Abstract
The cerebellum is an important structure for accurate control and timing of movement, and Purkinje neurons in the cerebellar cortex are key players in cerebellar motor control. Cerebellar dysfunction can result in ataxia, a disorder characterized by postural instability, gait disturbances and motor incoordination. Cerebellar ataxia is a symptom of a number of conditions, and the emerging evidence that Purkinje neuron dysfunction, in particular, abnormal Purkinje neuron repetitive firing, is a major driver of motor dysfunction in a subset of dominantly inherited ataxias is dicussed. Abnormalities in Purkinje neuron excitability that are observed in mouse models of each of these disorders, and where appropriate describe studies linking particular ion channels to aberrant excitability are also discussed. Common mechanisms of dysfunction and speculate about potential therapeutic targets, suggesting that Purkinje neuron firing abnormalities are a novel target for improving motor dysfunction in patients with some forms of dominantly inherited ataxia are proposed.
Keywords: atrophy, cerebellum, dominantly inherited ataxia, intrinsic excitability, ion channel, pacemaking, polyglutamine disorder, Purkinje neuron, spinocerebellar ataxia
The role of the cerebellum is to ensure that motor activity is performed precisely with accurately timed movements. A significant portion of the computation within the cerebellum is performed within the cerebellar cortex, a laminar, repetitive and highly organized structure. Within the cerebellar cortex, information ultimately converges on Purkinje neurons, the central computational integrators of the cerebellar system.
Purkinje neurons are tonically repetitively firing cells whose firing frequency changes during directed movement [1]. Purkinje neurons receive two main types of excitatory inputs: parallel fibers from granule cells (through the mossy fiber pathway) and climbing fibers from the inferior olive. In addition, Purkinje neurons receive feed-forward inhibitory input from molecular layer interneurons, which themselves are influenced by parallel fiber input [2]. Purkinje neurons integrate this information, and the processed information is then sent via inhibitory GABAergic synapses to the neurons of the deep cerebellar nuclei (DCN), a collection of paired nuclei (dentate nucleus, interposed nuclei and fastigial nucleus) embedded in the deep white matter of the cerebellum. With the exception of direct projections from the cerebellar flocculonodular lobe onto the vestibular nuclei, projections from Purkinje neurons in the remainder cerebellum are channeled through the neurons of the DCN and represent the sole output of the cerebellar cortex. The DCN, in turn, project to other targets in the CNS, including premotor and primary motor cortical areas (by way of the thalamus), the reticular nucleus and the red nucleus [3]. It is through these DCN outputs that the cerebellum communicates with the rest of the nervous system to exert control of movement [4].
Cerebellar dysfunction leads to ataxia, a syndrome characterized by gait disturbances, postural instability, degraded fine motor coordination, speech and swallowing difficulties, and vision abnormalities [5]. There are a wide variety of causes of cerebellar ataxia, including a number of dominantly inherited conditions that will serve as the focus for this review. For many of these heritable ataxic conditions, pathologic changes in Purkinje neurons are thought to largely account for the symptoms of the disease. Inherited cerebellar ataxias are associated with a wide variety of pathologic changes in Purkinje neurons, including alterations in morphology, physiology, cell number and, in some cases, the appearance of pathologic protein aggregates [6–9].
Mouse models of ataxia have suggested that functional abnormalities, in particular, abnormalities in Purkinje cell physiology, may play a significant role in the development of motor symptoms. Purkinje neurons are relatively physiologically unusual in that they exhibit autonomous high-frequency repetitive spiking [10], a property often referred to as pacemaking. Purkinje cell pacemaking is dependent upon the specific suite of ion channels that Purkinje neurons express in their cell membrane [11]. One would predict that disrupting ion channel function in Purkinje neurons may affect pacemaking, and it has indeed been shown that isolated ion channel defects in Purkinje cell produce dysfunctional pacemaking [12,13]. Interestingly, animals with these defects show profound signs of ataxia. This suggests that normal Purkinje neuron pacemaking is important for appropriate cerebellar regulation of movement, and points to ion channels as key intrinsic regulators of motor performance.
It has only recently been appreciated that abnormalities in the repetitive firing of Purkinje neurons may also play an important role in the development and progression of motor impairment in human inherited degenerative cerebellar ataxias. Mouse models of a number of degenerative ataxias have begun to demonstrate that substantial abnormalities in intrinsic pacemaking of Purkinje neurons accompany the development of ataxia and precede overt Purkinje neuron death. The timing of changes in intrinsic pacemaking suggests that physiologic changes in Purkinje neurons may be a primary event in these conditions, and raise the possibility that correcting abnormal Purkinje neuron pacemaking might be an important therapeutic priority.
This review aims to characterize the nature of abnormalities in Purkinje neuron intrinsic excitability, discusses their potential links with motor dysfunction and outlines therapeutic strategies targeting abnormal Purkinje neuron function for the symptomatic treatment of cerebellar ataxia. Using examples of specific mouse models of dominantly inherited degenerative ataxias that have abnormalities in Purkinje neuron pacemaking, the following sections attempt to show that a common pattern of Purkinje neuron dysfunction exists in these different ataxias, and that targeting these abnormalities has therapeutic potential. The ataxias that will be discussed in this review are outlined in Table 1.
Table 1.
