Abstract
Salmonella enteritidis14C-endotoxin was recovered predominantly from the nuclear and mitochondrial subcellular fractions of livers and spleens of mice and rats, 3.5 hr and 3 days after intravenous administration. Of the recovered radioactivity, 10 to 20% was present in the liver mitochondrial fraction as high-molecular-weight, biologically active material, suggesting the presence of intact endotoxin. Autoradiographic studies demonstrated nuclear and cytoplasmic labeling in the liver and at least nuclear label in spleen cells. The resistance of rats, as compared to mice, to the induction of amyloidosis does not appear to be based on a difference in subcellular localization of endotoxin within the reticuloendothelial system.
Full text
PDF

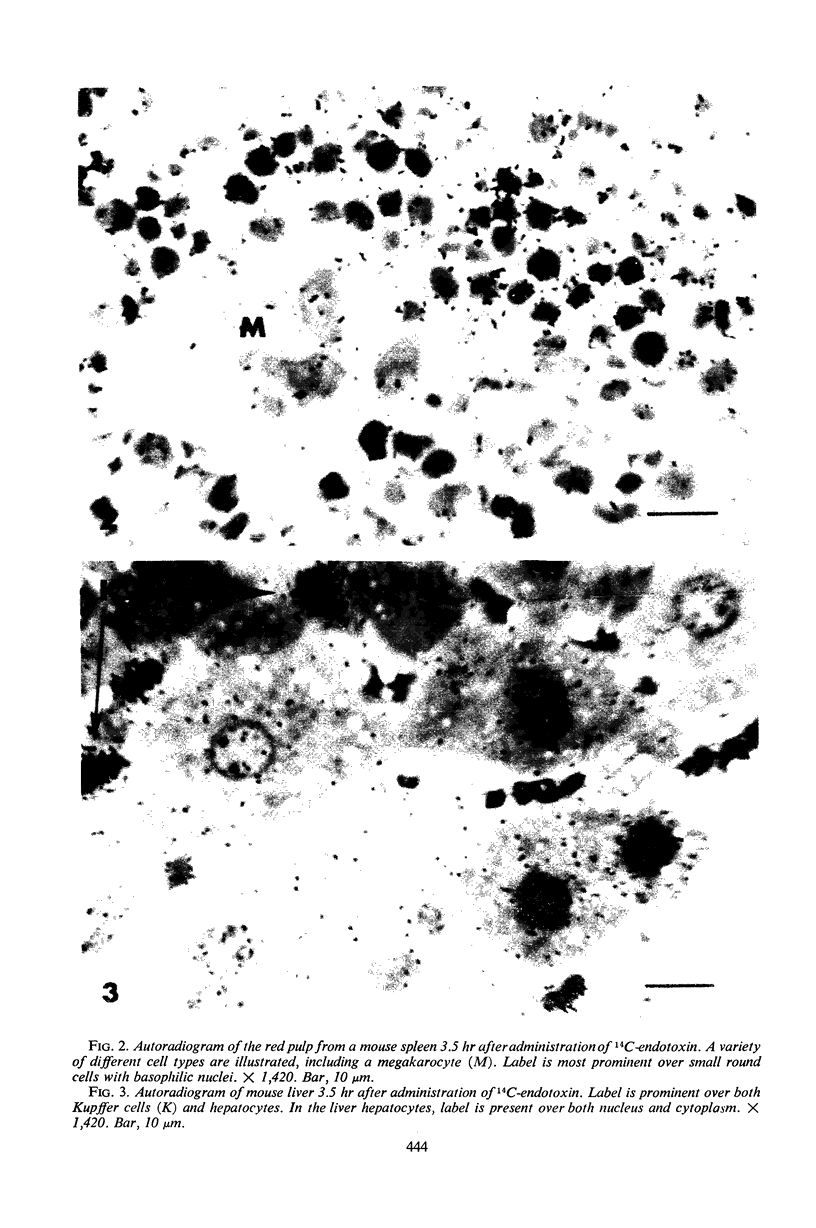

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth W. F., Gordon J. K., Willerson J. T. Amyloidosis induced in mice by Escherichia coli endotoxin. Science. 1968 Nov 8;162(3854):694–695. doi: 10.1126/science.162.3854.694. [DOI] [PubMed] [Google Scholar]
- CAREY F. J., BRAUDE A. I., ZALESKY M. Studies with radioactive endotoxin. III. The effect of tolerance on the distribution of radioactivity after intravenous injection of Escherichia coli endotoxin labeled with Cr51. J Clin Invest. 1958 Mar;37(3):441–457. doi: 10.1172/JCI103624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUNCE A. L., WITTER R. F., MONTY K. J., PATE S., COTTONE M. A. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J Biophys Biochem Cytol. 1955 Mar;1(2):139–153. doi: 10.1083/jcb.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- RIBI E., HASKINS W. T., LANDY M., MILNER K. C. Preparation and host-reactive properties of endotoxin with low content of nitrogen and lipid. J Exp Med. 1961 Nov 1;114:647–663. doi: 10.1084/jem.114.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF S. M., MULHOLLAND J. H., WARD S. B. QUANTITATIVE ASPECTS OF THE PYROGENIC RESPONSE OF RABBITS TO ENDOTOXIN. J Lab Clin Med. 1965 Feb;65:268–275. [PubMed] [Google Scholar]
- Willerson J. T., Gordon J. K., Talal N., Barth W. F. Murine amyloid. II. Transfer of an amyloid-accelerating substance. Arthritis Rheum. 1969 Jun;12(3):232–240. doi: 10.1002/art.1780120311. [DOI] [PubMed] [Google Scholar]