Abstract
Objective
The goal of the present study was to investigate an association between early gestational C-reactive protein (CRP), an established inflammatory biomarker, prospectively assayed in maternal sera, and schizophrenia in a large national birth cohort with an extensive serum biobank.
Methods
This study utilized a nested case-control design from the Finnish Prenatal Study of Schizophrenia cohort. 777 schizophrenia cases (630 with schizophrenia, 147 with schizoaffective disorder) that had maternal sera available for CRP testing were identified and matched to 777 controls in the analysis. Maternal CRP levels were assessed using a latex immunoassay from archived maternal serum specimens.
Results
Increasing maternal CRP levels, classified as a continuous variable, were significantly associated with schizophrenia in offspring (adjusted odds ratio (OR)=1.31, 95% confidence interval (CI)=1.10-1.56, p=0.003). This finding remained significant after adjusting for potential confounders including maternal and parental history of psychiatric disorders, twin/singleton birth, urbanicity, province of birth, and maternal socioeconomic status.
Conclusion
This finding provides the most robust evidence to date that maternal inflammation may play a significant role in schizophrenia, with possible implications for identifying preventive strategies and pathogenic mechanisms in schizophrenia and other neurodevelopmental disorders.
Keywords: schizophrenia, prenatal, C-reactive protein, infection, inflammation, cytokines
Introduction
Mounting epidemiological and preclinical evidence implicates prenatal infection and subsequent immune activation in the etiology of schizophrenia (for review, see (1)). The most convincing epidemiologic studies were based on birth cohorts in which maternal biomarkers of infection and inflammation were assayed from prospectively archived maternal serologic specimens drawn during pregnancy. These studies revealed associations between offspring schizophrenia and elevated maternal antibody to influenza, rubella, toxoplasma gondii, and herpes simplex virus-2 (1-6). Associations have also been found using ecologic designs and ascertainment of pregnancies complicated by clinical infections (7-11). Inflammation during pregnancy may represent a common pathway by which different infections, as well as other early environmental insults, increase risk for the disorder. In support of this, additional birth cohort studies found that levels of two pro-inflammatory cytokines, interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNFα), were significantly elevated in maternal serum samples from pregnancies that gave rise to offspring who later developed schizophrenia (12-13). Moreover, many autoimmune diseases that result in a chronic inflammatory state were found to be associated with schizophrenia (14). Risk of schizophrenia was further increased for persons with autoimmune diseases that also experienced a severe infection, suggesting that multiple perturbations that produce inflammation may act synergistically (14).
Additionally, a large number of investigations using in vivo models of maternal immune activation in rodents have found that prenatal infection and subsequent inflammation produce brain and behavioral changes in offspring analogous to those seen in patients with schizophrenia and other neuropsychiatric disorders (for reviews see (15-17)). Maternal immune activation during pregnancy, induced by either direct infection with influenza virus or indirect stimulation of the maternal immune system using a viral (Polyinositic polycytidylic acid, Poly IC) or bacterial (lipopolysaccharide, LPS) mimic, results in behavioral deficits as well as neurochemical, morphological and anatomical changes in the offspring brains similar to brain abnormalities reported in schizophrenia (for review see (17)). The ability of maternal immune activation in the absence of a pathogenic microbe to mimic brain and behavioral changes produced by direct infection with live influenza virus provides strong evidence that activation of the maternal immune system is responsible for many of the effects of prenatal infection on offspring brain and behavior.
To test whether maternal inflammation during pregnancy is associated with schizophrenia in offspring, we examined the relationship between maternal C-reactive protein (CRP) and schizophrenia in the Finnish Prenatal Study of Schizophrenia. The Finnish Prenatal Study of Schizophrenia capitalizes on a large and representative sample of pregnancies from a national birth cohort with prospectively collected and archived maternal serum specimens from an extensive biobank and well-validated offspring diagnoses of virtually all schizophrenia cases in Finland from national registries of both hospital admissions and outpatient treatment. We chose to measure maternal CRP as it is a well-established and reliable general marker of inflammation from both infectious and non-infectious exposures (18). Thus, we tested the hypothesis that maternal inflammation, as indicated by increased levels of CRP in maternal serum during early to middle gestation, is related to an increased risk of schizophrenia in offspring.
Materials/Subjects and Methods
The Finnish Prenatal Study of Schizophrenia is based on a nested case-control design. This study is part of a larger program of research known as the Finnish Prenatal Studies, which aim to examine prenatal exposures in relation to major psychiatric outcomes including schizophrenia and autism. The sampling frame was defined so that all members of the cohort were within the age of risk for schizophrenia. For this purpose, the sampling frame consisted of all offspring born in Finland from 1983 (the beginning of the Finnish Maternity Cohort, noted in the next section) to 1998. Subjects were followed up until 2009 (see “Case and control identification”).