Purkinje cell pacemaking defects in dominantly inherited ataxic disorders.
| Disease | Mutated gene | Functional deficit | Molecular targets |
|---|---|---|---|
| Dravet syndrome | SCN1A | Reduced excitability, reduced firing frequency | Voltage-gated sodium channels (activation) Glutamatergic synaptic transmission (potentiation) |
| Episodic ataxia type 2 | CACNA1A | Irregular spiking, poor encoding of parallel fiber synaptic input | Calcium-activated potassium channels (activation) |
| Huntington’s disease | HTT | Reduced firing frequency | – |
| Spinocerebellar ataxia type 1 | ATXN1 | Reduced firing frequency | – |
| Spinocerebellar ataxia type 2 | ATXN2 | Increased excitability, reduced firing frequency and bursting | Calcium-activated potassium channels (activation) |
| Spinocerebellar ataxia type 3 | ATXN3 | Increased excitability, reducing firing frequency | Calcium-activated potassium channels (activation) |
| Spinocerebellar ataxia type 5 | SPTNB2 | Reduced excitability, reduced firing frequency | Voltage-gated sodium channels (activation) Glutamatergic synaptic transmission (potentiation) FGF14/spectrin system (activation) |
| Spinocerebellar ataxia type 27 | FGF14 | Reduced excitability, reduced firing frequency | Voltage-gated sodium channels (activation) Glutamatergic synaptic transmission (potentiation) FGF14/spectrin system (activation) |
List of dominantly inherited ataxic disorders where dysfunctional Purkinje neuron pacemaking has been described. The gene that is mutated in the disease, the nature of any deficits in Purkinje neuron pacemaking observed in these models and potential therapeutic targets for clinical intervention are outlined for each condition.
Altered intrinsic Purkinje neuron pacemaking as a primary driver of ataxia
• Spinocerebellar ataxia type 2
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant disorder caused by polyglutamine expansion of the ATXN2 gene on the long arm of chromosome 12. In normal subjects, the gene has the sequence (CAG)8(CAA)1(CAG)4(CAA)1(CAG)8, coding for 22 consecutive glutamine residues near the N-terminal end of the protein. In individuals with SCA2, the length of the repeat generally exceeds 32 repeats. As is the case with other polyglutamine diseases, increasing repeat number inversely correlates with age of onset [14].
Recent work in mouse models of SCA2 has established a close relationship between abnormal repetitive firing in Purkinje neurons and the emergence of motor dysfunction. Purkinje neurons in the ATXN2(Q127) model of SCA2 show a progressive, age-dependent decrease in firing frequency. Notably, the progressive reduction in firing frequency was shown to accompany a progressive worsening of motor performance. The impairment of motor performance was observed to begin before any apparent Purkinje neuron atrophy. Since symptoms were first seen in the absence of atrophy, but were correlated with changes in firing frequency, these data suggested that abnormal Purkinje neuron pacemaking may be a much more important driver of pathology in SCA2 than had previously been appreciated [15].
The importance of Purkinje neuron pacemaking in SCA2 is further underscored by the efficacy of small molecule modulators of ion channel function that correct pacemaking and restore motor function. In a transgenic mouse model of SCA2 expressing ATXN2 containing 58 polyglutamine repeats, Purkinje neurons exhibit an abnormal burst pattern of firing [16]. Application of NS13001, a positive modulator of the calcium-activated potassium channel (SK) 2/3, rescues Purkinje neurons from an abnormal bursting pattern and returns them to a normal rate of repetitive spiking. The parallel improvement in motor performance when applied in vivo suggests a possible causal link between abnormal Purkinje neuron firing and ataxia in SCA2. In particular, the fact that activation of the hyperpolarizing current carried by SK2/3 is necessary to correct firing suggests that the abnormal pacemaking activity in these Purkinje neurons is a consequence of intrinsic Purkinje neuron hyperexcitability [16].
• Spinocerebellar ataxia type 27
Spinocerebellar ataxia type 27 (SCA27) is a disorder characterized by early-onset tremor, dyskinesia and slowly progressive cerebellar ataxia, and was first identified to be caused by mutations in FGF14 in 2003 [17]. The FGF14 gene, located at chromosome 13q34, encodes the FGF14 member of the FGF family. To date, few mutations in the FGF14 gene that have been identified as causing SCA27, notably a point mutation (F145S) and a frameshift mutation (p.Asp163fsX12).
SCA27 may be a disorder of aberrant Purkinje neuron pacemaking resulting from reduced activity of excitatory sodium channels. Studies in cultured hippocampal neurons heterozygous for the FGF14 F145S mutation show that F145S FGF14 associates with wild-type FGF14 and disrupts the interaction between wild-type FGF14 and NaV channel α subunits, causing impaired neuronal excitability [18]. The role of FGF14 in sodium channel binding and regulation of neuronal excitability seems to be functionally relevant in Purkinje neurons, since many Purkinje neurons from the Fgf14(−/−) mice demonstrate a failure to fire spontaneously and show degraded responsiveness to depolarizing current injection [19]. Purkinje neurons in the Fgf14(−/−) mice show disordered surface expression of NaV1.1 and NaV1.6 in the axon initial segment, and this likely accounts for the reduced excitability of the Purkinje neurons [20].
