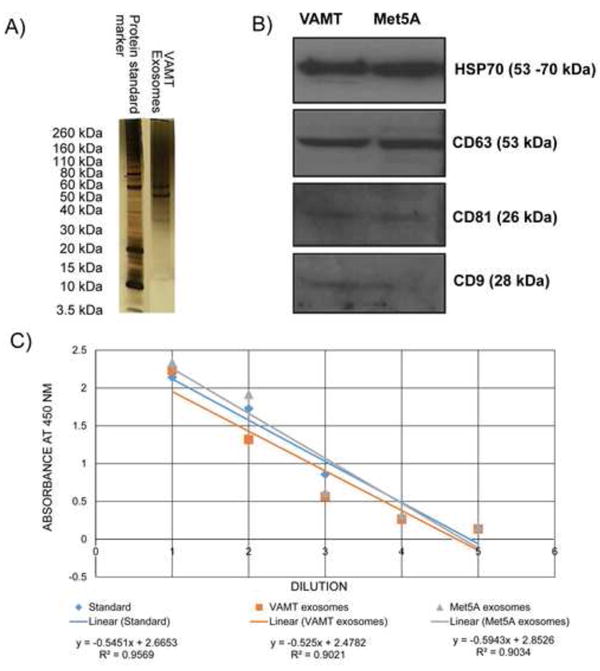
A) SDS-PAGE analysis of VAMT exosomal proteins. Exosomes were isolated from the supernatant of VAMT sarcomatoid mesothelioma cells and analyzed by denaturing SDS-PAGE (10%) analysis. B) Western blot analysis of VAMT and Met5A exosomes. VAMT and Met5A exosomes were subjected to Western blot analysis with exosome-verified antibodies as described in the Methods section. C) ELISA confirm of exosome purification. Quantification of VAMT and Met5A exosomes was performed by ELISA, using the exosome standard provided by the manufacturer (System Biosciences) for comparison. Exosome specific rabbit anti-human-CD9 antibody were used for the assay. Exosome standards provided by the vendor were assayed using CD9 antibodies. Visual confirmation of exosomes using EM is shown in Figure 2.
TnTs facilitate direct cell-to-cell transport of exosomes
To visualize exosomes and their spatial association with TnTs, we performed scanning and transmission electron microscopy (EM). Following careful fixation and preparation, TnTs were readily identified using scanning (Figure 2A) and transmission (Figure 2, panels B, C, D, & E) electron microscopy. Upon examination of the transmission EM images, we identified exosomes not only at the base of TnTs in the external cellular environment, but also in the submembrane region of these TnT-forming cells (Figure 2E). These exosomes were visually identical to speckled cup-shaped exosomes identified using EM in a prior study of mesothelioma cells.[15] We added DiI-labeled exosomes to unstained, non-fluorescing MSTO-211H cells. We discovered that the exogenous exosomes were incorporated into the cytoplasm of cells within 18 hours of incubation (Supplementary Figure 1A and 1B). When the cells were allowed to grow, we found that exosomes were incorporated into TnTs (Supplementary Figure 2). This observation supports prior work suggesting an association between exosomes with TnTs in endothelial cells.[28, 30] Time-lapse microscopic imaging also demonstrated the transfer of labeled exosomes from one cell to another via TnTs (Figure 3) in approximately 20-30 minutes (Supplementary Movie S1). These data show that exosomes co-localize with TnTs and that TnTs facilitate direct cell-to-cell transport of exosomes.
Figure 2. Structural examination of exosomes in and around TnTs using scanning and transmission electron microscopy (EM).
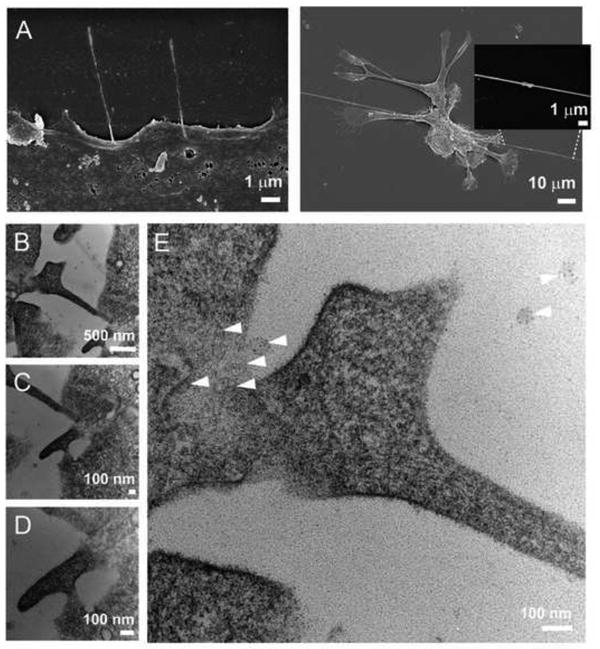
A) Scanning EM of TnTs as long, thin tubular structures emerging directly from the plasma membrane (left panel). TnTs have characteristic bulges (right panel), representing putative cellular cargo in transit (inset photograph). The indicated TnT is non-adherent and passes over short, thicker, adherent cellular extensions consistent with microvilli, which are also characteristic of mesothelioma.B) Two different TnTs providing either an ‘intercellular bridge’ between two cells or a partially-formed TnT stemming from one of the cells. C & D) The middle and lower panels provide closer views of panel B. E) The enlarged image to the right demonstrates exosomes in the extracellular environment and around the base of the connected TnT (arrowheads). These images are representative of the findings from this study. The speckled appearance of mesothelioma exosomes is identical to that demonstrated in a previous study by Hegmans et al.[15]
Figure 3. Movement of internalized exogenous VAMT exosomes across cells via TnTs.

