Abstract
Background
The aim of this study was to analyze the time-varying pattern of recurrence risk of early-stage (T1a-T2bN0M0) non-small cell lung cancer (NSCLC) after surgery using the hazard function and identify patients who might benefit from adjuvant chemotherapy.
Patients and Methods
This retrospective study enrolled 994 patients with early-stage NSCLC who underwent radical surgical resection between January 1999 and October 2009. Survival curves were generated using the Kaplan-Meier method, and the annual recurrence hazard was estimated using the hazard function.
Results
The median recurrence-free survival (RFS) was 8.8 years. The life table survival analysis showed that the 1-year, 3-year, 5-year and 10-year recurrence rates were 82.0%, 67.0%, 59.0% and 48.0%, respectively. Approximately 256 (25.7%) patients experienced relapse [locoregional: 32 (3.2%) and distant: 224 (22.5%)], and 162 patients died from cancer. The annual recurrence hazard curve for the entire population showed that the first major recurrence surge reached a maximum 1.6 years after surgery. The curve subsequently declined until reaching a nadir at 7.2 years. A second peak occurred at 8.8 years. An analysis of clinical-pathological factors demonstrated that this double-peaked pattern was present in several subgroups.
Conclusions
The presence of a double-peaked pattern indicates that there is a predictable temporal distribution of the recurrence hazard of early-stage NSCLC. The annual recurrence hazard may be an effective method of selecting patients at high risk of recurrence, who may benefit from adjuvant therapy.
Introduction
Screening with low-dose computed tomography (CT) has resulted in a growing number of early-stage non-small cell lung cancer (NSCLC) diagnoses [1]. Although curative surgical resection is the current treatment of choice for early-stage NSCLC, the risk of locoregional and distant relapse remains high, at 22%–40% [2]–[4]. Thus, understanding the clinical course of postoperative recurrent disease is essential for guiding effective treatment.
In most studies, the risk of recurrence is analyzed using survival curves rather than hazard functions [5], [6]. The hazard function, which depicts the rate of recurrence at any point in time among the remaining at-risk individuals, has been applied to provide insights into the patterns of recurrence of breast cancer [7], [8] and gastric cancer [9]. The hazard function describes not only the magnitude of the recurrence rate but also how it changes over time [7], [10].
To our knowledge, the present work is the first study of the temporal distribution of tumor recurrence hazard conducted with NSCLC patients. The temporal distribution of the recurrence hazard of early-stage NSCLC may shed light on the pattern of recurrence of NSCLC and may help identify patients who might benefit from adjuvant chemotherapy.
Patients and Methods
Institutional Review Board (IRB)
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center for the use of tumor samples and patients’ clinical history.
Patients’ selection
A total of 994 primary NSCLC patients who underwent radical resection at the Sun Yat-sen University Cancer Center between January 1999 and October 2009 were eligible for inclusion in this study. All patients’ were staged or restaged as T1a-2bN0M0 according to the seventh edition of the International Union Against Cancer Staging System for Lung Cancer that was released in 2007 [11]. Non-smokers were defined as having smoked fewer than 100 cigarettes in their life time [12]. The cases selected for this study fulfilled the following criteria: (1) histological confirmed primary NSCLC; (2) no evidence of metastatic disease, as determined by history, physical examination and routine computed tomography (CT); (3) complete surgical resection (R0) at our cancer center; and (4) at least 3 months of follow-up information concerning disease recurrence and death. Patients who underwent non-curative resection (R1) or neo-adjuvant therapy and those who died of postoperative complications were excluded from the study.
There were 147 patients received platinum-based adjuvant chemotherapy within 4 to 8 weeks after surgical resection. Among these patients, 91 patients were in stage Ib and 56 patients in stage IIa. The average length of time between surgery and start of chemotherapy was 5.7 weeks. Among the patients who received adjuvant chemotherapy, four cycles of cisplatin (75 mg/m2) with paclitaxel or vinorelbine chemotherapy were performed in 94 (63.9%) patients and four cycles of carboplatin (an area under the curve dose of 6 mg/mL per minute over 60 minutes) with paclitaxel or vinorelbine were performed in the remaining 53 (36.1%) patients.
All participants had signed the written informed consent for their clinical records to be used in future study before starting initial treatment.
Definition of recurrence
Local-regional recurrences were defined as those at the surgical site, at the anastomotic or bronchial stump, or in the local-regional lymph nodes (levels 1–14, including supraclavicular). Distant recurrences were defined as hematogenous metastases within solid organs, such as the lung, liver, brain and bone. Cervical or abdominal lymph node disease was considered a distant recurrence. Recurrences were diagnosed histologically, cytologically, and radiologically. A combined recurrence was defined as the detection of both locoregional and distant recurrences either simultaneously or within 30 days [13].
Follow-up of patients
The patients were routinely followed up at the Cancer Center of Sun Yat-sen University every 3 months during the first year after surgery, every 6 months during the next 2 years and once per year thereafter. Follow-up was maintained through the retrieval of follow-up medical records stored in the outpatient department database or followed by personal contact with the patients by our professional follow-up institution, including requests for information regarding tumor recurrences and survival status. Recurrence or its absence was diagnosed by queries to the patient, chest CT, abdominal CT, bone scans, whole-brain CT/MRI or PET/CT. If a tumor had recurred, additional information, including sites of recurrence and therapy, was requested. All of the patients were contacted again in January 2013 to determine their vital status.