Description of the cohort and biobank
All offspring in the Finnish Prenatal Study of Schizophrenia were derived from the Finnish Maternity Cohort which consists of virtually all pregnancies with archived prenatal serum specimens that were drawn beginning in 1983. Sera were drawn during the first and early second trimesters from over 98% of pregnant women in Finland, following informed consent, for screening of HIV, syphilis, and hepatitis. One maternal serum sample was obtained for each pregnancy. Over the years of births in the study, sera from over one million pregnancies were drawn. After the screening, serum samples were stored as one aliquot at –25°C in a single, centralized biorepository at the National Institute of Health and Welfare (THL) in Oulu, Finland. All of the serum samples in the Finnish Maternity Cohort can be linked with offspring by a unique personal identification number (PIN), which has been assigned to all residents of Finland since 1971.
Case and control identification
In order to identify cases for the present study, we utilized the nationwide Finnish Hospital Discharge and Outpatient Registry. The Finnish Hospital Discharge and Outpatient Registry contains all recorded diagnoses for all psychiatric hospital and outpatient admissions. The registry was established in 1963; computerized data are available from 1987 to the present. All Finnish citizens are entitled to Finland's national health insurance, which is maintained by the state and financed through tax revenues. This registry covers all mental and general hospitals, as well as all inpatient wards of local health centers, military wards, prison hospitals, and private hospitals. The registry contains the hospital identification code, dates and length of stay, and primary diagnoses at discharge. Cases in the Finnish Maternity Cohort with a diagnosis of schizophrenia (ICD-10 F20) or schizoaffective disorder (ICD-10 F25) were followed up from 1998-2009 (heretofore these cases are referred to as “schizophrenia”). All diagnoses were made in accord with the WHO International Classification of Diseases (ICD), 10th revision (ICD-10). The age at onset was dated by the first recorded contact with a psychiatric facility with a diagnosis of schizophrenia or schizoaffective disorder. Diagnostic validity of schizophrenia based on the Finnish Hospital Discharge and Outpatient Registry was found to be excellent; in a previous validation study, 93% of patients with a Finnish registry diagnosis of schizophrenia were assigned a consensus diagnosis of schizophrenia or a schizophrenia spectrum disorder (19). These cases were linked to the Finnish Maternity Cohort by the PINs in order to identify the corresponding maternal serum specimens. We identified a total of 1,232 cases of schizophrenia. Among these, 777 schizophrenia cases (630 with schizophrenia, 147 with schizoaffective disorder) had sufficient maternal sera available to perform CRP testing. These cases were matched 1:1 to controls drawn from the birth cohort who were without schizophrenia, other nonaffective psychotic disorders, and bipolar disorder on date of birth (+/– 1 month), sex, and residence in Finland at the time of diagnosis.
Finnish Population Registry
The computerized Finnish Population Registry was created in 1971 when the nationwide centralized population registry was established. The registry contains comprehensive data on place of birth, twin/singleton birth, date of emigration, date of death, subject's place of residence, and biological parents including their birth dates.
The study was approved by the ethical committees of the hospital district of Southwest Finland, THL (which also included register linkage approval), and the Institutional Review Board of the New York State Psychiatric Institute. Informed consent was obtained prior to acquisition of all maternal serum specimens after the nature and possible consequences of the procedure and data derived from serum analyses were explained.
CRP assay
CRP measurements were carried out blind to case/control status. CRP was measured on the clinical chemistry analyzer Architect c8200 (Abbott Laboratories, Abbott Park, IL, USA) using a latex immunoassay (Sentinel, Milan, Italy) in the THL laboratory in Helsinki Finland, under the supervision of Dr. Leiviskä. During the course of the study, the precision between series expressed as the coefficient of variation (mean ± SD) was 5.1% ± 2.3% and the systematic error (bias) (mean ± SD) was 2.7% ± 7.4. Assay sensitivity was 0.10 mg/L.
Covariates
The covariates included maternal age, paternal age, number of previous births, socioeconomic status (based on maternal education), maternal and parental history of schizophrenia, other nonaffective psychotic disorders, affective or other psychiatric disorders (for a list of ICD codes used see footnote in Table 1), gestational week of the maternal blood draw, twin/singleton birth, urban/semi-urban/rural birth, and province at birth. All covariates except for gestational week of the blood draw were obtained from the Finnish Population Registry; gestational week was obtained from the Finnish Maternity Cohort. Each covariate was classified as in Tables 1 and 2.
Table 1.
Relationship between covariates and maternal C-reactive protein (CRP) levels (≥median, <median) in controls.