MSTO-211H cells were co-cultured with DiI-labeled VAMT-derived exosomes and imaged by time-lapse microscopy. TnTs (arrows) and a moving DiI-labeled VAMT exosome (top arrow) are shown. The VAMT exosome was transported from one cell to another within 20-30 minutes (Supplementary Movie S1).
Exogenous tumor-derived exosomes stimulate TnT formation among mesothelioma cells
We then examined whether exosomes act as chemotactic or stimulatory factors of TnT formation. We collected exosomes from the supernatant of VAMT mesothelioma cells and Met5A mesothelial cells. MSTO-211H mesothelioma cells were cultured in standard or low-serum, hyperglycemic medium, with or without addition of exogenous VAMT-derived exosomes. Separately, Met5A exosomes were added to MSTO-211H cells as a non-cancer control. To determine the role of pH on exosomes and their potential ability to affect TnT formation, we also cultured cells under acidic (pH 6.6) or normal (pH 7.6) conditions. We found that exogenous exosomes stimulated increased MSTO TnT formation in the early stages of cell culture, specifically within 24-48 hours (Figure 4). Cells treated with exosomes under low serum, hyperglycemic conditions (2.5% FBS, 50 mM glucose) at either pH 7.6 or 6.6 formed more TnTs/cell than cells not treated with exosomes at the 24, 48, and 72 hour time points (p = 0.012 for 2.5% FBS, 50 mM glucose, pH 7.6 at 72 hours; p < 0.01 for all other specified conditions and time points). In addition, the number of TnTs was higher when the cells were cultured in an acidic microenvironment (pH 6.6) than at pH (7.6) for all time points. The ratio of TnTs/cell in cultures containing exogenous exosomes vs. no addition ranged from 1.12 to 1.87 in low-serum, hyperglycemic conditions, with a peak difference occurring after 48 hours of incubation (Supplementary Table 1). For example, there was a 43% increase in TnTs/cell 24 hours after addition of exogenous VAMT, and 86% increase noted by 48 hours, for cultures grown in low-serum, hyperglycemic medium at normal pH 7.6. Using the same media under acidic conditions led to a 30% relative increase at 24 hours, and 74% increase by 48 hours, respectively. The differences for each of these time points was statistically significant as stated above (Figure 4). Furthermore, cells cultured in standard passage medium (10% FBS, 25mM glucose, pH 7.6) with or without VAMT or Met5A exosome treatment formed fewer TnTs/cell than cells cultured in low serum, acidified, hyperglycemic conditions (2.5% FBS, 50 mM glucose, pH 6.6) (p < 0.01). Notably, the addition of Met5A-derived exosomes did not elicit any significant changes in TnT formation. This finding was particularly interesting in light of our previously published observation that that MSTO-211H and Met5A cells did not form TnTs to each other when mixed and grown together in co-cultures, yet each cell type readily formed TnTs to the same cell type when mixed and grown together as well as separately.[2] In sum, the data from this experiment showed that exogenous tumor-derived exosomes stimulate increased TnT formation in mesothelioma cells when cells and exosomes are co-cultured in a low serum, hyperglycemic microenvironment, but exosomes derived from benign mesothelial cells did not have this effect.
Figure 4. Tumor-derived exosomes stimulate increased rate of formation and early development of TnTs.