Statistical analysis
Recurrence-free survival (RFS) was defined as the time from surgery to the earliest occurrence of relapse (locoregional or distant) or death from cancer or cancer-related disease. Patients who were lost to follow-up were censored at the time of last contact. Patients who were alive at the end of the study were censored for the purpose of data analysis. RFS was assessed using the Kaplan-Meier method and was compared using the log-rank test. The Cox regression model was used to perform a multivariate survival analysis of all of the variables that were significant in the univariate analysis. For the graphical display of RFS, the annual hazard rates were estimated using a kernel smoothing method. A two-sided probability value of less than 0.05 was considered statistically significant. All the statistical analyses were performed using the Stata statistical software package (release 9.0; Stata Corporation, College Station, TX, USA). The relative risks (RRs) are presented with their 95% confidence intervals (CIs).
Results
Patient characteristics
A total of 994 cases satisfied the inclusion criteria and were included in this study. The clinical-pathological characteristics of the 994 patients are listed in Table 1. All of these patients received rigorous follow-up, with a median follow-up time of 6.1 years.
Table 1. Kaplan-Meier postoperative survival analysis (log-rank test) according to clinical-pathological factors of patients with early-stage NSCLC.
| Variable | N | Recurrence-free survival | 95% CI | P |
| 994 | 8.784 | 8.389–9.179 | ||
| Sex | ||||
| Male | 702 | 8.604 | 8.133–9.076 | 0.193 |
| Female | 292 | 9.204 | 8.488–9.921 | |
| Age (years) | ||||
| ≤65 | 680 | 9.179 | 8.713–9.646 | 0.010 |
| >65 | 314 | 7.593 | 6.897–8.289 | |
| Smoking history | 0.037 | |||
| Non-smoker1 | 429 | 9.267 | 8.685–9.848 | |
| Smoker | 565 | 8.400 | 7.866–8.935 | |
| Initial symptoms | 0.015 | |||
| Examination | 419 | 9.359 | 8.761–9.957 | |
| Symptoms2 | 575 | 8.377 | 7.857–8.897 | |
| Pathological type | 0.663 | |||
| Squamous cell carcinoma | 319 | 8.859 | 8.259–9.214 | |
| Non-squamous cell carcinoma | 675 | 8.733 | 8.256–9.210 | |
| Visceral pleural invasion | 0.003 | |||
| Present | 392 | 8.249 | 7.598–8.901 | |
| Absent | 602 | 9.171 | 8.679–9.662 | |
| Tumor diameter | <0.001 | |||
| ≤4.0 cm | 759 | 9.138 | 8.691–9.585 | |
| >4.0 cm | 235 | 7.659 | 6.845–8.473 | |
| Stations of resected lymph nodes | 0.064 | |||
| <6 | 8.575 | 8.111–9.038 | ||
| ≥6 | 9.281 | 8.537–10.025 | ||
| Number of resected lymph nodes | 0.022 | |||
| <15 | 471 | 8.325 | 7.777–8.873 | |
| ≥15 | 523 | 9.265 | 8.700–9.830 | |
| Lobar bronchus invasion | 0.909 | |||
| Present | 181 | 8.922 | 7.844–10.000 | |
| Absent | 813 | 8.760 | 8.330–9.189 | |
| Histological grade | 0.012 | |||
| G1+G2 | 631 | 9.111 | 8.619–9.602 | |
| G3+G4 | 363 | 8.159 | 7.503–8.815 | |
| CEA | <0.001 | |||
| ≤5 g/l | 565 | 9.110 | 8.582–9.638 | |
| >5 g/l | 248 | 7.667 | 6.900–8.434 | |
| Adjuvant chemotherapy3 | 0.321 | |||
| Yes | 147 | 8.681 | 8.255–9.107 | |
| No | 847 | 9.574 | 8.618–10.530 | |
| Pathological T category | <0.001 | |||
| P T1a | 172 | 10.032 | 9.162–10.902 | |
| P T1b | 147 | 9.550 | 8.600–10.501 | |
| P T2a | 568 | 8.473 | 7.938–9.008 | |
| P T2b | 107 | 7.079 | 5.911–8.248 | |
| AJCC stage | <0.001 | |||
| P Ia stage | 319 | 9.832 | 9.186–10.478 | |
| P Ib stage | 568 | 8.473 | 7.938–9.008 | |
| P IIa stage | 107 | 7.079 | 5.911–8.248 |
Non- smokers were defined as having smoked fewer than 100 cigarettes in their lifetime; 2Common symptoms of lung cancer: cough, dyspnea, weight loss and chest pain; 3Postoperative cisplatin-based chemotherapy.
Overall recurrence patterns
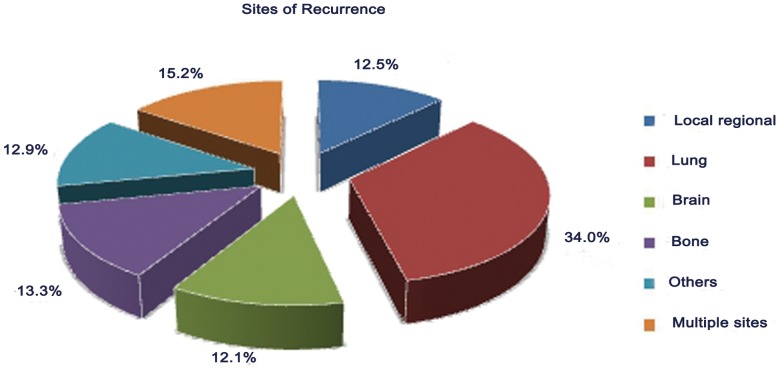
Approximately 256 patients experienced relapse (locoregional: 32 and distant: 224). The most common sites of failure were distant (87.5%, 224/256) in this group of patients. The most common site of distant metastasis was the lung (34.0%, 87/256), followed by the bone (13.3%, 34/256) (Figure 1). Overall, 162 patients died from cancer, and approximately 576 patients remained alive at the last follow-up.