• Huntington’s disease
Huntington’s disease is a fatal, progressive neurodegenerative disease that is characterized by cognitive dysfunction, psychiatric disturbance and impairment of motor coordination. Huntington’s disease is one of nine polyglutamine diseases, arising due to expansion of a trinucleotide CAG repeat in exon 1 of the HTT gene [21]. The most striking pathology of Huntington’s disease is observed in the neostriatum, where there is dramatic loss of medium spiny neurons [22]. Other neuronal populations are relatively spared until the disease is very advanced [23], despite the fact that most areas of the brain show robust expression of mutant HTT [24]. The cerebellum, like other non-neostriatal structures, has been traditionally viewed as largely unaffected in Huntington’s disease, but recent evidence suggests that there is a greater degree of cerebellar pathology in Huntington’s disease than had previously been appreciated [25]. A study carried out in the Hdh CAG knockin model of Huntington disease demonstrated that gene-expression changes of the cerebellum were qualitatively similar to those seen in the striatum, suggesting there may be a similar pathologic process that simply progresses more slowly in the cerebellum [26]. Notably, a recent human study demonstrated that cerebellar degeneration with Purkinje neuron loss occurs in patients with Huntington’s disease [27].
Recent evidence from several mouse models of Huntington’s disease suggests that dysfunctional pacemaking in Purkinje neurons may be responsible for some of the motor impairment that is observed in Huntington’s disease. In the R6/2 model of Huntington’s disease, there is a striking reduction in Purkinje neuron firing frequency by postnatal day 28. It should be noted that there are no Huntington inclusions in the Purkinje neurons until 12 weeks [28]. Similar results were obtained using the HdhQ200 knockin mouse model of Huntington’s disease, with loose patch recordings of Purkinje neurons showing a reduction in firing rate in the Huntington’s disease mice compared with control littermates [29].
• Spinocerebellar ataxia type 1
Spinocerebellar ataxia type 1 (SCA1) is an inherited cerebellar ataxia with autosomal dominant inheritance. This disease arises owing to an expanded CAG triplet repeat in the N-terminal coding region of the ATXN1 gene on chromosome 6p23. Ataxin-1, the protein encoded by ATXN1 has several putative endogenous functions, including as an RNA-binding protein [30] and as a transcriptional regulator [31]. Whether the expanded polyglutamine tract in mutant Ataxin-1 contributes to pathology by limiting an endogenous function, exaggerating an endogenous function, or by conferring some novel toxic function is not well understood. As with SCA2, pharmacologic manipulation of perturbed Purkinje neuron firing can improve ataxia in a mouse model of SCA1 [32]. A reduction in basal firing rates of Purkinje neurons was seen in acute cerebellar slices from 2–3-week-old SCA1 mice, a time point well before there is overt neurodegeneration. This reduction in firing rate could be corrected through the application of 4-aminopyridine, and the treatment of SCA1 animals in vivo with 3,4-aminopyridine was capable of improving motor function. Interestingly, prolonged 3,4-aminopyridine treatment could protect Purkinje neurons from atrophy, raising the possibility that abnormal pacemaking may not only play a role a role in degraded motor performance, but might also be a driver of neurodegeneration in SCA1.
• Spinocerebellar ataxia type 3
Spinocerebellar ataxia type 3 (SCA3), also known as Machado–Joseph disease, is a dominantly inherited cerebellar ataxia resulting from a CAG repeat expansion in the ATXN3 gene. The age of onset varies considerably, although on average repeat length is inversely correlated with age of onset. Patients typically present first with progressive gait imbalance and speech difficulties, eventually developing occulomotor difficulties, spasticity, dystonia and, eventually, severe dysarthria and dysphagia [33].
Only one study has addressed the role that aberrant Purkinje neuron pacemaking may play in SCA3 [34]. In this study, the activity of Purkinje neurons from SCA3 transgenic animals was assessed at 6–8 weeks, a time point where there was already motor dysfunction, but no apparent Purkinje neuron atrophy. Purkinje neurons at this time point were shown to have increased intrinsic excitability resulting in depolarization block and an inability to sustain repetitive firing. Notably, administration of SKA-31, an activator of the SK, can partially correct the neuronal and motor dysfunction.
• Episodic ataxia type 2
Episodic ataxia type 2 (EA2) is an autosomal dominant disorder that typically begins during the first two decades of life. EA2 is characterized by recurrent attacks of vertigo, imbalance and ataxia that last for hours to days and are sometimes provoked by exertion, stress or alcohol. Although the periods between attacks can be symptom-free for some EA2 patients, others may experience interictal migraine headaches, generalized weakness, limb ataxia or absence seizures [35]. In EA2, the causative mutations are in CACNA1A, the gene that codes for the Ca(v)2.1 subunit of the P/Q calcium channel complex. Most EA2 mutations disrupt the reading frame [36].
A postulated mechanism for ataxia in EA2 is an increased irregularity of pacemaking in Purkinje neurons. In ducky and tottering mutant mouse models EA2, irregularity in the intrinsic pacemaking activity of Purkinje neurons is reported [37]. Purkinje neurons from the ducky mice also showed irregularity of spiking in response to parallel fiber stimulation, suggesting that Purkinje neurons in these mutant animals were less accurately encoding synaptic input. These results suggest a potential mechanism by which high spiketo- spike variability could degrade motor performance signals in the cerebellum, and also point to interspike interval variability as a potential therapeutic target. The interspike interval variability of Purkinje neurons could be rescued using the SK channel activator 1-ethyl-2-benzimidazolinone, and treatment of ducky mice with 1-ethyl- 2-benzimidazolinone provided symptomatic improvement in motor performance.