| CRP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariates | ≥median | <median | |||||||
| Mean | SD | Mean | SD | t | DF | p | |||
| Maternal age | 28.7 | 5.2 | 27.9 | 5.5 | −2.15 | 775 | 0.03 | ||
| Gestational week of blood draw1 | 11.3 | 3.9 | 10.2 | 4.9 | −2.4 | 670 | <0.001 | ||
| N | % | N | % | X2 | DF | p | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.48 | 1 | 0.49 | ||||||
| Male | 218 | 56.0 | 227 | 58.5 | 1.11 | 0.83-1.47 | |||
| Female | 171 | 44.0 | 161 | 41.5 | Ref | Ref | |||
| Previous births | 36.0 | 1 | <0.0001 | ||||||
| 0 | 109 | 28.0 | 190 | 49.0 | Ref | Ref | |||
| ≥1 | 280 | 72 | 198 | 51.0 | 0.4 | 0.30-0.55 | |||
| Maternal education2 | 0.46 | 3 | 0.93 | ||||||
| Less than high school | 87 | 22.5 | 87 | 22.5 | 1.02 | 0.72-1.73 | |||
| High school graduate | 222 | 57.4 | 217 | 56.2 | Ref | Ref | |||
| Bachelors degree | 52 | 13.4 | 58 | 15.0 | 1.14 | 0.75-1.73 | |||
| Masters/PhD degree | 26 | 6.7 | 24 | 6.2 | 0.94 | 0.53-1.70 | |||
| Family history | |||||||||
| Maternal SSD* | 5 | 1.3 | 9 | 2.3 | 1.17 | 1 | 0.28 | 1.82 | 0.61-5.49 |
| Parental SSD* | 12 | 3.1 | 13 | 3.4 | 0.04 | 1 | 0.83 | 1.09 | 0.49-2.42 |
| Maternal affective** | 31 | 8.0 | 30 | 7.7 | 0.02 | 1 | 0.90 | 0.97 | 0.57-1.63 |
| Parental affective** | 46 | 11.8 | 53 | 13.7 | 0.59 | 1 | 0.44 | 1.18 | 0.77-1.80 |
| Any maternal psychiatric disorder*** | 47 | 12.1 | 49 | 12.6 | 0.05 | 1 | 0.82 | 1.05 | 0.69-1.61 |
| Any parental psychiatric disorder*** | 80 | 20.6 | 89 | 23.0 | 0.64 | 1 | 0.42 | 1.15 | 0.82-1.62 |
| Twinning2 | 21 | 5.4 | 16 | 4.1 | 0.70 | 1 | 0.40 | 0.75 | 0.39-1.47 |
| Urbanicity | 9.35 | 2 | 0.01 | ||||||
| Urban | 206 | 59.8 | 232 | 53.0 | 1.57 | 1.14-2.15 | |||
| Semi-urban | 44 | 11.31 | 56 | 14.4 | 1.77 | 1.10-2.83 | |||
| Rural | 139 | 35.7 | 100 | 25.8 | Ref | Ref | |||
| Province of Birth | 1.56 | 3 | 0.67 | ||||||
| Southern Finland | 132 | 33.9 | 145 | 37.4 | 1.23 | 0.88-1.72 | |||
| Eastern Finland | 59 | 15.2 | 59 | 15.2 | 1.12 | 0.73-1.72 | |||
| Western Finland | 148 | 38.1 | 132 | 34.0 | Ref | Ref | |||
| Northern Finland | 50 | 12.9 | 52 | 13.4 | 1.17 | 0.74-1.84 |
SSD = Schizophrenia spectrum disorder and other nonaffective psychoses (ICD10: F20-25, F28-29; ICD9: 295, 297, 298.9X, 301.2C; ICD8: 295, 297, 298.20, 298.30, 298.99, 299)
ICD10: F30-34, F38-39; ICD9: 296, 300.4, 298.8A; ICD8: 296, 298.00, 298.10, 300.41
In addition to the above this includes: ICD10: F84, F40-45, F48, F50-53, F55, F59-66, F68-69, F99, F10-19; ICD9: 299, 300-300.3, 300.5-301.1, 301.2 excluding 301.2C, 301.3-301.9, 302, 307.1A, 307.4A, 307.4F, 307.4H, 307.5A, B, C & E, 307.8A, 307.9X, 309-309.1, 309.2 excluding A&B, 309.2D, E & F, 309.3-309.9 excluding 309.3A & 309.4A, 312.0A, 312.1-312.2, 312.3 excluding 312.3D, 312.4-312.9, 291-292, 303-305; ICD8: 308, 300.0-300.3, 300.4, 300.5-302.9, 305, 306.40, 306.50, 306.98, 307.99, 291, 303-304
105 missing (46≥median, 59<median)
4 missing (2≥median, 2<median)
Table 2.