Figure 1. Relative frequencies for recurrence sites for postoperative early-stage NSCLC.
Survival analysis according to clinical-pathological factors
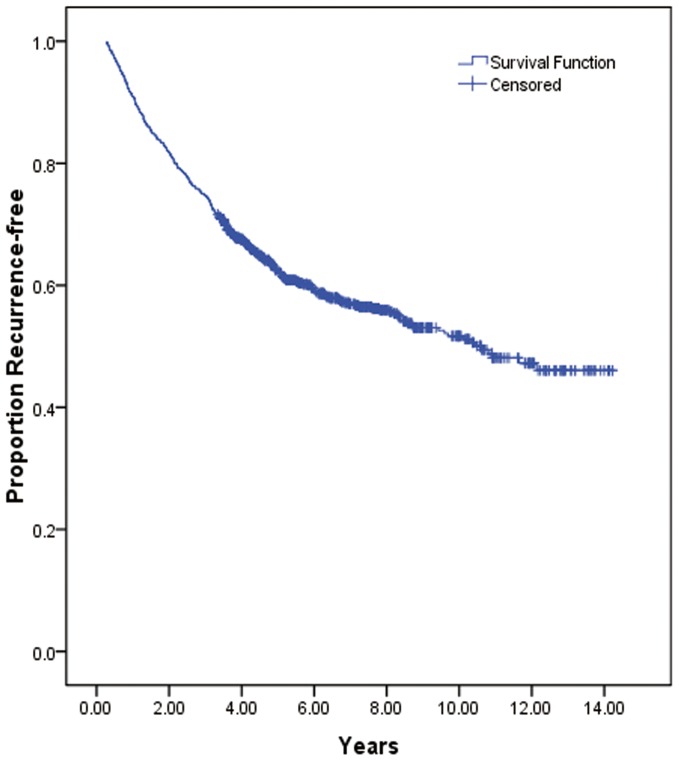
The survival curve for the entire population declined the most rapidly between 0.5 year and 2.5 years after surgery, with an extremely steep slope during this period. Subsequently, the entire curve continued a downward trend, albeit with a gentler slope, and it reached a virtual plateau by 10 years (Figure 2).
Figure 2. Recurrence-free survival for patients in early-stage NSCLC.
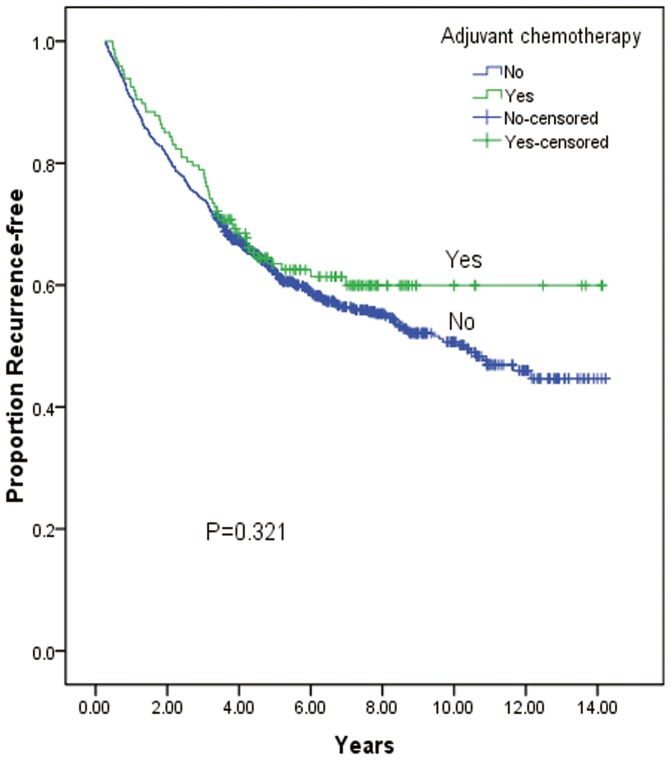
The median RFS was 8.8 years. The life table survival analysis showed that the 1-year, 3-year, 5-year, and 10-year recurrence rates were 82.0%, 67.0%, 59.0% and 48.0%, respectively. The univariate analysis revealed that the following variables were significantly correlated with RFS: patient age, smoking history, initial symptoms, visceral pleural invasion, tumor diameter, number of resected lymph nodes, histological grade, level of carcinoembryonic antigen (CEA), and pathological T category (Table 1). The patients who received platinum-based adjuvant chemotherapy showed a longer RFS compared with those who did not (median 9.6 years versus 8.7 years); however, this difference was not statistically significant (Table 2) (Figure 3).