• Spinocerebellar ataxia type 5
Spinocerebellar ataxia type 5 (SCA5) generally begins in the third of fourth decade of life, and is characterized by incoordination of extremities, slurred speech and ataxic gait. Unlike some of the other degenerative cerebellar ataxias, SCA5 generally does not significantly shorten lifespan, probably since neurodegeneration in SCA5 is restricted to the cerebellum and the brainstem is largely unaffected [38]. First described in an American kindred and subsequently in German and French families, SCA5 was determined to be caused by a mutation in SPTNB2, which codes for the β-III spectrin protein [39]. The American and French families have separate inframe deletions in the third of 17 spectrin repeat motifs, while the German family has a mutation in the in the second calponin homology domain, a region known to bind actin and Arp1.
SCA5, like SCA27, is a disorder that appears to show abnormal Purkinje neuron pacemaking secondary to dysfunctional sodium channel function. A mouse model of SCA5 with partial deletion of the gene for β-III spectrin showed reduced Purkinje neuron firing rate that progressively worsened with age. Slice recordings from the βIII-spectrin(−/−) mice showed that in addition to the reduced spontaneous firing, the Purkinje neurons showed reduced total sodium current and reduced resurgent sodium current [40]. In Purkinje neurons, the resurgent sodium current is almost entirely mediated by NaV1.6 [12], suggesting that Purkinje neurons lacking β-III spectrin have reduced NaV1.6 function, which may contributing to the abnormal pacemaking.
• Severe myoclonic epilepsy in infancy
Severe myoclonic epilepsy in infancy, also known as Dravet syndrome, is an autosomal dominant form of epilepsy, which most commonly arises due to de novo germline mutations in SCN1A, the gene encoding the voltage-gated sodium channel NaV1.1. Patients with Dravet syndrome experience an array of neurologic and psychiatric complications, including a variety of different types of seizures, movement disorders and cognitive deficits. Notably, there are prominent changes in cerebellar structure and function in Dravet syndrome: 60% of patients with Dravet syndrome experience ataxia [41], and there is evidence to suggest that some Dravet syndrome patients show significant Purkinje neuron atrophy and death [42].
An Scn1a exon 25 deletion mutant mouse recapitulates the ataxia seen in some Dravet syndrome patients [43]. Recordings from the Purkinje neurons of the Scn1a mutant mouse revealed that the firing rates of Purkinje neurons from mutant mice were substantially reduced, with no effect on threshold for action potential generation. The reduced firing frequency was understood to be largely due to loss of excitatory sodium current, since the amplitudes of whole-cell peak, persistent and resurgent sodium currents in Purkinje neurons were reduced.
Shared mechanisms & therapeutic targets
• Dysfunction of the FGF14/spectrin system for sodium channel membrane localization (SCA5 & SCA27)
In the ataxias described above, shared patterns of dysfunction are apparent. SCA5 and SCA27 are both conditions where Purkinje neurons have inappropriately low excitability owing to reduced expression or activation of voltage-gated sodium channels. This shared dysfunction probably represents a mechanistically connected defect in voltage-gated sodium channel docking, potentially as a result of dysfunction in sodium channel membrane insertion system that relies on both FGF14 and spectrin family members. The connection between FGF14, spectrin proteins and sodium channels is well established for the axon initial segment, with recent data showing that dynamic regulation of excitability relies on a system that includes FGF14 and βIV-spectrin that localizes sodium channels to the axon initial segment [20]. Although a similar link between FGF14 and βIII-spectrin is not as well established, several lines of evidence suggest that βIII-spectrin may play a similar role to βIV-spectrin for membrane protein insertion in the soma. βIII-spectrin is localized to the cell body and dendrites of Purkinje neurons and associates with ankyrin and cytoskeletal elements in much the same way as other members of its family [44,45], and βIII-spectrin mutations lead to defective localization of a number of membrane proteins in the soma and dendrites [40]. If it is the case that βIII-spectrin recapitulates the role of βIV-spectrin but does so for the soma, then it is also likely that it would rely on FGF14 for membrane insertion of sodium channels in the somatic membrane in much the same way that βIV-spectrin relies on FGF14 for insertion of sodium channels into the axon initial segment. This raises the possibility that SCA5 and SCA27 actually represent dysfunction of the same pathway for sodium channel membrane insertion, a possibility that is supported by the defective sodium currents that are seen in mouse models for both of these disorders.
The fact that this pathway is dysregulated in both SCA5 and SCA27 suggests that it may be an ideal system to target therapeutically in order to restore normal Purkinje pacemaking and improve symptoms of ataxia. Conceivably, treatment with FGF14 in both contexts may be sufficient to restore normal excitability. In SCA27, it replaces the absent FGF14 and takes advantage of the other components of the machinery to restore appropriate membrane localization of sodium channels in the axon initial segment. In SCA5, the elevated FGF14 may be capable of increasing the activity of non-βIII isoforms of the spectrin family, and although this will not necessarily provide spatially-appropriate restoration of sodium currents (since not all spectrin family members traffic to the soma and dendrites), it will still increase the available sodium current density and hopefully may improve excitability to the point where it restores normal spiking.