The average number of TnTs per cells at 24, 48, and 72 hours are shown for cells treated with VAMT exosomes (A) or Met5A exosomes (B). Purified exosomes were added to MSTO-211H cells in culture under various media conditions in the presence or absence of exogenous VAMT or Met5A-derived exosomes. An Olympus IX70 inverted microscope was used to count TnTs in 10 fields per cell for each medium condition at regular time intervals (0, 24, 48, and 72 hours) using a 20x objective lens. The number of cells was also counted, and data were graphed and reported as average number of TnTs per cell (TnTs/cell). Experiments were performed in duplicate, with measurements averaged. Statistically significant differences were calculated and P values are indicated by asterisks.
Lipid rafts are quantifiably higher in TnT-forming cells
We previously characterized TnT composition by staining for critical actin-associated proteins, and determined that fascin and ezrin were expressed in areas of TnT formation.[2] To explore the potential role of other cellular components in TnT formation, we hypothesized that lipid rafts are highly expressed in TnTs because of their ability to stabilize and form structural anchors for membrane entry points. We stained cells with lipid raft/cholera toxin B and performed confocal fluorescence microscopy (Supplementary Movie S2). We consistently identified enrichment of lipid rafts in mesothelioma cells connected via TnTs in three mesothelioma cell lines: MSTO-211H, VAMT, H2052. To test whether lipid rafts are greater enriched in TnT-forming cells than in non-TnT-forming cells, we cultured three mesothelioma cells lines (MSTO-211H, VAMT, and H2052) and a non-malignant mesothelial cell line (Met5A) separately on cover glass slides for 48 hours in low-serum, hyperglycemic medium. We then fixed the cells and stained them for lipid rafts using cholera toxin. Microscopic images were taken using a wide-field Zeiss Axio200M microscope and included brightfield and fluorescent-channel images, which were then combined to produce overlaid images (Figure 5). Images included representative fields containing cells with and without TnTs. Using ImageJ software, we measured the areas and integrative densities of TnT-forming and non-TnT-forming cells. The average CTCF/area was higher for all cells with intact TnTs than for cells without TnTs (Figure 6). The ratios of CTCF/area between TnT-forming and non-TnT forming cells were as follows: 1.48 for VAMT, 2.06 for H2052, 1.76 for MSTO-211H, and 1.82 for Met5A. These data demonstrated that for all three mesothelioma cell lines analyzed, lipid rafts were quantifiably higher in TnT-forming cells than in non-TnT-forming cells. Additionally, the non-malignant mesothelial cell line Met5A (which also formed TnTs in low-serum, hyperglycemic medium) demonstrated similar enrichment of lipid rafts in cells forming TnTs, indicating that this characteristic is inherent to TnTs in general but not specific to TnTs in cancer specifically.
Figure 5. Representative images of confocal microscopy showing enrichment of lipid rafts in TnT-forming VAMT and MSTO-211H cells.
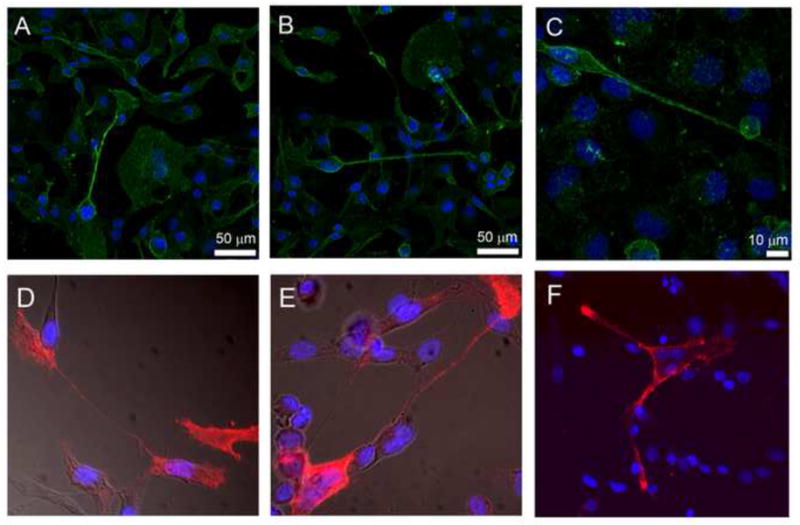
Cells were cultured for 48-72 hours in low-serum (2.5% FBS), hyperglycemic (50 mM glucose) RPMI medium. After microscopic confirmation of TnT formation, cells were fixed as described in “Methods”. Biotinylated-cholera toxin B was used to stain and identify lipid rafts. The top row demonstrates VAMT cells (panels A, B, & C); the bottom row demonstrates MSTO-211H cells (panels D, E, & F). Images were obtained using a Leica SP5 confocal microscope using either 20x/0.7 water immersion objective (panels A and B), 63x/1.4 oil immersion objective (panel C), or 40 x (panels D, E, & F) lenses.