Table 2. Results of multivariate survival analyses of Recurrence-free survival (RFS) according to the Cox regression model.
| Variable | N | RR | 95% CI | P value |
| Tumor diameter | 1.323 | 1.000–1.750 | 0.050 | |
| ≤4.0 cm | 759 | |||
| >4.0 cm | 235 | |||
| CEA | 1.414 | 1.131–1.768 | 0.002 | |
| ≤5 g/l | 565 | |||
| >5 g/l | 248 | |||
| Number of dissected lymph nodes | 0.781 | 0.630–0.968 | 0.024 | |
| <15 | 471 | |||
| ≥15 | 523 | |||
| Age (years) | 1.191 | 0.949–1.495 | 0.132 | |
| ≤65 | 680 | |||
| >65 | 314 | |||
| Smoking history | 1.003 | 0.795–1.264 | 0.983 | |
| Non-smoker | 429 | |||
| Smoker | 565 | |||
| Initial symptoms | 1.137 | 0.904–1.429 | 0.273 | |
| Examination | 419 | |||
| Symptoms | 575 | |||
| Histological grade | 0.824 | 0.655–1.036 | 0.097 | |
| G1+G2 | 631 | |||
| G3+G4 | 363 | |||
| Visceral pleural invasion | 1.160 | 0.896–1.503 | 0.260 | |
| Present | 392 | |||
| Absent | 602 | |||
| Pathological T category | 1.191 | 1.026–1.384 | 0.022 | |
| PT1a | 172 | |||
| PT1b | 147 | |||
| PT2a | 568 | |||
| PT2b | 107 |
RR: relative risk; 95%CI: 95% confidence interval.
Figure 3. Recurrence-free survival for patients in early-stage NSCLC according to adjuvant chemotherapy.
When the above variables were included in the multivariate analysis, the results suggested that tumor diameter, number of lymph nodes, CEA level, and pathological T category were independent factors that affected RFS (Table 2).
Recurrence hazard analysis according to clinical-pathological factors
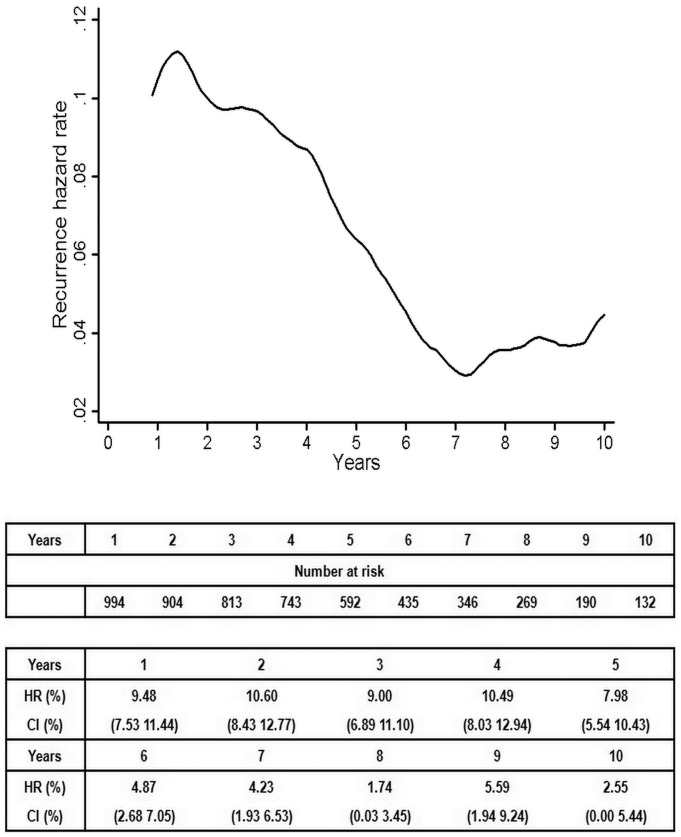
The annual recurrence hazard curve for the entire population showed that the first major recurrence surge peaked 1.6 years after surgery. Subsequently, the curve declined until reaching a nadir at 7.2 years. A second peak occurred at 8.8 years (Figure 4). The time-varying patterns of recurrence after surgery are listed in Table 3.
Figure 4. Annual recurrence hazard rates for patients in early-stage NSCLC.
Table 3. Patterns of recurrence after surgery.
| Years after surgery | n | N | Type of recurrence | |||
| Locoregional | Distant | Death | ||||
| 0–1 | 90 | 994 | 7 | 59 | 24 | 0 |
| 1–2 | 91 | 904 | 8 | 44 | 39 | 0 |
| 2–3 | 70 | 813 | 7 | 36 | 27 | 0 |
| 3–4 | 70 | 743 | 4 | 38 | 28 | 81 |
| 4–5 | 41 | 592 | 4 | 22 | 15 | 116 |
| 5–6 | 19 | 435 | 1 | 9 | 9 | 70 |
| 6–7 | 13 | 346 | 1 | 6 | 6 | 64 |
| 7–8 | 4 | 269 | 0 | 2 | 2 | 75 |
| 8–9 | 9 | 190 | 0 | 1 | 8 | 49 |
| 9–10 | 3 | 132 | 0 | 2 | 1 | 26 |
| ≥10 | 8 | 103 | 0 | 5 | 3 | 95 |
| 418 | 32 | 224 | 162 | 576 |
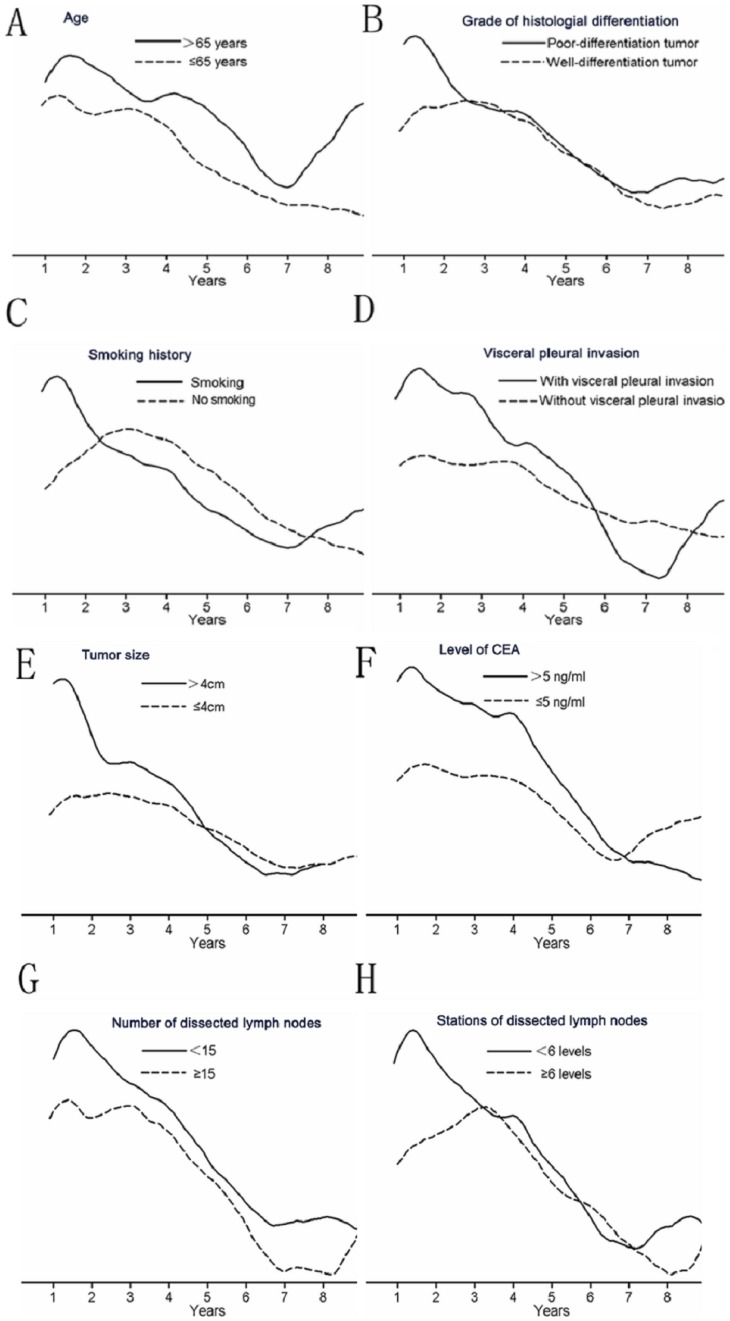