• Reduction in Purkinje neuron excitability (SCA5, SCA27 & Dravet syndrome)
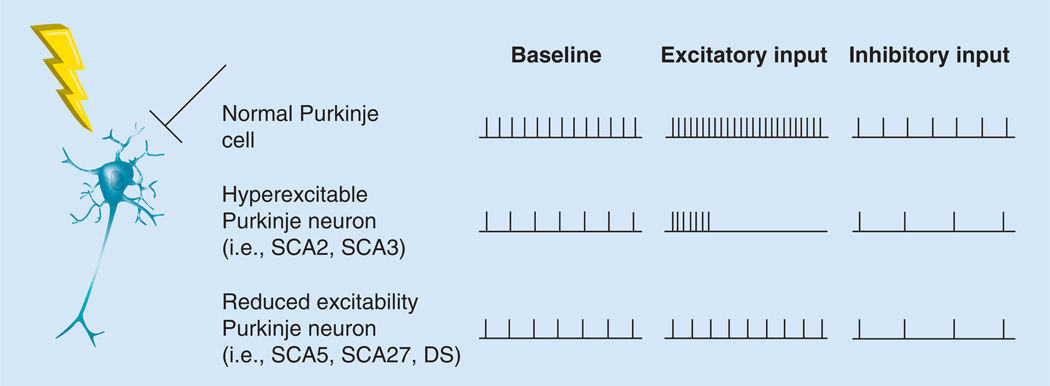
Although not necessarily through the same mechanism, SCA5, SCA27 and Dravet syndrome share an ataxic phenotype that is likely derived from reduced excitability of Purkinje neurons. For any condition where there is reduced excitability of Purkinje neurons, the input-output curve for Purkinje neurons may be flattened, leading to defective modulation of Purkinje neuron firing frequency and, therefore, degraded bandwidth for motor control signaling at the Purkinje neuron-to-DCN neuron synapse (Figure 1). In these conditions, one would hope to develop a strategy that could improve the input–output curve for these Purkinje neurons.
Figure 1. Purkinje neurons show abnormal firing frequency patterns in ataxia.
A schematic diagram of a Purkinje neuron with excitatory synaptic input (lightning bolt) and inhibitory synaptic input (bar-headed line). To the right is the firing pattern of Purkinje neurons at baseline, as well as the firing pattern in response to excitatory synaptic input and inhibitory synaptic input. The rows represent the firing frequency under each of these conditions from three different types of Purkinje neurons: unaffected Purkinje neurons, hyperexcitable Purkinje neurons (e.g., those seen in SCA2 and SCA3), and reduced excitability Purkinje neurons (e.g., those seen in DS, SCA5 and SCA27). DS: Dravet syndrome; SCA2: Spinocerebellar ataxia type 2; SCA3: Spinocerebellar ataxia type 3; SCA5: Spinocerebellar ataxia type 5; SCA27: Spinocerebellar ataxia type 27.
The reduced Purkinje neuron excitability, which is observed in these conditions, may be amenable to pharmacologic manipulation. One promising strategy is to increase the strength of the granule cell-to-Purkinje neuron synapse, taking advantage of the fact that Purkinje neuron firing frequency increases linearly with the strength granule cell synaptic input [46]. The strength of this synapse can be increased by pharmacologically potentiating glutamatergic signaling, and this potentiation will increase the firing frequency change in postsynaptic Purkinje neurons that occur for a given strength of granule cell synaptic input. Overall, this is likely to broaden the firing frequency range across which Purkinje neurons operate, which would improve Purkinje neuron firing frequency bandwidth. This would translate to improved bandwidth on motor control signaling and improvement of patient motor symptoms.
Another therapeutic strategy aimed at restoring Purkinje neuron excitability may involve taking advantage of other available sodium channels to facilitate appropriate excitability. Work from computational modeling suggests that there is remarkable homeostatic flexibility in the use of sodium conductances by neurons, and by adjusting these conductances neurons have some capacity to maintain intrinsic homeostasis [47]. There is evidence that these mechanisms are active even in the face of severe pathology: in a mouse model of SMEI there is partial upregulation of NaV1.6 to compensate for defective NaV1.1, although the upregulation is insufficient to restore normal function [43]. It is conceivable that a similar phenomenon might occur in SCA5 or SCA27, where there appears to be a defect in NaV1.6: a study of the optic nerve in Scn8a knockout mice showed that NaV1.2 can compensate for NaV1.6 by replacing it at sites where the two channels compete for membrane insertion [48]. Despite the fact that all of these conditions are likely to show some degree of compensation, the compensation is incomplete, and the remaining deficiency accounts for dysfunction that is observed. Although the mechanism behind this compensation is not well understood, it is likely that using small molecule modulators to potentiate this process in Purkinje neurons would allow for relatively specific correction of dysfunction in only the affected cells. This type of strategy would be much preferred to strategies that aim to correct defective sodium currents and reduced excitability through global sodium channel activation, since global activation of sodium channels would almost certainly produce intolerable consequences of excess excitability elsewhere in the brain. Alternatively, one may bypass the cell’s intrinsic compensatory mechanisms and improve excitability through viral delivery of channel cDNA with the intent of driving expression of those sodium channels whose function is lost.
• Disorders of Purkinje neuron hyperexcitability (SCA2 & SCA3)
In mouse models of SCA2 and SCA3, aberrant Purkinje neuron spiking (bursting, low-frequency pacemaking or both) seem to be a result of Purkinje neuron hyperexcitability. In these models, the data suggest that intrinsic hyperexcitability is reducing the effective firing frequency, either by inducing depolarization block (in SCA3) or by causing bursting (in SCA2). The shared hyperexcitability of these two conditions is further supported by the therapeutic efficacy of SK activators that would act to reduce membrane excitability.
