Abstract
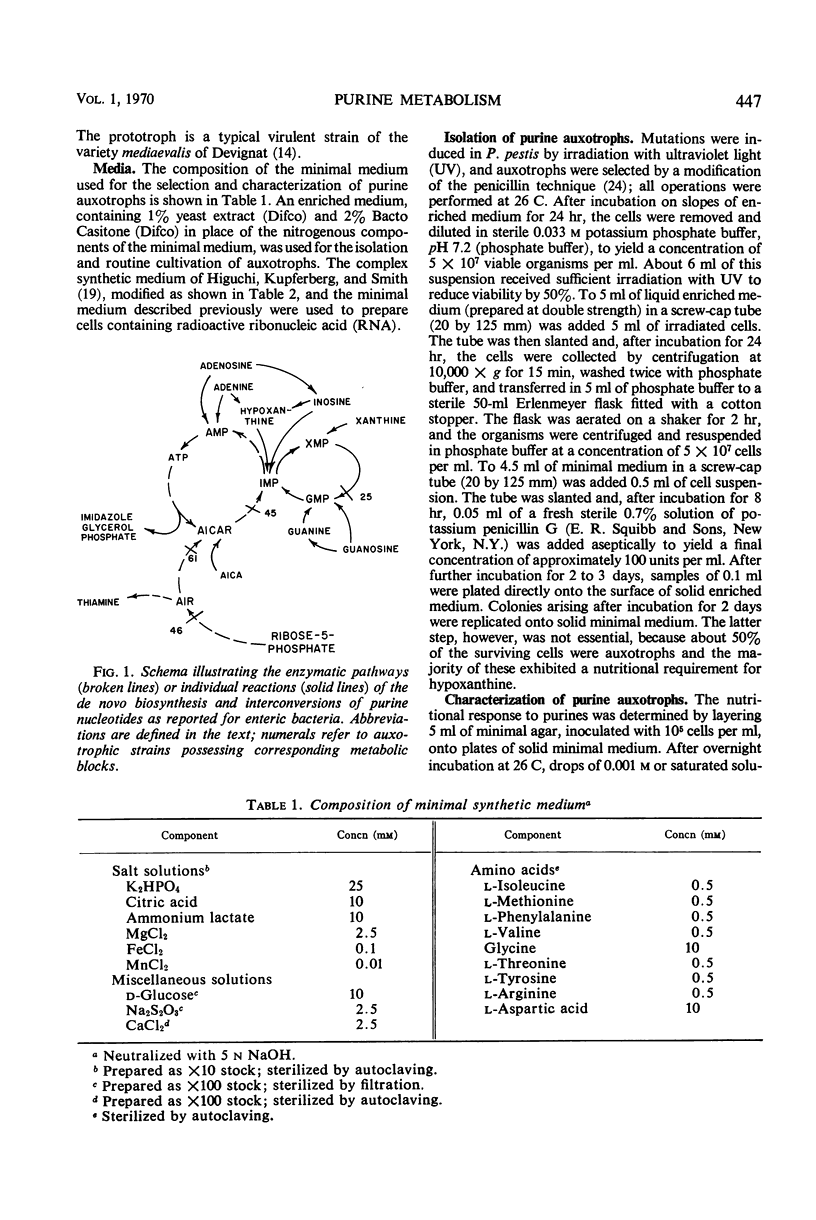
Included among the five established determinants of virulence in Pasteurella pestis are the abilities of cells to accomplish the de novo biosynthesis of purines and to grow as dark pigmented (P+) colonies on a solid synthetic medium containing hemin. P+ isolates of P. pestis strain KIM-10 (mouse intraperitoneal ld50 < 10 cells) failed to convert exogenous guanine-8-14C to adenine residues of ribonucleic acid (RNA) when cultivated in a minimal medium which favored the pigmentation reaction. This conversion occurred in P+ cells grown in an enriched medium which did not support the pigmentation reaction and was observed in P− mutants cultivated in both types of media. Both P+ and P− isolates converted exogenous adenine-8-14C but not adenine-2-14C at a significant rate to guanosine residues of RNA when grown under a variety of conditions. This difference appeared to reflect a deficiency of adenine deaminase. The mouse intraperitoneal ld50 of purine-auxotrophs was about 102 cells when the metabolic block occurred prior to the de novo formation of inosine monophosphate (IMP). In contrast, the corresponding value for a mutant blocked between IMP and guanine monophosphate was > 107 cells in mice and > 108 cells in guinea pigs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., HUTCHISON D. J. REPRESSION BY ADENINE OF THE FORMYLTETRAHYDROFOLATE SYNTHETASE IN AN ANTIFOLIC-RESISTANT MUTANT OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1964 Apr;87:792–798. doi: 10.1128/jb.87.4.792-798.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Genotypic alterations associated with avirulence in streptomycin-resistant Pasteurella pestis. J Bacteriol. 1962 Oct;84:615–624. doi: 10.1128/jb.84.4.615-624.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W. Genetics of virulence in bacteria. Br Med Bull. 1962 Jan;18:69–73. doi: 10.1093/oxfordjournals.bmb.a069938. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. VIRULENCE OF PASTEURELLA PESTIS AND IMMUNITY TO PLAGUE. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1963;37:59–113. doi: 10.1007/978-3-662-36742-1_2. [DOI] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J Infect Dis. 1967 Dec;117(5):403–417. doi: 10.1093/infdis/117.5.403. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVIGNAT R. Variétés de l'espèce Pasteurella pestis; nouvelle hypothèse. Bull World Health Organ. 1951;4(2):247–263. [PMC free article] [PubMed] [Google Scholar]
- Garber E. D., Hackett A. J., Franklin R. The Virulence of Biochemical Mutants of Klebsiella Pneumoniae. Proc Natl Acad Sci U S A. 1952 Aug;38(8):693–697. doi: 10.1073/pnas.38.8.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen J W, Stadler J, Plough H H, Miller H N. Virulence and Immunizing Capacity of Salmonella Typhimurium as Related to Mutations in Metabolic Requirements. Genetics. 1953 Nov;38(6):531–549. doi: 10.1093/genetics/38.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., CARLIN C. E. Studies on the nutrition and physiology of Pasteurella pestis. II. A defined medium for the growth of Pasteurella pestis. J Bacteriol. 1958 Apr;75(4):409–413. doi: 10.1128/jb.75.4.409-413.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivánovics G., Marjai E. Die Virulenz der Purin-Auxotrophen des Bacillus anthracis. Zentralbl Bakteriol Orig. 1964 Jul;193(3):363–375. [PubMed] [Google Scholar]
- Ivánovics G., Marjai E., Dobozy A. The growth of purine mutants of Bacillus anthracis in the body of the mouse. J Gen Microbiol. 1968 Sep;53(2):147–162. doi: 10.1099/00221287-53-2-147. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., de Haan P. G., Nijkamp H. J. Mapping of purine markers in Escherichia coli K 12. Genet Res. 1965 Nov;6(3):442–453. doi: 10.1017/s0016672300004328. [DOI] [PubMed] [Google Scholar]