The temporal distribution of the recurrence risk varied with age. The older (≥65 years) patients exhibited a relatively pronounced and variable pattern compared with the younger (<65 years) ones. The smoothed hazard plots of these two subgroups were parallel to each other, with lower hazard rates in the younger (<65 years) patients (Figure 5A). The curve of the older (≥65 years) patients increased almost linearly after 7 years.
Figure 5. Recurrence hazard rates according to clinic-pathological characteristics.
A: age; B: grade of histological differentiation; C: smoking history; D: visceral pleural invasion; E: tumor diameter; F: level of CEA; G: number of dissected lymph nodes; H: stations of dissected lymph nodes.
A visual inspection of the hazard curves for different grades of histological differentiation (Figure 5B) suggested that the patients with poorly differentiated tumors exhibited earlier and higher first peaks compared with the patients with well-differentiated tumors. However, the curves of the two groups were nearly identical after 2.8 years.
The hazard rate curves of the smokers and non-smokers were virtually superimposable (Figure 5C). The first peak of the smokers was lower than that of the non-smokers. The risk of recurrence was significantly higher for the smokers than for the non-smokers after 7.5 years. A similar phenomenon was observed among the patients with visceral pleural invasion(Figure 5D). The first peak of the patients without visceral pleural invasion appeared lower and later than that of the patients with visceral pleural invasion.
The analysis according to tumor diameter (≤4.0 cm versus >4.0 cm) demonstrated that the first recurrence peak was significantly lower and later for the patients with a tumor diameter of ≤4.0 cm compared with those with a tumor diameter of>4.0 cm(Figure 5E). The curves of both subgroups tended to intersect after 5.0 years, and there was no clear second peak in either group. The analysis according to CEA level (Figure 5F) revealed that although the patients with abnormal CEA level exhibited a higher first peak, the patients with normal CEA level tended to have a greater risk of recurrence after 6.8 years compared with the patients with abnormal level of CEA.
When the hazard rate was analyzed in terms of the number of dissected mediastinal lymph nodes, a double-peaked temporal distribution of recurrence was again revealed. The smoothed hazard plots of these two subgroups were parallel to each other, and the annual recurrence hazard curve (Figure 5G) demonstrated that the patients with <15 dissected lymph nodes tended to experience more relapses at all the analyzed time periods. In terms of the stations of the dissected mediastinal lymph nodes, the patients who were dissected at <6 stations exhibited both peaks at earlier time points(Figure 5H).