For cells that have aberrant pacemaking and are susceptible to depolarization block, synaptic input may produce changes in Purkinje neuron signaling, which will dramatically degrade a cell’s participation in the network (Figure 1). Therefore, it is plausible that mitigating hyperexcitability through the activation of modulatory hyperpolarizing currents may normalize the input/output function for that cell so that it is able to respond appropriately to synaptic input. One way to do this is by pharmacologic modulation of SKs. Studies on several mouse models of SCA2 and SCA3 have suggested that the hyperexcitability can be mitigated by activation of SK2, which in turn results in improved firing and improved motor function. In the mouse model of SCA3, use of SKA-31, an activator of SKs, can correct dysfunctional Purkinje neuron pacemaking by allowing nonspiking cells to begin and firing, as well as by preventing depolarization block in affected Purkinje neurons. These changes are accompanied by an improvement in motor dysfunction [34]. In the mouse model of SCA2, application of an SK2 channel activator in the slice restores normal repetitive spiking in Purkinje neurons. Treatment of SCA2 mice with a selective SK2 activator alleviates symptoms of ataxia in vivo [16].
• Relationship between aberrant Purkinje neuron physiology & neuropathology
It is important to note that there is emerging evidence that links abnormal pacemaking to Purkinje neuron atrophy and cell death. Studies from animal models of two autosomal dominant degenerative cerebellar ataxias have shown that drugs, which are capable of correcting Purkinje neuron pacemaking, not only improve motor performance, but also limit neurodegeneration when initiated early in the course of disease. A study in a mouse model of SCA1 demonstrated that treatment with aminopyridines could improve Purkinje neuron pacemaking in vitro and could also restore BDNF expression and protect Purkinje neurons from atrophy in vivo when treatment was initiated at a time point before there is detectable cellular pathology [32]. A study in SCA2 yielded comparable results, demonstrating that in vitro modulation of SK channels could improve pacemaking and in vivo modulation of SK channels could improve neuropathology [16].
Both of these studies have raised the possibility that aberrant pacemaking may be more than just an epiphenomenon of degenerative cerebellar ataxias and may, in fact, be a driver of cellular pathology. These results suggest that normal pacemaking in Purkinje neurons plays a critical trophic function that is disrupted in these diseases. This speculation is quite plausible in the SCA1 model, since aminopyridine treatment improves expression of the growth factor BDNF, and BDNF levels are known to be regulated in an activity-dependent manner in some types of neurons [49]. Although more studies need to be carried out in order to gain a deeper mechanistic understanding of the connection between pacemaking and atrophy in autosomal dominant degenerative ataxias, it represents a promising avenue for research for both mechanistic understanding and therapeutic treatment of these conditions.
Conclusion & future perspective
Research in inherited cerebellar ataxias is progressing rapidly, and the body of work highlighted in this review demonstrates that abnormal Purkinje neuron function may account for impaired motor performance in a subset of dominantly inherited cerebellar ataxias. Future research should help clarify whether there is a causal relationship between abnormal Purkinje neuron physiology and motor dysfunction. Furthermore, evidence for aberrant pacemaking described above reflects only a fraction of the conditions that present with cerebellar ataxia, and as such it is not yet clear to what extent aberrant Purkinje neuron pacemaking is a key contributor to motor dysfunction in other cerebellar ataxias.
More research needs to be carried out in order to understand changes in Purkinje neuron physiology in cerebellar ataxias, and active investigation in this area is not only likely to yield important insights into the mechanisms of pathology, but may also produce novel therapeutics. To this point, the work on Purkinje neuron pacemaking dysfunction in cerebellar ataxias is still largely descriptive, and greater focus on mechanism will yield important insights into what cellular and molecular changes underlie the changes in physiology that are typically measured. In particular, carrying out thorough investigations of any changes in ion channel expression or function in each of these conditions is likely to help explain the aberrant pacemaking seen in Purkinje neurons, since Purkinje neurons rely primarily on the ion channels that they express for autonomous pacemaking activity. Understanding these changes will not only make clear which ion channels are dysregulated so as to produce aberrant pacemaking, but also which ion channels’ activity may be modulated in such a way that the Purkinje neuron excitability is tuned appropriately and normal pacemaking restored. One may then be able to design a therapeutic strategy that is specific for a particular disease entity and that aims to modulate dysregulated channels, unaffected channels or both in such a way that appropriate excitability is restored.
Emerging evidence suggests that there may be relationship between aberrant physiology and the development of neuropathologic changes in Purkinje neurons. If this is the case, restoring normal Purkinje neuron pacemaking would be an attractive target not only as symptomatic therapy, but also as a neuroprotective strategy. The etiology for ataxia in patients would need to identified early and appropriately treated during a period where there is Purkinje neuron dysfunction but not yet substantial loss of Purkinje neurons. For those patients who are identified early, appropriate intervention would prove doubly beneficial, since it would not only correct motor performance acutely but could also delay the onset of serious disability.
EXECUTIVE SUMMARY.