Figure 6. Quantification of lipid raft fluorescence assessed by average CTCF/area of TnT-forming and non-TnT-forming cells.
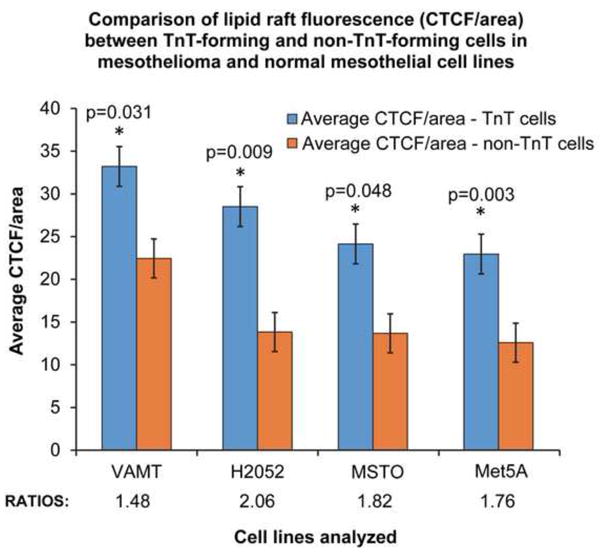
ImageJ software was used to measure the integrative density and area of cells and TnTs (measured as pixels). Using this information, we calculated the average CTCF/average area of TnT-forming cells and non-TnT forming cells in three mesothelioma (MSTO-211H, VAMT, H2052) cell lines and one mesothelial (Met5A) cell line.
Discussion
Intercellular communication between cancer cells is crucial to the progression of invasive cancers[31], but the mechanisms by which communication occurs between distant and proximal cells in the tumor matrix remain poorly understood. Our results suggest that exosomes and TnTs are not mutually exclusive modes of intercellular communication and may in fact work in a cooperative manner. Electron microscopic imaging demonstrated exosomes in close proximity to the site of origin of TnTs. These findings support a spatial association between exosomes and TnTs. It is conceivable that exosomes can transmit signals to recipient cells, and this signaling may directly or indirectly stimulate TnT formation. We also speculate that exosomes may further act as chemotactic agents that act to guide TnT growth in specific directions toward other cells. Our study implicates exosomes not only as potential mediators of TnT formation, but also provides additional evidence that these nano-sized vesicles package vital cellular contents, such as proteins and microRNAs, for directed intercellular trafficking via TnTs. Other studies support the notion that exosomes are capable of transporting microRNAs, albeit by extracellular diffusion of exosome packages.[32-40] Examination of the ultrastructural characteristics of exosomes using field emission scanning EM has demonstrated that they themselves may also connect to each other via intervesicular connections.[41] Further studies are required to characterize the connections cited in that study and to determine whether they may in fact be TnTs or structures more similar to lamellopodia or filopodia.
The concept that chemoattractants facilitate and direct TnTs has been explored in non-malignant cells. For example, mast cells have been shown to induce chemokine stimulation (eg, antigen and macrophage inflammatory protein 1-α) leading to TnT formation at areas of calcium accumulation. Exosomes themselves may not only act as intercellular signals but also as chemoattractants.[42] One potential mechanism is that exosomes can serve as chemoattractants for filopodia or invadopodia, guiding cells and allowing them to ‘sense’ the presence of nearby cells. Subsequent TnT formation could then promote intercellular transfer of a wide variety of cellular material. This concept is supported by time-lapse imaging data from our group and others that filipodial extensions extend but then retract, followed closely by TnT formation.[2, 43] We postulate that exosomes and these extensions serve as initial ‘environmental probes’ that seek out and identify locations of neighboring cells, allowing the cell to form TnTs in a regulated manner. Furthermore, studies by other groups have demonstrated that tumor exosomes can be transported from cell-to-cell directly via TnTs connecting cancer cells to endothelial cells.[28, 30] Our work confirms that this directed transport of exosomes can also occur via TnTs, and further demonstrates exosome transport via TnTs specifically connecting malignant cells, confirming what others have speculated.[44]
Our study shows that elevated levels of exosomes in a hyperglycemic, low pH tumor microenvironment stimulate an increased rate of TnT formation within the first 24-48 hours of in vitro culture. Exosome secretion and uptake is activated under acidic conditions,[45] and low pH secondary to increased glycolysis is a well-established property of proliferating and metastatic cancer cells. Indeed, acidic pH has been reported to enhance the invasive potential of cancer cells.[46] We previously reported that acidic pH in combination with hyperglycemia and a low-serum or serum-free environment stimulated an increased rate of TnT formation among MSTO cells.[2] Thus, the difference in TnTs that we observed in this study cannot be attributed to an increase in cell number. In fact, our conditions that stimulated TnT formation also decreased the proliferation rate. Taken together, these data suggest that elevated levels of exosomes in the hyperglycemic, low pH tumor microenvironment stimulate increased TnT production in MSTO cells. The acidic environment in a low serum, hyperglycemic setting may promote the release of endogenous exosomes or increase their fusion with cell membranes of recipient cells, which in turn may increase TnT production. It was recently reported that disruption of F-actin polymerization in hepatocarcinoma and ovarian cancer cells led to significantly decreased release of cellular microparticles (MPs) from these cells.[47] The authors also found that MPs did not interact or co-localize with lysosomes, the endoplasmic reticulum, or Golgi apparatus, and suggested that an alternate mechanism for tumor uptake of microparticles (or exosomes) exists independent of endocytosis. To this end, our data support the notion that actin-based TnTs provides a plausible alternative and additional mode for uptake and transfer of exosomes or similar microvesicles by malignant cells.