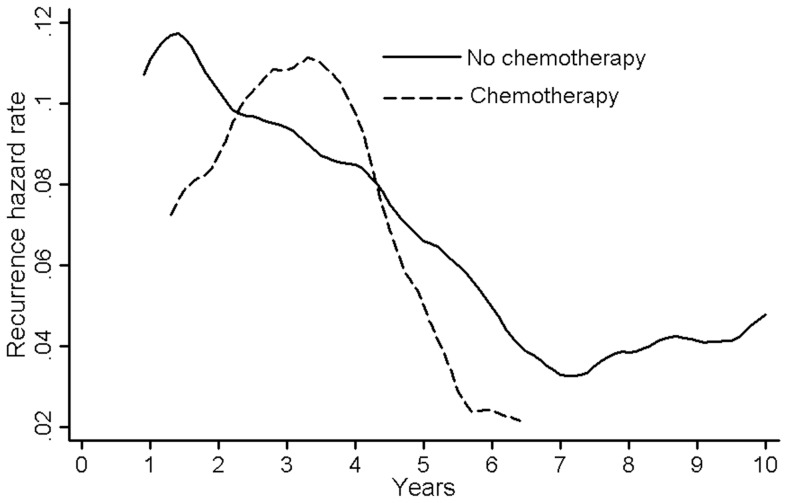
The type of treatment also affected the shape of the curves. Compared with those without adjuvant chemotherapy, the patients who received platinum-based adjuvant chemotherapy exhibited an later major recurrence surge. The first peak of the adjuvant chemotherapy-treated subgroup was 3.5 years after surgery, and the corresponding peak of their without chemotherapy-treated counterparts occurred at 1.5 years. After 4.2 years, the curve of the patients who received adjuvant chemotherapy was lower than that of the patients without adjuvant chemotherapy, and this pattern continued for the remainder of the study period (Figure 6).
Figure 6. Recurrence hazard rates for patients in early-stage NSCLC according to adjuvant chemotherapy.
Discussion
Patients with early-stage (T1a-T2bN0M0) disease represent approximately 20%–30% of all patients with non-small cell lung cancer (NSCLC). Currently, surgery is the preferred treatment for early-stage NSCLC, and it is considered the only procedure with the potential to cure this condition [14]. However, the long-term survival of patients with early-stage NSCLC is still not optimistic. Despite surgical resection, approximately 20–40% of these patients die from local recurrence or distant metastasis within 5 years [2]. The present study is the first to demonstrate the presence of a double-peaked recurrence hazard pattern among early-stage (T1a-T2bN0M0) non-small cell lung cancer (NSCLC) patients after surgery. Our outcome of a double-peaked recurrence hazard pattern provides support for the theory of tumor dormancy [15], [16], which postulates that micrometastatic foci may exist in different biologic steady states, most of which do not promote tumor growth. However, this orderly and stable process may be perturbed by surgery, which stimulates a transition from dormancy to growth, resulting in a sudden acceleration of the metastatic process and eventually leading to recurrence [17]. This phenomenon may account for the first peak of recurrence risk of malignant carcinoma after surgery.
The site of recurrence of early-stage NSCLC after surgical resection was also investigated in this study. Among patients with early-stage NSCLC, most tumors recurred as distant metastases, rather than local-regional recurrences (22.5% versus 3.2%). In early-stage NSCLC after surgical resection, the rate of distant metastasis has been reported to be between 14.0% and 23.0% [18], [19], and the local-regional recurrence was 5.0% [20]. The patterns of tumor recurrence affect the therapy and survival of NSCLC patients. Based on the present study, the adjuvant treatment for early-stage NSCLC should be systemic therapy, rather than local therapy, due to the recurrence pattern of this disease.
High-risk factors, include poorly differentiated tumors [21], [22], vascular invasion [23], wedge resection [24], tumors >4.0 cm [25], visceral pleural involvement [26], [27], and incomplete lymph node sampling (Nx) [28], [29], are prognostic factors strongly associated with increased risks of recurrence and death among early-stage NSCLC patients. In our study, a double-peaked pattern was observed in a variety of patient subgroups, regardless of histological differentiation grade, tumor diameter, visceral pleural involvement or the number of dissected mediastinal lymph nodes; the patients with high-risk factors exhibited earlier and higher peaks. These factors should be considered when pondering treatment with adjuvant chemotherapy.
There is controversy concerning whether age affects the treatment and prognosis of lung cancer, particularly for patients with early lung cancer. Mery et al. [30] reported that age was an important prognostic factor for the survival of patients with stage I–II NSCLC after controlling for factors such as gender, histological type, clinical stage and type of surgery. Agarwal et al. [31] also confirmed that the mortality rate increased sharply with age in patients with stage I–II NSCLC: a one-year increase in age was associated with a nearly 6% increase in HR. However, some researchers have reported that age is not an important prognostic factor for the survival of early-stage lung cancer patients because elderly patients experience complications, organ impairment and other non-cancer-related factors [32], [33]. In the current study, older (≥65 years) patients exhibited a relatively pronounced and variable pattern compared with younger (<65 years) ones. The smoothed hazard plots of these two subgroups were parallel to each other, with lower hazard rates in the younger (<65 years) patients. The curve of the older (≥65 years) patients increased almost linearly after 7.0 years. Older age (≥65 years) may be an adverse prognostic indicator in early-stage NSCLC, especially among patients who live longer than 5 years after surgery.
In the present study, we verified that the double peaks were more prominent among patients who were smokers. The primary risk factor for NSCLC is tobacco smoking, which is involved in more than 85–90% of all lung cancer-related deaths [34], [35]. Although a history of smoking is well established as a risk factor for NSCLC, it remains controversial as a prognostic factor [36], [37]. In our data, the hazard rate curves of the non-smokers and smokers were virtually superimposable. The smokers showed an earlier major recurrence surge, with the first peak occurring 1.2 years after surgery, while the corresponding peak for their non-smoking counterparts occurred at 3.2 years. The risk of recurrence was significantly higher for the smokers than the non-smokers after 7.5 years.