Altered intrinsic Purkinje neuron pacemaking as a primary driver of ataxia
In many dominantly inherited ataxias, Purkinje neuron dysfunction accompanies worsening motor performance. These changes in function and motor performance often precede histologic changes in Purkinje neurons.
In spinocerebellar ataxia type 2 and 3, Purkinje neurons show reduced firing frequency or abnormal bursting activity secondary to increased intrinsic excitability.
In spinocerebellar ataxia type 5 and 27, and Dravet syndrome, Purkinje neurons show reduced firing frequency secondary to reduced intrinsic excitability.
In spinocerebellar ataxia type 1, Purkinje neurons show reduced firing frequency. A pharmacologic agent that corrects firing frequency also improves motor performance.
In episodic ataxia type 2, Purkinje neurons fire irregularly, and improving this irregular firing with a calcium-activated potassium channel channel activator improves motor performance.
Shared mechanisms & therapeutic targets
Purkinje neurons demonstrate reduced excitability in spinocerebellar ataxia type 5 and 27, probably owing to dysfunction of an FGF14/spectrin system that targets sodium channel to the membrane. Using FGF14 to increase the function of this system could restore Purkinje neuron excitability and improve symptoms in these conditions.
Purkinje neurons show reduced excitability in spinocerebellar ataxia type 5 and 27, and Dravet syndrome that may be improved by potentiating glutamatergic synapses onto Purkinje neurons or by increasing voltage-gated sodium channel function in Purkinje neurons.
Purkinje neurons are hyperexcitable in spinocerebellar ataxia type 2 and 3, and reducing excitability by activating calcium-activated potassium channels could be a useful strategy to improve motor symptoms in these conditions.
Acknowledgments
Funding was provided through the NIH 1K08NS072158 (VG Shakkottai). Funding was provided through the University of Michigan Medical Scientist Training Program NIH Grant T32GM007863 (R Chopra).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• of considerable interest
- 1.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J. Neurophysiol. 1968;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 2.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin, Germany: Springer; 1974. [Google Scholar]
- 3.Snell RS. Clinical Neuroanatomy (7th Edition) PA, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 4.Ito M. The Cerebellum and Neural Control. NY, USA: Raven Press; 1984. [Google Scholar]
- 5.Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–351. doi: 10.1007/s12311-011-0247-4. [DOI] [PubMed] [Google Scholar]
- 6.Taroni F, Didonato S. Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 2004;5(8):641–655. doi: 10.1038/nrn1474. [DOI] [PubMed] [Google Scholar]
- 7.Magana JJ, Velazquez-Perez L, Cisneros B. Spinocerebellar ataxia type 2: clinical presentation, molecular mechanisms, and therapeutic perspectives. Mol. Neurobiol. 2013;47(1):90–104. doi: 10.1007/s12035-012-8348-8. [DOI] [PubMed] [Google Scholar]
- 8.Hersheson J, Haworth A, Houlden H. The inherited ataxias: genetic heterogeneity, mutation databases, and future directions in research and clinical diagnostics. Hum. Mutat. 2012;33(9):1324–1332. doi: 10.1002/humu.22132. [DOI] [PubMed] [Google Scholar]
- 9.Rub U, Schols L, Paulson H, et al. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog. Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J. Neurosci. 1999;19(5):1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausser M, Raman IM, Otis T, et al. The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J. Neurosci. 2004;24(42):9215–9219. doi: 10.1523/JNEUROSCI.3375-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin SI, Khaliq ZM, Aman TK, et al. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J. Neurophysiol. 2006;96(2):785–793. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Kovalchuk Y, Adelsberger H, et al. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc. Natl Acad. Sci. USA. 2010;107(27):12323–12328. doi: 10.1073/pnas.1001745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auburger GW. Spinocerebellar ataxia type 2. Handb. Clin. Neurol. 2012;103:423–436. doi: 10.1016/B978-0-444-51892-7.00026-7. [DOI] [PubMed] [Google Scholar]
- 15. Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum. Mol. Genet. 2013;22(2):271–283. doi: 10.1093/hmg/dds427. •• Very thorough longitudinal study of functional, structural and molecular changes in Purkinje cells in a mouse model of spinocerebellar ataxia type 2, demonstrating clearly that there are a number of functional changes in Purkinje cells that coincide with the development of early symptoms and precede morphologic changes
- 16.Kasumu AW, Hougaard C, Rode F, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem. Biol. 2012;19(10):1340–1353. doi: 10.1016/j.chembiol.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Swieten JC, Brusse E, De Graaf BM, et al. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia [corrected] Am. J. Hum. Genet. 2003;72(1):191–199. doi: 10.1086/345488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laezza F, Gerber BR, Lou JY, et al. The FGF14 (F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability. J. Neurosci. 2007;27(44):12033–12044. doi: 10.1523/JNEUROSCI.2282-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakkottai VG, Xiao M, Xu L, et al. FGF14 regulates the intrinsic excitability of cerebellar Purkinje neurons. Neurobiol. Dis. 2009;33(1):81–88. doi: 10.1016/j.nbd.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao M, Bosch MK, Nerbonne JM, Ornitz DM. FGF14 localization and organization of the axon initial segment. Mol. Cell. Neurosci. 2013;56:393–403. doi: 10.1016/j.mcn.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat. Rev. Neurosci. 2013;14(10):708–721. doi: 10.1038/nrn3570. [DOI] [PubMed] [Google Scholar]