Relationship between covariates and schizophrenia
| Covariates | Controls | Cases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | DF | p | |||
| Maternal age | 28.2 | 5.1 | 28.5 | 5.6 | −1.21 | 1552 | 0.23 | ||
| Gestational week of blood draw1 | 10.9 | 4.1 | 11.3 | 4.2 | −1.44 | 1340 | 0.18 | ||
| N | % | N | % | X2 | DF | p | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.00 | 1 | 1.0 | ||||||
| Male | 445 | 57.3 | 445 | 57.3 | 1.0a | - | |||
| Female | 332 | 42.7 | 332 | 42.7 | Ref | Ref | |||
| Previous births | 0.27 | 1 | 0.60 | ||||||
| 0 | 299 | 38.5 | 289 | 37.2 | Ref | Ref | |||
| ≥1 | 478 | 61.5 | 488 | 62.8 | 1.06 | 0.86-1.29 | |||
| Maternal education2 | 7.51 | 3 | 0.06 | ||||||
| Less than high school | 174 | 22.5 | 197 | 25.5 | 1.08 | 0.85-1.38 | |||
| High school graduate | 439 | 56.8 | 454 | 58.7 | Ref | Ref | |||
| Bachelors degree | 110 | 14.2 | 78 | 10.1 | 0.70 | 0.51-0.96 | |||
| Masters/PhD degree | 50 | 6.5 | 44 | 5.7 | 0.86 | 0.56-1.32 | |||
| Family history | |||||||||
| Maternal SSD* | 14 | 1.8 | 84 | 10.8 | 53.4 | 1 | <0.0001 | 8.0 | 4.15-15.44 |
| Parental SSD* | 25 | 3.2 | 129 | 16.6 | 78.0 | 1 | <0.0001 | 6.2 | 3.87-9.94 |
| Maternal affective | 61 | 7.9 | 162 | 20.9 | 53.4 | 1 | <0.0001 | 2.98 | 2.17-4.09 |
| Parental affective | 99 | 12.7 | 237 | 30.5 | 72.3 | 1 | <0.0001 | 2.92 | 2.32-3.81 |
| Any maternal psychiatric disorder | 96 | 12.4 | 251 | 32.3 | 89.14 | 1 | <0.0001 | 3.46 | 2.61-4.58 |
| Any parental psychiatric disorder | 169 | 21.8 | 386 | 49.7 | 131.98 | 1 | <0.0001 | 3.61 | 2.83-4.61 |
| Twinning3 | 37 | 4.8 | 15 | 1.9 | 9.63 | 1 | 0.002 | 0.31 | 0.21-0.72 |
| Urbanicity | 7.84 | 2 | 0.02 | ||||||
| Urban | 438 | 56.4 | 490 | 63.1 | 1.39 | 1.10-1.75 | |||
| Semi-urban | 100 | 12.9 | 93 | 12.0 | 1.15 | 0.82-1.61 | |||
| Rural | 239 | 30.8 | 194 | 25.0 | Ref | Ref | |||
| Province of Birth | 28.4 | 3 | <0.0001 | ||||||
| Southern Finland | 277 | 35.7 | 380 | 48.9 | 1.76 | 1.39-2.23 | |||
| Eastern Finland | 118 | 15.2 | 93 | 12.0 | 1.04 | 0.76-1.44 | |||
| Western Finland | 280 | 36.0 | 216 | 27.8 | Ref | Ref | |||
| Northern Finland | 102 | 13.1 | 88 | 11.3 | 1.13 | 0.80-1.60 |
SSD = Schizophrenia spectrum disorder and other nonaffective psychoses
OR is 1 by definition because the groups are matched on sex
212 missing (107 cases, 105 controls)
10 missing (4 cases, 6 controls)
8 missing (4 cases, 4 controls)
Statistical analysis
The analysis was based on a nested case-control design in which the controls for each case were matched from the population at risk (the Finnish Prenatal Study of Schizophrenia birth cohort) on selected factors, elaborated in “Case and control identification.” In the main analysis, we examined maternal CRP as a continuous measure. Given the skewed distribution of CRP, the variable was log-transformed before analysis.
In order to further facilitate interpretation of the data, we conducted an additional analysis with maternal CRP as a categorical variable. CRP levels ≥10 mg/L are considered clinically abnormal (18). Therefore, we examined the risk of developing schizophrenia among offspring of mothers with CRP levels ≥10 mg/L in relation to those with levels <10 mg/L.
Appropriate to the nested case-control study design, point and interval estimates of odds ratios were obtained by fitting conditional logistic regression models for matched sets. Statistical significance was judged at p<0.05. After examining the main effects, we then investigated whether the effect of maternal CRP on schizophrenia risk was modified by sex. For this purpose, sex and sex by CRP interaction terms were added to the statistical model. The interaction terms were deemed to be statistically significant based on p< 0.05. Statistical analyses were performed with SAS software (SAS 9.2, SAS Institute, Cary, NC, USA).
Results
The 777 schizophrenia cases that were assayed for maternal CRP did not differ from the 737 cases that were not assayed on maternal age (p=0.36), gestational week of blood draw (p=0.29), previous number of births (p=0.70), maternal socioeconomic status (p=0.50), maternal psychiatric disorders (p=0.36), parental psychiatric disorders (p=0.20), number of twin births (p=0.49), or urbanicity (p=0.89). There was a trend for a difference in province of birth (p=0.07), with a slight underrepresentation of the Northern Finland Province among those included in the sample.
Covariates
Increased maternal age, increased number of previous births, greater gestational week of the blood draw, and birth in a rural area were all significantly associated with increased CRP (Table 1). As expected, maternal and parental history of several psychiatric disorders, including schizophrenia and affective disorders, were significantly associated with schizophrenia in offspring (Table 2). In addition, twin/singleton birth, urbanicity, and province of birth were significantly associated with schizophrenia in offspring. Maternal education was associated with schizophrenia at the trend level (p=0.06, Table 2).