Our data show that mesothelioma cell cultures to which VAMT exosomes were added formed TnTs, beyond any potential effects of self-derived exosomes. Our goal was to minimize secretion of endogenous MSTO-derived exosomes prior to the addition of exogenous VAMT-derived exosomes. Endogenous exosome release was suppressed via a 4-hour incubation of MSTO cells at 4°C per prior protocols[25] and served as a critical control. In fact, several experimental approaches designed to block exosome secretion in vitro have been reported by blocking sphingomyelinase in neurons[48] and by the use of chemical agents such as amiloride, which inhibits H+/Na+ and Na+/Ca2+ calcium channels.[26] However, what is unclear is whether these agents are cell-specific or randomly effective. Based on a limited number of studies, we chose to combine several approaches including pre-incubation of MSTO cells at 4°C to decrease secretion of endogenous exosomes; pre-culture in mTeSR serum-free/vesicle-free medium [24] for 24 hours prior to performing experiments; and use of exosomes derived from a separate cell line, VAMT. These approaches ensured that the observed changes in TnT numbers were due to the addition of exogenous exosomes.
Identification of TnT-specific biomarkers would aid in the visual confirmation and molecular-based study of TnTs. Cancer represents an excess of normal activity leading to an increased rate of cellular proliferation, and a biomarker upregulated in TnTs may represent a potential indicator of malignancy. Lipid rafts may represent one potential candidate, in part, via intracellular signaling and activation of the MAPK pathway.[21] We have previously demonstrated that drugs that directly or indirectly affect this pathway (mTOR inhibitors or metformin acting via stimulation of adenosine monophosphate kinase or AMPK) also suppress TnT formation in mesothelioma.[2] Recent studies by other groups have confirmed the ability of an additional mTOR inhibitor (rapamycin) to decrease nanotube formation between benign mesothelial cells, as well as the association of cholesterol/lipid raft microdomains with TnTs.[9, 22, 23] Others have further demonstrated that depletion of cellular cholesterol using agents such as methyl-β-dextrin decreased the density of nanotubes between cells from a urothelial cancer cell line.[23] The same drug effectively reduced cholesterol concentrations and nanotube formation in human mesothelial cells cultivated in vitro.[9] Paradoxically, the use of the HMG-CoA reductase inhibitor simvastatin - a drug in common medical use for treatment of hypercholesterolemia – led to significant increases in numbers of nanotubes.[9] Nonetheless, as the authors postulate, the distribution of cholesterol across individual cells may account at least in part for this finding. As anti-cholesterol drugs are increasingly being investigated for supplemental use in the treatment of a variety of cancers, it is worth investigating the role of cholesterol microdomains in direct cell-to-cell communication via TnTs in cancer. Taken together, this body of information suggest that lipid rafts are associated with, or intersect, similar pathways implicated in TnT formation. Lipid rafts are quantifiably increased in mesothelioma and mesothelial cells forming TnTs, and thus merit further exploration as a potential biomarker for TnTs.