- 22.Graveland GA, Williams RS, Difiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227(4688):770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- 23.Cowan CM, Raymond LA. Selective neuronal degeneration in Huntington’s disease. Curr. Top. Dev. Biol. 2006;75:25–71. doi: 10.1016/S0070-2153(06)75002-5. [DOI] [PubMed] [Google Scholar]
- 24.Bhide PG, Day M, Sapp E, et al. Expression of normal and mutant huntingtin in the developing brain. J. Neurosci. 1996;16(17):5523–5535. doi: 10.1523/JNEUROSCI.16-17-05523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fennema-Notestine C, Archibald SL, Jacobson MW, et al. In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology. 2004;63:989–995. doi: 10.1212/01.wnl.0000138434.68093.67. [DOI] [PubMed] [Google Scholar]
- 26.Fossale E, Seong IS, Coser KR, et al. Differential effects of the Huntington’s disease CAG mutation in striatum and cerebellum are quantitative not qualitative. Hum. Mol. Genet. 2011;20(21):4258–4267. doi: 10.1093/hmg/ddr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rub U, Hoche F, Brunt ER, et al. Degeneration of the cerebellum in Huntington’s disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013;23(2):165–177. doi: 10.1111/j.1750-3639.2012.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dougherty SE, Reeves JL, Lucas EK, Gamble KL, Lesort M, Cowell RM. Disruption of Purkinje cell function prior to huntingtin accumulation and cell loss in an animal model of Huntington disease. Exp. Neurol. 2012;236(1):171–178. doi: 10.1016/j.expneurol.2012.04.015. • Demonstrates very early changes in Purkinje neuron pacemaking from a Huntington’s disease model with in vivo recording, arguing that Purkinje neuron dysfunction probably contributes to motor impairment
- 29.Dougherty SE, Reeves JL, Lesort M, Detloff PJ, Cowell RM. Purkinje cell dysfunction and loss in a knock-in mouse model of Huntington disease. Exp. Neurol. 2013;240:96–102. doi: 10.1016/j.expneurol.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue S, Serra HG, Zoghbi HY, Orr HT. The spinocerebellar ataxia type 1 protein, ataxin-1, has RNA-binding activity that is inversely affected by the length of its polyglutamine tract. Hum. Mol. Genet. 2001;10(1):25–30. doi: 10.1093/hmg/10.1.25. [DOI] [PubMed] [Google Scholar]
- 31.Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 2010;6(7):e1001021. doi: 10.1371/journal.pgen.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hourez R, Servais L, Orduz D, et al. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J. Neurosci. 2011;31(33):11795–11807. doi: 10.1523/JNEUROSCI.0905-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012;103:437–449. doi: 10.1016/B978-0-444-51892-7.00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shakkottai VG, do Carmo Costa M, Dell’Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J. Neurosci. 2011;31(36):13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. • Work in spinocerebellar ataxia type 3 that demonstrates a strong link between altered Purkinje neuron pacemaking and degraded motor performance, and also identifies molecular changes in ion channels that could account for the changes in physiology
- 35.Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics. 2007;4(2):267–273. doi: 10.1016/j.nurt.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Baloh RW. Episodic ataxias 1 and 2. Handb. Clin. Neurol. 2012;103:595–602. doi: 10.1016/B978-0-444-51892-7.00042-5. [DOI] [PubMed] [Google Scholar]
- 37. Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 2006;9(3):389–397. doi: 10.1038/nn1648. •• One of the first studies looking at a dominantly-inherited ataxia, which suggested that altered pacemaking in Purkinje neurons may be the cause of degraded motor performance
- 38.Dick KA, Ikeda Y, Day JW, Ranum LP. Spinocerebellar ataxia type 5. Handb. Clin. Neurol. 2012;103:451–459. doi: 10.1016/B978-0-444-51892-7.00028-0. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda Y, Dick KA, Weatherspoon MR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 40.Perkins EM, Clarkson YL, Sabatier N, et al. Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J. Neurosci. 2010;30(14):4857–4867. doi: 10.1523/JNEUROSCI.6065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl. 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 42.Catarino CB, Liu JY, Liagkouras I, et al. Dravet syndrome as epileptic encephalopathy: evidence from long-term course and neuropathology. Brain. 2011;134(Pt 10):2982–3010. doi: 10.1093/brain/awr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from NaV1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J. Neurosci. 2007;27(41):11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J. Cell Biol. 1986;102(6):2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohara O, Ohara R, Yamakawa H, Nakajima D, Nakayama M. Characterization of a new beta-spectrin gene which is predominantly expressed in brain. Brain. 1998;57(2):181–192. doi: 10.1016/s0169-328x(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 46.Walter JT, Khodakhah K. The linear computational algorithm of cerebellar Purkinje cells. J. Neurosci. 2006;26(50):12861–12872. doi: 10.1523/JNEUROSCI.4507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Leary T, Williams AH, Caplan JS, Marder E. Correlations in ion channel expression emerge from homeostatic tuning rules. Proc. Natl Acad. Sci. USA. 2013;110(28):E2645–E2654. doi: 10.1073/pnas.1309966110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vega AV, Henry DL, Matthews G. Reduced expression of NaV1.6 sodium channels and compensation by NaV1.2 channels in mice heterozygous for a null mutation in Scn8a. Neurosci. Lett. 2008;442(1):69–73. doi: 10.1016/j.neulet.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 49.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002;22(23):10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]