Some studies have shown that level of CEA has prognostic significance in NSCLC [38], [39]. This finding was supported by our survival analysis, which showed that the risk of recurrence among patients with normal CEA level was significantly higher than that of the patients with abnormal CEA level before 7.0 years. However, the opposite pattern was observed after 7 years, indicating that the usefulness of CEA as a prognostic factor for recurrence risk may change over time. Thus, the CEA level tested before surgery may have significance for early recurrence, but not for late recurrence.
Many randomized clinical trials have reported the efficacy of platinum-based adjuvant chemotherapy after surgical resection in stage II-IIIA lung cancer [40]–[42]. However, the efficacy of platinum-based adjuvant chemotherapy in stage IB cancer is controversial [43], [44]. Pignon et al. performed a meta-analysis of the large adjuvant trials for NSCLC conducted since 1995 (excluding CALGB 9633) [45]. Their stage IB subset analysis (1,371 patients) trended toward showing a benefit of adjuvant treatment (HR, 0.93) but failed to reach statistical significance (95% CI, 0.78–1.10). In the current study, compared with those without adjuvant chemotherapy, the patients who received platinum-based adjuvant chemotherapy exhibited a first peak that appeared lower and later. After 4.2 years, the curve of the patients who received adjuvant chemotherapy was lower than that of the patients without adjuvant chemotherapy, and this pattern continued for the remainder of the study period. The results of our study demonstrate that for early-stage NSCLC patients, platinum-based adjuvant chemotherapy may reduce and delay the recurrence hazard.
The mechanisms for recurrence and metastasis of early stage NSCLC was unclear. Driver gene mutations were associated with the carcinogenesis and response to targeted therapies and prognosis of NSCLC. Some studies had reported that identification the driver gene mutation can select patients with early-stage disease who are at high risk of recurrence [46]–[47]. Brock et al. [48] also confirmed that methylation of the promoter region of the cyclin-dependent kinase inhibitor 2A gene p16, the H-cadherin gene CDH13, the Ras association domain family 1 gene RASSF1A, and the adenomatous polyposis coli gene APC in patients with stage I NSCLC treated with curative intent by means of surgery is associated with early recurrence.
Previous study reported that about 5.0% non small cell lung cancer have the risk of developing metachronous second primary lung cancer [49]. The standard diagnosis of second primary lung cancer was controversial, the different histological type from that of the first tumor was considered as the key criteria to define the second lung cancer. In our study, the initial data showed about 3.54% (39/1103) patients developed second malignant tumor during the flow-up after surgical resection: 10 patients developed second primary lung cancer, and 29 patients developed the other site malignant tumor. All these patients were diagnosed as secondary malignant tumor in histological type and were excluded from the present study. However, for patients with multiple tumors after surgical resection, our study was limited to distinguish the second malignant tumor from metastatic disease.
As a retrospective study, this study has other several potential limitations. The median follow-up time of 6.1 years was not sufficiently long; 576 patients were still alive at the last follow-up. Because there were only 147 patients who received platinum-based adjuvant chemotherapy, the subgroup analysis was unable to identify patients who might benefit from adjuvant chemotherapy.
In conclusion, we confirmed the validity of a double-peaked pattern of recurrence risk of early-stage (T1a-T2bN0M0) non-small cell lung cancer. The main pattern of tumor recurrence was distant metastasis, rather than local-regional recurrence. The hazard function may be useful for selecting patients at high risk of recurrence to receive postoperative therapy, which offers the possibility of decreasing and/or delaying the recurrence hazard.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Our data are available from the Sun Yat-sen Cancer Center Data Access/Ethics Committee for researchers who meet the criteria for access to confidential data.
Funding Statement
This research was supported by national and provincial Science and technology projects (No. 2012AA021502 and No.2012B031800295). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, et al. (2013) Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 369: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelsey CR, Marks LB, Hollis D, Hubbs JL, Ready NE, et al. (2009) Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 115: 5218–5227. [DOI] [PubMed] [Google Scholar]
- 3. Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, et al. (2009) The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 4: 792–801. [DOI] [PubMed] [Google Scholar]
- 4. Detterbeck FC, Boffa DJ, Tanoue LT (2009) The new lung cancer staging system. Chest 136: 260–271. [DOI] [PubMed] [Google Scholar]
- 5. Tantraworasin A, Saeteng S, Lertprasertsuke N, Arreyakajohn N, Kasemsarn C, et al. (2013) Prognostic factors of tumor recurrence in completely resected non-small cell lung cancer. Cancer Manag Res 5: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kobayashi N, Toyooka S, Soh J, Ichimura K, Yanai H, et al. (2007) Risk factors for recurrence and unfavorable prognosis in patients with stage I non-small cell lung cancer and a tumor diameter of 20 mm or less. J Thorac Oncol 2: 808–812. [DOI] [PubMed] [Google Scholar]
- 7. Yin W, Di G, Zhou L, Lu J, Liu G, et al. (2009) Time-varying pattern of recurrence risk for Chinese breast cancer patients. Breast Cancer Res Treat 114: 527–535. [DOI] [PubMed] [Google Scholar]
- 8. Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 9. Feng XY, Chen YB, Wang W, Guan YX, Li YF, et al. (2013) Time-varying pattern of recurrence risk for gastric cancer patients. Med Oncol 30: 514. [DOI] [PubMed] [Google Scholar]
- 10. Simes RJ, Zelen M (1985) Exploratory data analysis and the use of the hazard function for interpreting survival data: an investigator’s primer. J Clin Oncol 3: 1418–1431. [DOI] [PubMed] [Google Scholar]