We determined that lipid rafts were localized to cancer cells with intact TnTs and greater enriched in cells with TnTs than those without. However, it is also recognized that TnTs are dynamic structures that are not always associated with lipid raft domains. For this reason, we assessed a number of representative images and calculated the average fluorescence per area across a spectrum sample size. Our findings suggested that these lipid microdomains do play a role in TnT formation and could potentially serve as biomarkers for cancer progression. Enrichment of lipid rafts in and around TnTs is not surprising considering the demonstrated ability of TnTs to transport lipid-raft based cargos such as prion proteins[12] or lipophilic components of cytosol between connected cells.[2]
Previous studies have shown that the interplay between lipid rafts and exosomes is critical for the propagation of cellular processes, although this characteristic is not necessarily exclusive to cancer cells.[16, 21, 42] In fact, as exosomes reach a recipient cell, they might be anchored by lipid rafts and then able to transmit signals that induce or guide TnT formation in a precise manner. In this model, enrichment of lipid rafts would create a ‘point of entry’ for signals transmitted by exosomes. Lipid rafts have been studied in reticulocytes and shown to facilitate release of exosomal contents to the extracellular environment.[49] It is conceivable that they also facilitate the fusion of exosomes with cells, and the formation of TnTs for the specific transport of those exosomes. It is important to consider that in addition to the capacity of exosomes to transport cellular cargo, they also contain cytosolic and plasma membrane proteins and express cell recognition molecules that mediate selective cellular uptake.[50] Future studies will undoubtedly examine the potential mechanisms that facilitate interactions between exosomes, TnTs, and lipid rafts, and their role in cancer growth.
Conclusions and Future Directions
Exosomes and lipid rafts represent two potential cellular components that might work in concert with malignant cells to facilitate intercellular communication by TnTs. It is critical that future studies investigate the functions of TnTs and the mechanisms by which they are selectively initiated, formed, maintained, and de-constructed, including key protein components specific to each of these processes. The fact that exosomes stimulate TnT formation in the early stages of cell culture implies a level of co-dependence for effective intercellular communication. Understanding these underlying mechanisms and functions has potentially large-scale implications across multiple fields of cell biology. We will continue to explore how exosomes and TnTs interface to carry out intercellular communication critical to tumor progression and survival.
Supplementary Material
Supplementary Movie S1. Time-lapse microscopic imaging of exosome transport via TnTs connecting malignant mesothelioma cells. MSTO-211H cells (5×104) were cultured with 5×105 fluorescent DiI (red)-labeled exosomes on Nunc Labtek-II 4-chamber microplates. Cells were grown at 37°C with 5% CO2 for 16 hours and transferred to the live cell recorder (BioStationIM-Q, Nikon Instruments Inc). Phase and red fluorescent images were collected once every 5 minutes for 48 hours using a 20× objective lens. Data were analyzed and compiled using NIS elements AR software (version 4.00.07).
Supplementary Movie S2. 3-dimensional depiction of cells stained for lipid rafts. MSTO-211H cells were cultured for 48 hours then fixed and stained for lipid rafts as described in “Methods” (red = lipid raft/cholera toxin stain; blue = DAPI/nuclear stain). Confocal imaging was performed with z-stacking. 3-dimensional imaging as demonstrated in this movie illustrates enrichment of lipid rafts in a TnT connecting two cells.
Highlights.
Exosomes derived from malignant cells can stimulate an increased rate in the formation of tunneling nanotubes.
Tunneling nanotubes can serve as conduits for intercellular transfer of these exosomes.
Most notably, exosomes derived from benign mesothelial cells had no effect on nanotube formation.
Cells forming nanotubes were enriched in lipid rafts at a greater number compared with cells not forming nanotubes.
Our findings suggest causal and potentially synergistic association of exosomes and tunneling nanotubes in cancer.
Acknowledgments
We wish to thank T. Tong and J-C Wang, Ph.D. of the Molecular Cytology Core Facility at MSKCC for their helpful suggestions, expertise, and assistance with imaging and immunofluorescence; M. Turkekul and N. Fan for assistance with immunofluorescent staining; N. Lampen for assisting with preparation of cells and imaging for electron microscopy evaluation; and K. DeBeer for excellent administrative assistance and support. We also thank C. Taubert in designing the figures; and M. Franklin for excellent editorial suggestions and critical review of the manuscript.
Research Funding: This research was kindly supported by the Baker Street Foundation; the Minnesota Medical Foundation; the Masonic Cancer Center and Department of Medicine, Division of Hematology, Oncology and Transplantation, University of Minnesota; American Cancer Society, Institutional Research Grant #118198-IRG-58-001-52-IRG94 from the American Cancer Society; the National Pancreas Foundation; Women's Health Interdisciplinary Seed Grant, Powell Women's Health Center, University of Minnesota; and the University of Minnesota Clinical and Translational Science Institute (CTSI) KL2 Scholar Award (to E.L.).