Maternal CRP and schizophrenia
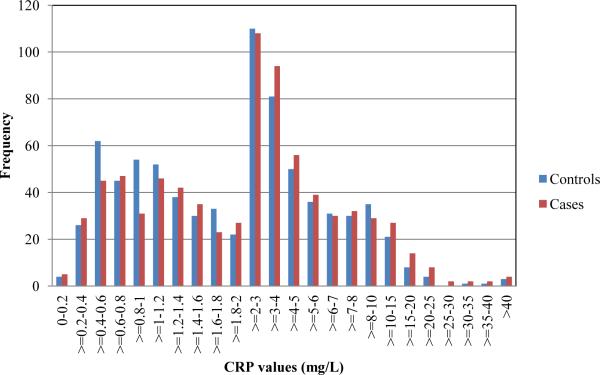
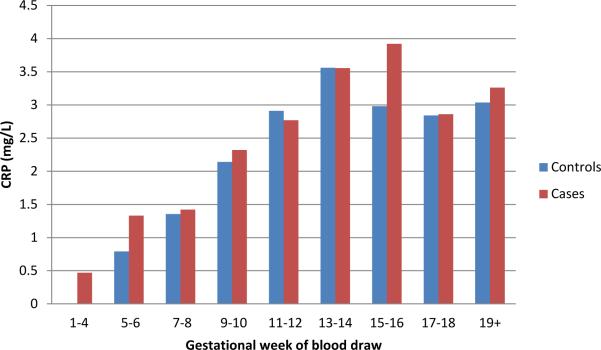
In the unadjusted analysis, there was a significant association between increasing maternal CRP and risk of schizophrenia (OR=1.12, 95% CI=1.02-1.24, p=0.019). Inspection of the distribution of maternal CRP values for cases and controls revealed that in most of the sampling intervals in the highest 50th percentile of CRP values, there was an over-representation of cases, relative to controls, and, as expected, in most of the sampling intervals in the lowest 50th percentile of CRP values, there was an under-representation of cases, relative to controls. This indicates that the relationship was not driven by a difference between cases and controls only among the most highly elevated values (Figure 1). Overall, median maternal CRP level for cases was 2.47 mg/L and for controls was 2.17 mg/L. Although no covariate was associated with both maternal CRP and schizophrenia, for further reassurance, we adjusted for each covariate following the unadjusted analysis. The magnitude of the association was increased following adjustment for maternal age, previous births, maternal education, parental psychiatric disorders, urbanicity of birth, province of birth, twin/singleton birth, and gestational week of the blood draw (OR=1.28, 95% CI=1.07-1.54, p=0.007). This finding indicates that for every 1 mg/L increase in maternal CRP, the risk of schizophrenia was increased by 28%. Additionally, maternal CRP levels rise with increasing gestational week of the blood draw for both cases and controls (Figure 2). There appears to be an elevation in maternal CRP levels in cases, relative to controls, at both earlier and later gestational sampling points.
Figure 1.
Distribution of CRP values for cases and controls. The graph depicts the frequency of cases and controls with CRP values (mg/L) in the given intervals.
Figure 2.
Median CRP values for cases and controls by gestational week of the blood draw. The graph shows the median CRP value (mg/L) for cases and controls with maternal serum obtained in the indicated range of gestational weeks.
In a secondary analysis, we examined whether offspring of mothers with CRP levels ≥10 mg/L (a level that is considered clinically abnormal (18)) had an increased risk of developing schizophrenia relative to those with maternal levels <10 mg/L. We found that offspring of mothers with CRP levels ≥10 mg/L had an increased risk of developing schizophrenia (OR=1.58, 95% CI=1.04-2.40, p=0.03). Adjusted for maternal age, previous births, maternal education, parental psychiatric disorders, urbanicity of birth, province of birth, twin/singleton birth, and gestational week of the blood draw, the magnitude of this effect was modestly attenuated, but lost statistical significance (OR=1.43, 95% CI=0.83-2.46, p=0.20).
Maternal CRP and schizophrenia by sex of offspring
Given the established differences in risk of schizophrenia by sex, we conducted a supplementary analysis to assess effect modification by sex on the relationship between maternal CRP and risk of schizophrenia. There was no sex by CRP interaction in predicting schizophrenia (p=0.13)
Discussion
Elevated maternal CRP during pregnancy is associated with an increased risk of schizophrenia in offspring. This finding was not confounded by maternal age, previous births, maternal education, parental psychiatric disorders, urbanicity of birth, province of birth, twin/singleton birth, and gestational week of the blood draw. Given the design advantages elaborated below, this study provides the most robust evidence to date that maternal inflammation during pregnancy is related to the risk of schizophrenia in the offspring, and is consistent with many preclinical studies (reviewed in the Introduction) that have suggested a causal association.