- 11. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, et al. (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2: 706–714. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Fang R, Sun Y, Han X, Li F, et al. (2011) Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 6: e28204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amini A, Lou F, Correa AM, Baldassarre R, Rimner A, et al. (2013) Predictors for locoregional recurrence for clinical stage III-N2 non-small cell lung cancer with nodal downstaging after induction chemotherapy and surgery. Ann Surg Oncol 20: 1934–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, et al. (2006) Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 355: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 15. Gimbrone MJ, Leapman SB, Cotran RS, Folkman J (1972) Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 136: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varani J, Lovett ER, Lundy J (1981) A model of tumor cell dormancy: effects of anesthesia and surgery. J Surg Oncol 17: 9–14. [DOI] [PubMed] [Google Scholar]
- 17. Demicheli R, Retsky MW, Hrushesky WJ, Baum M (2007) Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol 4: 699–710. [DOI] [PubMed] [Google Scholar]
- 18. Al-Kattan K, Sepsas E, Fountain SW, Townsend ER (1997) Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 12: 380–384. [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, et al. (2008) Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg 34: 499–504. [DOI] [PubMed] [Google Scholar]
- 20.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, et al.. (2013) Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 145: 75–81, 81–82. [DOI] [PubMed]
- 21. Sun Z, Aubry MC, Deschamps C, Marks RS, Okuno SH, et al. (2006) Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 131: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 22.Jones DR, Daniel TM, Denlinger CE, Rundall BK, Smolkin ME, et al.. (2006) Stage IB nonsmall cell lung cancers: are they all the same? Ann Thorac Surg 81: 1958–1962, 1962. [DOI] [PubMed]
- 23. Shimada Y, Saji H, Yoshida K, Kakihana M, Honda H, et al. (2012) Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 7: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 24. Ou SH, Zell JA, Ziogas A, Anton-Culver H (2008) Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumor size. Chest 133: 662–669. [DOI] [PubMed] [Google Scholar]
- 25. Ou SH, Zell JA, Ziogas A, Anton-Culver H (2007) Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 110: 1532–1541. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, et al. (2005) Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg 130: 160–165. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa T, Okumura N, Kokado Y, Miyoshi K, Matsuoka T, et al. (2007) Clinical relevance of intraoperative pleural lavage cytology in non-small cell lung cancer. Ann Thorac Surg 83: 204–208. [DOI] [PubMed] [Google Scholar]
- 28. Park SY, Lee JG, Kim J, Byun GE, Bae MK, et al. (2013) Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang WL, Shen-Tu Y, Wang ZQ (2011) Prognostic Factors for Survival of Stage IB Upper Lobe Non-small Cell Lung Cancer Patients: A Retrospective Study in Shanghai, China. Chin J Cancer Res 23: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mery CM, Pappas AN, Bueno R, Colson YL, Linden P, et al. (2005) Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 128: 237–245. [DOI] [PubMed] [Google Scholar]
- 31. Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP (2010) Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer 68: 398–402. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto K, Padilla AJ, Calvo MV, Garcia-Zarza A, Pastor GJ, et al. (2003) Surgical results of stage I non-small cell lung cancer: comparison between elderly and younger patients. Eur J Cardiothorac Surg 23: 21–25. [DOI] [PubMed] [Google Scholar]
- 33. Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, et al. (2005) Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol 55: 231–240. [DOI] [PubMed] [Google Scholar]
- 34. Secretan B, Straif K, Baan R, Grosse Y, El GF, et al. (2009) A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 10: 1033–1034. [DOI] [PubMed] [Google Scholar]
- 35. Doll R, Peto R (1976) Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J 2: 1525–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, et al. (2011) Poor prognostic factors in patients with stage IB non-small cell lung cancer according to the seventh edition TNM classification. Chest 139: 855–861. [DOI] [PubMed] [Google Scholar]
- 37. Jin Y, Zhao L, Peng F (2013) Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics (Sao Paulo) 68: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molina R, Filella X, Auge JM, Fuentes R, Bover I, et al. (2003) Tumor markers (CEA, CA 125, CYFRA 21–1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol 24: 209–218. [DOI] [PubMed] [Google Scholar]
- 39. Gaspar MJ, Diez M, Rodriguez A, Ratia T, Martin DA, et al. (2003) Clinical value of CEA and CA125 regarding relapse and metastasis in resectable non-small cell lung cancer. Anticancer Res 23: 3427–3432. [PubMed] [Google Scholar]
- 40. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, et al. (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350: 351–360. [DOI] [PubMed] [Google Scholar]
- 41. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, et al. (2005) Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 42. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, et al. (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 7: 719–727. [DOI] [PubMed] [Google Scholar]
- 43. Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, et al. (2004) A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350: 1713–1721. [DOI] [PubMed] [Google Scholar]
- 44. Strauss GM, Herndon JN, Maddaus MA, Johnstone DW, Johnson EA, et al. (2008) Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26: 5043–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, et al. (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26: 3552–3559. [DOI] [PubMed] [Google Scholar]
- 46. Kratz JR, He J, Van Den Eeden SK, Zhu ZH, Gao W, et al. (2012) A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 379: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu ZH, Sun BY, Ma Y, Shao JY, Long H, et al. (2009) Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol 27: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 48. Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, et al. (2008) DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 358: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 49. Ishigaki T, Yoshimasu T, Oura S, Ota F, Nakamura R, et al. (2013) Surgical treatment for metachronous second primary lung cancer after radical resection of primary lung cancer. Ann Thorac Cardiovasc Surg 19: 341–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Our data are available from the Sun Yat-sen Cancer Center Data Access/Ethics Committee for researchers who meet the criteria for access to confidential data.