Abbreviations
- TnTs
Tunneling nanotubes
- nm
nanometers
- ATCC
American Type Culture Collection
- FBS
fetal bovine serum
- MAPK
mitogen-activated protein kinase
- AMPK
adenosine monophosphate kinase
- mTOR
mammalian target of rapamycin
- CTCF
corrected total cell fluorescence
- DIC
differential interference contrast
- RT
room temperature
- SMACs
supramolecular activation clusters
- ELISA
enzyme-linked immunosorbent assay
- MPs
microparticles
Footnotes
Conflict-of-interest disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular Highways for Intercellular Organelle Transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 2.Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA. Tunneling Nanotubes Provide a Unique Conduit for Intercellular Transfer of Cellular Contents in Human Malignant Pleural Mesothelioma. PLoS One. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou E, Fujisawa S, Barlas A, Romin Y, Manova-Todorova K, Moore MAS, Subramanian S. Tunneling Nanotubes: A New Paradigm for Studying Intercellular Communication and Therapeutics in Cancer. Communicative & Integrative Biology. 2012;5 doi: 10.4161/cib.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZG, Liu SL, Tian ZQ, Zhang ZL, Tang HW, Pang DW. Myosin-Driven Intercellular Transportation of Wheat Germ Agglutinin Mediated by Membrane Nanotubes between Human Lung Cancer Cells. ACS Nano. 2012;6:10033–10041. doi: 10.1021/nn303729r. [DOI] [PubMed] [Google Scholar]
- 5.Watkins SC, Salter RD. Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H. M-Sec Promotes Membrane Nanotube Formation by Interacting with Ral and the Exocyst Complex. Nat Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 7.Eugenin EA, Gaskill PJ, Berman JW. Tunneling Nanotubes (Tnt) Are Induced by Hiv-Infection of Macrophages: A Potential Mechanism for Intercellular Hiv Trafficking. Cell Immunol. 2009;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane Nanotubes Physically Connect T Cells over Long Distances Presenting a Novel Route for Hiv-1 Transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 9.Ranzinger J, Rustom A, Abel M, Leyh J, Kihm L, Witkowski M, Scheurich P, Zeier M, Schwenger V. Nanotube Action between Human Mesothelial Cells Reveals Novel Aspects of Inflammatory Responses. PLoS One. 2011;6:e29537. doi: 10.1371/journal.pone.0029537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, Knowles DM, Chiu A, Sanders RW, Chen K, Cerutti A. Hiv-1 Evades Virus-Specific Igg2 and Iga Responses by Targeting Systemic and Intestinal B Cells Via Long-Range Intercellular Conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galkina SI, Stadnichuk VI, Molotkovsky JG, Romanova JM, Sud'ina GF, Klein T. Microbial Alkaloid Staurosporine Induces Formation of Nanometer-Wide Membrane Tubular Extensions (Cytonemes, Membrane Tethers) in Human Neutrophils. Cell Adh Migr. 2010;4:32–38. doi: 10.4161/cam.4.1.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C. Prions Hijack Tunnelling Nanotubes for Intercellular Spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 13.Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling Nanotube (Tnt)-Like Structures Facilitate a Constitutive, Actomyosin-Dependent Exchange of Endocytic Organelles between Normal Rat Kidney Cells. Exp Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda K, Park HC, Ratliff B, Addabbo F, Hatzopoulos AK, Chander P, Goligorsky MS. Adriamycin Nephropathy: A Failure of Endothelial Progenitor Cell-Induced Repair. Am J Pathol. 2010;176:1685–1695. doi: 10.2353/ajpath.2010.091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic Analysis of Exosomes Secreted by Human Mesothelioma Cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of Breast Tumor Cells Induces Rapid Secretion of Exosomes Which Subsequently Mediate Cellular Adhesion and Spreading. PLoS One. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taner SB, Onfelt B, Pirinen NJ, McCann FE, Magee AI, Davis DM. Control of Immune Responses by Trafficking Cell Surface Proteins, Vesicles and Lipid Rafts to and from the Immunological Synapse. Traffic. 2004;5:651–661. doi: 10.1111/j.1600-0854.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 18.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous Cell-to-Cell Transmission of Human Immunodeficiency Virus to Multiple Targets through Polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta N, DeFranco AL. Visualizing Lipid Raft Dynamics and Early Signaling Events During Antigen Receptor-Mediated B-Lymphocyte Activation. Mol Biol Cell. 2003;14:432–444. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic Analysis of Hiv-1 Nef Cellular Binding Partners Reveals a Role for Exocyst Complex Proteins in Mediating Enhancement of Intercellular Nanotube Formation. Retrovirology. 2012;9:33. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi K, Parolini I, Malavasi F, Peschle C, Sargiacomo M, Testa U. Tfr2 Localizes in Lipid Raft Domains and Is Released in Exosomes to Activate Signal Transduction Along the Mapk Pathway. J Cell Sci. 2006;119:4486–4498. doi: 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 22.Kabaso D, Lokar M, Kralj-Iglic V, Veranic P, Iglic A. Temperature and Cholera Toxin B Are Factors That Influence Formation of Membrane Nanotubes in Rt4 and T24 Urothelial Cancer Cell Lines. Int J Nanomedicine. 