There are several biologically plausible hypotheses to account for this association. First, CRP may be acting as a proxy for the inflammatory cytokine, interleukin-6 (IL-6) (20). Preclinical studies indicate that IL-6 may mediate some of the effects of maternal immune activation on schizophrenia-related behavioral phenotypes in rodents (21-23). Following maternal injection of Poly IC or LPS, levels of several pro-inflammatory cytokines, including IL-6, are elevated in the maternal serum, the placenta and possibly in the brain of the developing offspring, although this last finding remains controversial (for review see (17)). Indeed, Smith and colleagues elegantly demonstrated that the pro-inflammatory cytokine, interleukin-6 (IL-6), is both necessary and sufficient to produce the behavioral abnormalities in sensorimotor gating and latent inhibition observed in adult offspring of mothers given an injection of Poly IC during pregnancy (23). We were not able to quantify IL-6 in the present study given the sample volume requirements for this assay. In addition to acting as a proxy for IL-6, CRP might also affect fetal development through certain mediating factors. Clinical studies have shown that increased CRP in pregnant women is associated with conditions such as preeclampsia as well as with pre-term birth and lower birth weight (24-26), each of which are related to adverse early or later reproductive outcomes. In fact, it has been hypothesized that CRP itself may contribute to placental dysfunction in preeclampsia by eliciting endothelial dysfunction, resulting in vascular damage and impaired placental development (27-28). Finally, CRP may directly affect brain development. CRP is involved in the complement cascade, activation of which is known to play a role in normal synaptic pruning and refinement during development (29). If elevated levels of maternal CRP result in increased levels of CRP in the developing offspring brain, it could conceivably alter synaptic connectivity in a way that increases risk for the development of psychopathology.
Though no previous study has examined maternal CRP in relation to schizophrenia in offspring, maternal cytokines have been investigated in two prior birth cohort studies. Maternal tumor necrosis factor-alpha (TNFα) levels were related to a significantly increased risk of schizophrenia and other psychotic disorders (N=27 cases) in offspring from the National Collaborative Perinatal Project (13). Significantly elevated maternal interleukin-8 (IL-8) levels were related to an elevated risk of schizophrenia and other schizophrenia spectrum disorders (consisting mostly of schizoaffective disorder) (N=59 cases) in the Child Health and Development Study birth cohort (12).
Other study strengths included CRP levels from prospectively drawn archived maternal serum specimens. Moreover, the specimens were drawn during early to middle pregnancy, rather than at delivery or in the neonate, allowing for a greater focus on prenatal influences. In addition, case ascertainment was facilitated by the socialized health care system of Finland, which covers all individuals who seek treatment for schizophrenia, encoded in national psychiatric registries. This allowed us to obtain nearly all schizophrenia cases diagnosed in Finland in a national population-based birth cohort. In addition, the Finnish population registry permitted the identification of controls who are representative of the source population that gives rise to the cases. These methodological features minimize the potential for selection bias.
There are multiple exposures that could elevate levels of maternal serum CRP, including both infectious and non-infectious insults. The fact that risk of developing schizophrenia was elevated among offspring of mothers with CRP ≥10 mg/L (relative to offspring of mothers with CRP <10 mg/L) argues that elevated CRP may reflect a recent infection or an active inflammatory process, as CRP ≥ 10 mg/L is considered clinically abnormal (18). This interpretation is consistent with an extensive literature documenting an increased risk of schizophrenia among offspring whose mothers experienced an infection during pregnancy (for review see (1)). However, the fact that we also found a significant increase in risk for schizophrenia for CRP levels expressed as a continuous variable (following log transformation) may indicate that even mildly elevated levels of CRP, which might reflect a low-grade inflammatory process, are related to schizophrenia in offspring. In either case, it seems likely that elevated levels of maternal CRP, and the underlying immunological activation they likely reflect, interact with other environmental insults to give rise to this disorder. This hypothesis is supported by a recent preclinical study in rodents demonstrating that mild maternal immune activation during pregnancy interacted with subsequent peri-pubertal stress in the offspring to give rise to behavioral abnormalities in adulthood considered to be analogous to those found in schizophrenia (30). Alternatively, elevated maternal CRP levels may reflect genetic, rather than environmental, factors. Several common polymorphisms in the CRP gene have been associated with elevated levels of serum CRP (31). Future work will be necessary to assess interactions between maternal infection, peri-pubertal stress and other postnatal environmental risk factors, as well as with genetic susceptibility, in schizophrenia.
Limitations of this study include the following. First, although there was no evidence of confounding following extensive testing of many covariates, residual confounding by unmeasured factors may have occurred. Second, due to the fact that the sample was born in 1983 and the last follow-up year was 2009, our sample was relatively young, with a mean age for cases and controls of 22.8 years (standard deviation = 2.2 years) and a mean age at first treatment for cases of 19.0 years (standard deviation = 2.7 years). Thus, it is possible that CRP is a risk factor for earlier-onset cases of schizophrenia. Additionally, CRP may be a risk modifier rather than a risk factor for schizophrenia; in this scenario, elevated CRP would be related to an earlier onset of the disease in persons who were at risk for other reasons.
