The letter by Bergmann and collaborators1 questions our comments2 regarding their work on 14C birth dating of human cardiomyocytes with aging.3 Three points are made in an attempt to challenge the issues we have raised, which cast serious doubts on the validity of their data on myocyte turnover with physiological aging: 1. Appropriateness of the myocardial samples examined; 2. Specificity of the protocol employed for the isolation of myocyte nuclei; and 3. Correct interpretation of the 14C measurements.
Myocardial Samples
As acknowledged in their letter, 6 of the 12 hearts studied, i.e., 50% of the cases, had overt myocardial pathology. Surprisingly, samples collected from a patient who died of acute myocardial infarction were considered representative of normal cardiac aging. Additionally, no histological analysis was performed in the 6 “non pathological” hearts; gross examination at autopsy was deemed to be sufficient to define the structural integrity of the myocardium. Specific clinical, anatomical and histological criteria have to be met for the inclusion of human hearts in studies of physiological aging (Table 1).4,5 The approach employed by Bergmann and collaborators is far from any sensible standard needed to determine the biology of human cardiac aging.
Table 1. Inclusion Criteria for the Analysis of Physiological Myocardial Aging.
| Clinical Criteria |
|
| Anatomical Criteria |
|
| Histological Criteria |
|
Isolation of Myocyte Nuclei
The authors defend the findings reported in Science3 by citing the manuscript which subsequently appeared in Experimental Cell Research.6 We have been criticized in the letter for ignoring this hastily published paper. Our reasons for not quoting this second report need to be discussed. In this work, a series of images illustrating different degrees of nuclear troponin staining have been obtained with a variety of antigen retrieval methods. These findings have been interpreted as unequivocal documentation of the expression of troponin I in myocyte nuclei, independently from the age of the cells, rather than the consequence of alterations in nuclear pore complexes in senescent cardiomyocytes with nuclear translocation of proteins physiologically restricted to the cytoplasm.7 However, all these stainings were done in formalin-fixed, paraffin-embedded tissue. These protocols do not correspond to the preparation of unfixed nuclei that was used in the paper in Science.3
Importantly, controls for specificity of the staining for cardiac troponin T and troponin I were not performed in this6 and in the previous study.3 The conviction of the authors that mixing antibodies with donkey serum “prevents nonspecific antibody reactions” is questionable.6 This approach may decrease the unspecific binding of the secondary antibody generated in donkeys, but it will not prevent the unspecific interaction of the primary antibody with nuclear proteins. Controls with omission of the primary antibody, irrelevant primary antibody and immunoprecipitation of primary antibody with troponin proteins were not performed. Similarly, spectral analysis to confirm the specificity of the recorded signals was not introduced. These limitations apply also to labeling of the pericentriolar material-1 protein utilized to identify myocyte nuclei.6 Conversely, all these controls were done in our study7 which questioned the validity of troponin expression in young, intact cardiomyocyte nuclei.
Troponin I Positive Myocyte Nuclei
The data in Figure 2 of the manuscript published in Experimental Cell Research are misleading.6 Myocyte number decreases with age while the number of fibroblasts increases. Interstitial fibrosis and foci of replacement fibrosis are typically found in the aged human heart4,5,8 and comparable findings have been reported in rats and mice.9,10 This is highly relevant because the analysis of 14C was performed in 6 old diseased hearts3 in which myocardial scarring was a critical factor. Unfortunately, this variable was not introduced by Bergmann and collaborators, making their analysis invalid. By measuring only the percentage of troponin I positive myocyte nuclei without considering the increase in fibroblasts, their data are consistent with the notion that the percentage of troponin I positive myocyte nuclei increases with age.7 Relative measurements cannot be directly translated into absolute values.11
Figure 2. Analysis of 14C data in reference 2.
See text. Modified from reference 3.
Nuclear DNA Content
The 3-dimensional reconstruction illustrating the procedure used to measure DNA content in myocardial sections 30 μm in thickness is problematic.6 By necessity, the intensity of labeling of nuclei varied across the thickness of the section, becoming progressively weaker with the depth of the section. To compensate for this artifact, the signal intensity of the labeled DNA was amplified to levels saturating the photodetection system. This is apparent in the images shown in Figure 4.6 As a result, all nuclei had comparable brightness and the major variable was represented by the size of the nucleus, rather than by the actual DNA content. This makes the measurement of ploidy invalid.7
Mathematical Model
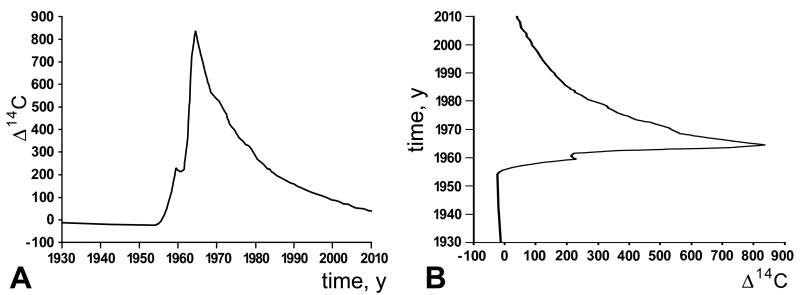
The atmospheric 14C curve (Figure 1A) shows the dependent variable, 14C concentration, as a function of time, which reflects the independent variable. This curve has the characteristics of a proper function: one independent variable yields one and only one dependent variable. However, in retrospective 14C birth dating of cells, 14C is used as the independent variable and time as the dependent variable (Figure 1B). This transformation produces a degenerate function, i.e., one independent variable yields more than one dependent variables. This fundamental principle has been overlooked in the analysis and interpretation of the data on 14C in myocyte nuclei.3 An arbitrary decision was made and the ascending limb of the 14C curve was employed to derive myocyte age in individuals born before the atmospheric 14C peak. There is no basis for this predetermined biased choice. No additional information concerning myocyte renewal in these samples was obtained to support this approach. If the descending limb of the 14C curve would have been considered, myocyte turnover rate would have been higher with aging, a phenomenon shown recently to be the case.5 The 12 mathematical scenarios listed in the online material3 are irrelevant when this critical variable is not resolved. Additionally, the exponential decay curve drawn arbitrarily to fit the data is statistically incorrect.2,7
Figure 1. Relation between atmospheric 14C and time.