2011;6:495–509. doi: 10.2147/IJN.S16982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokar M, Kabaso D, Resnik N, Sepcic K, Kralj-Iglic V, Veranic P, Zorec R, Iglic A. The Role of Cholesterol-Sphingomyelin Membrane Nanodomains in the Stability of Intercellular Membrane Nanotubes. Int J Nanomedicine. 2012;7:1891–1902. doi: 10.2147/IJN.S28723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and Uptake of Exosomes by Ovarian Cancer Cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobrie A, Colombo M, Raposo G, Thery C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 26.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane Associated Hsp72 from Tumor-Derived Exosomes Mediates Stat3-Dependent Immunosuppressive Function of Mouse and Human Myeloid-Derived Suppressor Cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatenby RA, Gawlinski ET. A Reaction-Diffusion Model of Cancer Invasion. Cancer Res. 1996;56:5745–5753. [PubMed] [Google Scholar]
- 28.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine Induction of Endothelium by Tumor Exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human Tumor Virus Utilizes Exosomes for Intercellular Communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, Kohn EC. Exosomes Released by K562 Chronic Myeloid Leukemia Cells Promote Angiogenesis in a Src-Dependent Fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny PA, Lee GY, Bissell MJ. Targeting the Tumor Microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory Mechanisms and Intercellular Transfer of Micrornas in Living Cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T. Human Villous Trophoblasts Express and Secrete Placenta-Specific Micrornas into Maternal Circulation Via Exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 34.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from Human Saliva as a Source of Microrna Biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional Transfer of Microrna-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, Mochizuki T. Let-7 Microrna Family Is Selectively Secreted into the Extracellular Environment Via Exosomes in a Metastatic Gastric Cancer Cell Line. PLoS One. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional Delivery of Viral Mirnas Via Exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma Microvesicles Transport Rna and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DD, Gercel-Taylor C. Microrna Signatures of Tumor-Derived Exosomes as Diagnostic Biomarkers of Ovarian Cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-Mediated Transfer of Mrnas and Micrornas Is a Novel Mechanism of Genetic Exchange between Cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Rasool HI, Palanisamy V, Mathisen C, Schmidt M, Wong DT, Gimzewski JK. Structural-Mechanical Characterization of Nanoparticle Exosomes in Human Saliva, Using Correlative Afm, Fesem, and Force Spectroscopy. ACS Nano. 2010;4:1921–1926. doi: 10.1021/nn901824n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-Containing Exosomes Are Released from Heat-Stressed Tumor Cells Via Lipid Raft-Dependent Pathway and Act as Efficient Tumor Vaccine. J Immunol. 2011;186:2219–2228. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- 43.Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH. Selective Block of Tunneling Nanotube (Tnt) Formation Inhibits Intercellular Organelle Transfer between Pc12 Cells. FEBS Lett. 2009;583:1481–1488. doi: 10.1016/j.febslet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 44.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, De Leo G, Alessandro R. Role of Exosomes Released by Chronic Myelogenous Leukemia Cells in Angiogenesis. Int J Cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental Ph Is a Key Factor for Exosome Traffic in Tumor Cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic Ph Enhances the Invasive Behavior of Human Melanoma Cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 47.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, Zhang G, Feng ZH, Ye D, Huang B. Delivery of Chemotherapeutic Drugs in Tumour Cell-Derived Microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 48.Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte-Neuron Communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid Raft-Associated Protein Sorting in Exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 50.Mincheva-Nilsson L, Baranov V. The Role of Placental Exosomes in Reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie S1. Time-lapse microscopic imaging of exosome transport via TnTs connecting malignant mesothelioma cells. MSTO-211H cells (5×104) were cultured with 5×105 fluorescent DiI (red)-labeled exosomes on Nunc Labtek-II 4-chamber microplates. Cells were grown at 37°C with 5% CO2 for 16 hours and transferred to the live cell recorder (BioStationIM-Q, Nikon Instruments Inc). Phase and red fluorescent images were collected once every 5 minutes for 48 hours using a 20× objective lens. Data were analyzed and compiled using NIS elements AR software (version 4.00.07).
Supplementary Movie S2. 3-dimensional depiction of cells stained for lipid rafts. MSTO-211H cells were cultured for 48 hours then fixed and stained for lipid rafts as described in “Methods” (red = lipid raft/cholera toxin stain; blue = DAPI/nuclear stain). Confocal imaging was performed with z-stacking. 3-dimensional imaging as demonstrated in this movie illustrates enrichment of lipid rafts in a TnT connecting two cells.