Although the primary goal of this analysis was to investigate an association between maternal CRP and later risk of schizophrenia in the offspring, in the course of testing for confounding we demonstrated associations in this cohort between family psychiatric history, twinning, urbanicity and province of birth and schizophrenia (Table 2). We wish to briefly discuss these covariates. The increased risk of schizophrenia for subjects with a parent with either a schizophrenia spectrum or non-affective disorder or a parent with any type of psychiatric diagnosis was expected given the prior literature. Specifically, it has been demonstrated that having a mother or a parent with schizophrenia increases the offspring risk of schizophrenia by 6 to 8-fold (32-33). Our finding of a decreased risk of schizophrenia in twin, relative to singleton pregnancies differs from a previous study in a Danish sample, which found that twins had a 26% increased risk of schizophrenia relative to offspring of singleton, pregnancies (34). Another study, in a West African population, found that twinning had no relationship to schizophrenia risk (35). Potential explanations for these varying results include an interaction of this variable with genetic background, or mediation by levels of prenatal care and obstetric complications, which could vary by the country of birth. The finding in this study of an association between urbanicity and schizophrenia has also been demonstrated in numerous prior epidemiologic studies (33, 36). While the reasons for this association are unknown, hypothesized mediators include increased risk of infections or other factors related to urbanicity, selective migration, and increased access to psychiatric services (37). Finally, our finding that living in the Southern Province of Finland increased risk of developing schizophrenia was unexpected. In fact, a previous study reported that the prevalence of psychoses was highest in Northern and Eastern Finland (38), so understanding the origin of our finding will require further studies.
While the covariates discussed above were associated with increased risk of schizophrenia, they did not confound the relationship between maternal CRP and schizophrenia as none of them were independently related to levels of maternal CRP (Table 1) and the association between maternal CRP and schizophrenia remained significant after adjusting for all of these covariates. It is still possible, however, that these covariates might be effect modifiers of the relationship between maternal CRP and schizophrenia. For example, maternal CRP might interact with familial risk or urbanicity to increase risk of this disorder. Although outside the scope of this paper, we aim to explore these questions in future work.
Moreover, elevated maternal CRP may not be a risk factor that is specific to schizophrenia. In the same Finnish national birth cohort that was investigated in the present study, our group demonstrated a significant increase in maternal CRP in pregnancies that gave rise to childhood autism (autistic disorder) cases (39). In support of an early inflammatory contribution to the pathophysiology of autism, several other studies have found evidence that severe maternal infection during pregnancy or elevated levels of inflammatory signaling molecules in the amniotic fluid or neonate are associated with an increased risk of autism (40-43), although there are also negative reports (44-45). While we have not yet investigated maternal CRP and bipolar or major affective disorder in this cohort, there is evidence that maternal infection is a risk factor for development of these disorders in offspring, suggesting that an inflammatory component may also play a role in these outcomes (46-48).
It is intriguing to speculate that maternal inflammation during pregnancy may ‘prime’ the brain to broadly increase risk for the later development of different types of psychiatric syndromes. This is consistent with preclinical studies which have demonstrated that maternal immune activation during pregnancy produces offspring with behavioral and brain phenotypes that are most likely relevant to multiple psychiatric disorders, especially schizophrenia and autism (15, 17). Interaction with specific genetic or environmental insults, during particular developmental windows, might then determine the specificity of the later disorder. This hypothesis can be tested in future studies.
In conclusion, we have demonstrated that elevated maternal CRP is related to an increased risk of schizophrenia in offspring. These findings are consistent with an extensive preclinical literature on maternal immune activation and brain and behavioral abnormalities in animal models, as well as with epidemiologic evidence of maternal infection and immunologic dysfunction as risk factors for schizophrenia. If replicated, these findings may have important implications for elaborating the role of immune system dysfunction in schizophrenia. Finally, our finding that maternal CRP is associated with an increased risk of schizophrenia in the offspring has important implications for disease prevention, given that many standard approaches already exist to reduce the incidence of infections, or lessen the severity of the inflammation that they produce (49).
Acknowledgements
This manuscript was supported by R01 MH082052-05 (A.S.B.), K02 MH065422-09 (A.S.B.) from the National Institute of Mental Health and the State Research Institute (THL), T32 MH16434-31 (S.E.C) from the National Institute of Mental Health, a Brain & Behavior Research Foundation Young Investigator Grant (S.E.C), and the Sackler Institute Fellowship (S.E.C.).
We wish to acknowledge the Finnish Maternity Cohort laboratory staff for retrieving and preparing the samples for analysis, and Jacky Chow for manuscript preparation.
Footnotes
Disclosures: None
Author Contributions
Dr. Canetta contributed to hypothesis generation, study design, data analysis, and manuscript writing.
Dr. Sourander contributed to hypothesis generation, study design, data collection, and manuscript writing.
Dr. Surcel contributed to hypothesis generation, study design, data collection, and manuscript writing.
Dr. Hinkka-Yli Salomäki contributed to the study design, data analysis, and manuscript writing.
Dr. Leiviskä led the CRP assay and contributed to manuscript writing.
Dr. Kellendonk contributed to study design, and manuscript writing.
Dr. McKeague contributed to the study design, data analysis, and manuscript writing.
Dr. Brown contributed to hypothesis generation, study design, data analysis, and manuscript writing.
No data are reported in any outside source.
The authors have no conflicts of interest.