A, Concentration of 14C, dependent variable, as a function of time, independent variable. B, Time, dependent variable, as a function of 14C concentration, independent variable.
Non-Myocyte Birth Date: 1000 A.D.
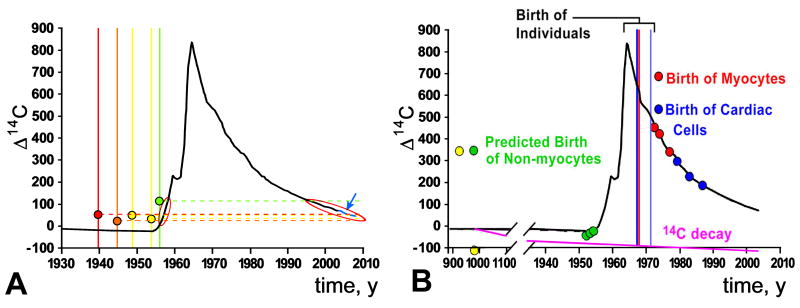
Some comments concerning the analysis of the results on atmospheric 14C are in order. Panel A in Figure 2 (Figure 1C in ref. 2) illustrates the data obtained from patients 50 to 73 years of age. In these hearts, it is not possible to indicate the age of the cells, since the measured 14C may reflect its incorporation during the rising or decaying part of the 14C curve (indicated by us with red ovals). The difference in the rate of myocyte renewal between young and old individuals may reflect an artifact in calculation due to assumptions of the model imposed and not a real biological phenomenon. Let us consider the patient shown by the green line in Panel A. If this subject had two bursts of myocyte formation, one in 1958 and one in 1997, identical values of 14C content would be obtained and incorrectly interpreted as reflecting myocytes born only in 1958. Also, the heart of the 4 oldest patients, born between 1939 and 1955 (bracket), could contain myocytes born before the sharp rise of atmospheric 14C, or myocytes formed shortly before death. In the original graph, the black line of the 14C curve was not drawn to the time of patients' death, late 2006/early 2007; this missing part is now shown in blue (arrow). Thus, the claim that myocyte renewal decreases with age has no basis.
Despite the attempt made to defend the data published in Science, to fit the model, 3 of the 6 subjects born after the 14C peak (Panel B: vertical lines within bracket) required non-myocytes to possess impossible 14C signatures from before the bomb pulse (Panel B: green dots). Importantly, the subject born before the bomb pulse, shown by a green line in Panel A and by a yellow dot in Panel B required non-myocyte DNA from 1000 A.D., depleted by radioactive decay, to fit the model. The Δ14C 42.76 value claimed in the letter does not correspond to any of those listed in Table S1 of the online material.3 Even if we accept the Δ14C 42.76 value, non-cardiomyocytes and cardiomyocytes would be of comparable age, 48 and 49 years old, respectively, sharply in contrast with the 18-40 fold higher turnover rate claimed by the authors for non-cardiomyocytes.3 Finally, the fraction of cardiomyocytes used in the original study2 varied from 29% to 60% of the cardiac cell pool (Table 3 online), confirming the lack of specificity of the nuclear markers used to collect myocyte nuclei. The 36% value indicated in the letter refers to the manuscript in Experimental Cell Research6 which has no relevance to retrospective 14C birth dating of human cardiomyocytes.
Conclusions
Unfortunately, the findings on myocyte turnover reported in Science in 2009 support the old view of the limited regenerative capacity of the adult and aging human heart. These results have often been used to challenge the notion that the heart is a self-renewing organ regulated by a stem cell compartment. Editorials12,13 and reviews14,15 have taken this information to question the existence of a resident stem cell and its critical role in cardiac homeostasis and pathology. Importantly, new clinical data are emerging in which cardiac stem cells have been isolated from very sick hearts, expanded in vitro and delivered back to the patients.16 The dramatic improvement in quality of life and cardiac performance offers the most compelling argument in favor of the regenerative capacity of the adult diseased human heart.
Footnotes
The Letter was handled by a Consulting Editor: James Willerson.
References
- 1.Bergmann O, Zdunek S, Bernard S, Druid H, Jovinge S, Frisen J. Comment on “Role od cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology.”. Circ Res. 2011;109:xxx–xxx. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 5.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisén J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res. 2010;317:188–194. doi: 10.1016/j.yexcr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D'Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 9.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 10.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 11.Anversa P, Olivetti G. Cellular basis of physiologic and pathologic myocardial growth. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology, Section 2: The Cardiovascular System: The Heart. Vol. 1. Oxford University Press; 2004. [Google Scholar]
- 12.Parmacek MS, Epstein JA. Cardiomyocyte renewal. N Engl J Med. 2009;361:86–88. doi: 10.1056/NEJMcibr0903347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murry CE, Lee RT. Development biology. Turnover after the fallout. Science. 2009;324:47–48. doi: 10.1126/science.1172255. [DOI] [PubMed] [Google Scholar]
- 14.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goihberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomized phase 1 trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)61590-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]