References
- 1.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AS, Schaefer CA, Quesenberry CP, Jr., Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–73. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 3.Brown AS, Cohen P, Greenwald S, Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry. 2000;157(3):438–43. doi: 10.1176/appi.ajp.157.3.438. [DOI] [PubMed] [Google Scholar]
- 4.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 5.Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63(8):809–15. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen PB, Pedersen CB, Hougaard DM, Nørgaard-Petersen B, Mors O, Børglum AD, et al. A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in thier offspring. Schizophr Res. 2010;122(1-3):257–63. doi: 10.1016/j.schres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–92. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 8.O'Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337(8752):1248–50. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 9.Sham PC, O'Callaghan E, Takei N, Murray GK, Hare EH, Murray RM. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–6. doi: 10.1192/bjp.160.4.461. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35(3):631–7. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166(9):1025–30. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 12.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161(5):889–95. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 13.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15(4):411–20. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- 14.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30- year population-based register study. Am J Psychiatry. 2011;168(12):1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 15.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33(7):1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24(6):881–97. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 19.Makikyro T, Isohanni M, Moring J, Hakko H, Hovatta I, Lonnqvist J. Accuracy of register-based schizophrenia diagnoses in a genetic study. Eur Psychiatry. 1998;13(2):57–62. doi: 10.1016/S0924-9338(98)80019-9. [DOI] [PubMed] [Google Scholar]
- 20.Karlovic D, Serretti A, Vrkic N, Martinac M, Marcinko D. Serum concentrations of CRP, IL-6, TNF-alpha and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012;198(1):74–80. doi: 10.1016/j.psychres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–15. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11(1):47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 23.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst GD, de Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA, et al. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. Am J Obstet Gynecol. 2011;205(2):132, e1–12. doi: 10.1016/j.ajog.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma IL-4, IL-8, IL-12, interferon-gamma and CRP levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birth weight. J Matern Fetal Neonatal Med. 2012;25(9):1569–73. doi: 10.3109/14767058.2011.648233. [DOI] [PubMed] [Google Scholar]
- 26.Mihu D, Costin N, Mihu CM, Blaga LD, Pop RB. C-reactive protein, marker for evaluation of systemic inflammatory response in preeclampsia. Rev Med Chir Soc Med Nat Iasi. 2008;112(4):1019–25. [PubMed] [Google Scholar]
- 27.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 28.Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol. 2004;24(6):565–70. doi: 10.1016/s0270-9295(04)00127-5. [DOI] [PubMed] [Google Scholar]
- 29.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 30.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–9. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 31.Flores-Alfaro E, Fernandez-Tilapa G, Salazar-Martinez E, Cruz M, Illades- Aguiar B, Parra-Rojas I. Common variants in the CRP gene are associated with serum C-reactive protein levels and body mass index in healthy individuals in Mexico. Genetics and molecular research : GMR. 2012;11(3):2258–67. doi: 10.4238/2012.May.14.5. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen PB, Pedersen MG, Pedersen CB. Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol Med. 2010;40(2):201–10. doi: 10.1017/S0033291709990419. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340(8):603–8. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 34.Klaning U, Mortensen PB, Kyvik KO. Increased occurrence of schizophrenia and other psychiatric illnesses among twins. Br J Psychiatry. 1996;168(6):688–92. doi: 10.1192/bjp.168.6.688. [DOI] [PubMed] [Google Scholar]
- 35.Sirugo G, Ashenbrenner J, Odunsi K, Morakinyo O, Page G. No evidence of association between the genetic predisposition for dizygotic twinning and schizophrenia in West Africa. Schizophr Res. 2004;70(2-3):343–4. doi: 10.1016/j.schres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Lewis G, David A, Andreasson S, Allebeck P. Schizophrenia and city life. Lancet. 1992;340(8812):137–40. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 37.Maki P, Veijola J, Jones PB, Murray GK, Koponen H, Tienari P, et al. Predictors of schizophrenia--a review. Br Med Bull. 2005;73-74:1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- 38.Lehtinen V, Joukamaa M, Lahtela K, Raitasalo R, Jyrkinen E, Maatela J, et al. Prevalence of mental disorders among adults in Finland: basic results from the Mini Finland Health Survey. Acta psychiatrica Scandinavica. 1990;81(5):418–25. doi: 10.1111/j.1600-0447.1990.tb05474.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 41.Grether JK, Croen LA, Anderson MC, Nelson KB, Yolken RH. Neonatally measured immunoglobulins and risk of autism. Autism Res. 2010;3(6):323–32. doi: 10.1002/aur.160. [DOI] [PubMed] [Google Scholar]
- 42.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2013;14(7):528–38. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- 44.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252(1-2):75–82. doi: 10.1016/j.jneuroim.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–54. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machon RA, Mednick SA, Huttunen MO. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1997;54(4):322–8. doi: 10.1001/archpsyc.1997.01830160040006. [DOI] [PubMed] [Google Scholar]
- 47.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational Influenza and Bipolar Disorder in Adult Offspring. JAMA Psychiatry. 2013:1–8. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- 48.Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, Terajima M, et al. Serological Documentation of Maternal Influenza Exposure and Bipolar Disorder in Adult Offspring. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suvisaari JM, Haukka JK, Tanskanen AJ, Lonnqvist JK. Decline in the incidence of schizophrenia in Finnish cohorts born from 1954 to 1965. Arch Gen Psychiatry. 1999;56(8):733–40. doi: 10.1001/archpsyc.56.8.733. [DOI] [PubMed] [Google Scholar]