Abstract
The mammalian transient receptor potential melastatin channel 8 (TRPM8) is highly expressed in trigeminal and dorsal root ganglia. TRPM8 is activated by cold temperature or compounds that cause a cooling sensation, such as menthol or icilin. TRPM8 may play a role in cold hypersensitivity and hyperalgesia in various pain syndromes. Therefore, TRPM8 antagonists are pursued as therapeutics. In this study we explored the feasibility of blocking TRPM8 activation with antibodies. We report the functional characterization of a rabbit polyclonal antibody, ACC-049, directed against the third extracellular loop near the pore region of the human TRPM8 channel. ACC-049 acted as a full antagonist at recombinantly expressed human and rodent TRPM8 channels in cell based agonist-induced 45Ca2+ uptake assays. Further, several poly-and monoclonal antibodies that recognize the same region also blocked icilin activation of not only recombinantly expressed TRPM8, but also endogenous TRPM8 expressed in rat dorsal root ganglion neurons revealing the feasibility of generating monoclonal antibody antagonists. We conclude that antagonist antibodies are valuable tools to investigate TRPM8 function and may ultimately pave the way for development of therapeutic antibodies.
Introduction
The transient receptor potential melastatin 8 (TRPM8) channel is a non-selective cation channel that is activated by cold temperature (below ∼23°C) or compounds that cause a cooling sensation, such as menthol and icilin [1]–[3]. TRPM8 is expressed in a subpopulation of small to medium diameter neurons in the trigeminal and dorsal root ganglia that confer sensitivity to innocuous cold in the somatosensory system [4]. Three independently generated mouse models lacking functional TRPM8 channels exhibit attenuated cold sensation at a discrete temperature range in behavioral assays [5]–[7]. TRPM8 channels not only mediate behavioral, but also autonomic responses to innocuous cold, including the regulation of body temperature [8]–[10] and potentially cutaneous vascular tone [11]. Supporting these findings, TRPM8 expression was reported in other tissues, including the respiratory tract, urinary system, and vasculature [11], [12]. Thus, TRPM8 may play multiple functional roles, likely to be in a tissue-dependent manner, not only under innocuous conditions, but also in disease states. Cold hypersensitivity and hyperalgesia are symptoms of several neuropathic conditions [13], including painful bladder syndrome [14], and chemotherapy-induced neuropathy [15]. Genetic ablation of TRPM8 in mice abolishes cold-evoked behaviors after peripheral inflammation or nerve injury [6] and in models of chemotherapy-induced neuropathy [16]. Similarly, selective ablation of TRPM8 positive neurons in mice results in reduced sensitivity to innocuous cold, attenuated cold hypersensitivity and loss of cooling-mediated analgesia after injury [17]. Lastly, small molecule antagonists are efficacious in animal models of neuropathy [18] and overactive bladder [19], thus supporting a potential therapeutic benefit of TRPM8 antagonists.
As an alternative to small molecules, antibodies that bind near the pore regions of ion channels have been shown to antagonize channel activation [20]–[22]. Antibodies are known to exhibit exquisite specificity and unlimited diversity and could therefore provide advantages over small molecules. Due to their long plasma half-life, antibodies may represent better therapeutic agents for chronic disease conditions, including neuropathic pain. In addition, antibodies are generally peripherally restricted and therefore devoid of central side-effects.
To explore the possibility to target TRPM8 with antagonist antibodies, we have characterized commercially available poly- and monoclonal antibodies directed against the pore loop of TRPM8 as antagonists of cold as well as chemical ligand activation.
Materials and Methods
Reagents
TRPM8 positive control antagonist, compound M8-B [9], TRPV1 positive control antagonist, AMG6451 [23], and TRPA1 positive control antagonist, AMG9090 [24] all were synthesized at Amgen, Inc. A list of the antibodies used is shown in Table 1 and the amino acid homology of the third extracellular loop of different TRP channels is shown in Figure 1. ACC-049, a rabbit polyclonal TRPM8 antibody generated against an epitope in the third extracellular loop near the pore region of human TRPM8 was purchased from Alomone labs (Jerusalem, Israel). Its cognate peptide (SDVD GTTYDFAHC corresponding to amino acid residues 917-929 of human TRPM8) was also purchased from Alomone labs. Other rabbit polyclonal antibodies generated against the third extracellular loop near the pore region were purchased from Thermo Scientific (Waltham, MA), Antibodies online (Atlanta, GA) and Enzo Lifesciences (Farmingdale, NY). Rabbit monoclonal antibodies generated against the third extracellular loop near the pore region were purchased from MyBiosource (San Diego, CA), Creative Diagnostics (Shirley, NY) and Lifespan Biosciences (Seattle, WA). Reagents used in the study were purchased from the following companies: Icilin and menthol were purchased from Sigma-Aldrich (St. Louis, MO). Ham's F-12 nutrient mixture, DMEM, 1x glutamine-penicillin-streptomycin, 1x non-essential amino acids, dialyzed fetal bovine serum, genetecin, blasticidin-S-HCl, zeocin; Alexa fluor 488 and Hoechst 33342 were purchased from Invitrogen (Carlsbad, CA). Tetracycline-free fetal bovine serum was purchased from Hyclone (Logan, UT), Tetracycline hydrochloride from Cellgro Mediatech Inc. (Hemdon, VA). Neurobasal medium with 1X B-27 Supplement and 1X Glutamax was purchased from Life technologies (Grand Island, NY), Insulin was from Sigma (St. Louis, MO). Cytostar-T plates, poly-D-lysine coated Cytostar-T plates, calcium-45-radionuclide (45Ca2+) and microplate scintillation counter TopCount NXT-Packard were purchased from PerkinElmer life and analytical sciences (Waltham, MA). Control IgG was purchased from Jackson ImmunoResearch Laboratories, Inc. (Westgrove, PA). Fatal-Plus Solution was purchased from Vortech Pharmaceuticals (Dearborn, MI), the Papain Dissociation System kit from Worthington Biochemical Corp. (Lakewood, NJ)
Table 1. Source and characteristics of the antibodies.
| Source | Catalog number | Lot number(s) | Clonality | Epitope lies within |
| Alomone labs | ACC-049 | AN0010, 0011, 0012 | Polyclonal | 917–929 |
| MyBioSource | MBS609041 | 13100131 | Monoclonal | 925–975 |
| Creative Diagnostics | CDB890 | CABT37242RH | Monoclonal | 920–960 |
| Thermo Scientific | OST00133W | PB1818654 | Polyclonal | 900–950 |
| Antibodies Online | ABIN351226 | Rb0496-290608W | Polyclonal | 900–950 |
| Lifespan Biosciences | LS-B6668 | 53021 | Monoclonal | 900–1000 |
| EnzoLifesciences | BML-SA664 | 02261432 | Polyclonal | 917–929 |
Except for ACC-049, the antigen sequence used for the antibodies was not disclosed by the vendors.
Figure 1. Alignment of extracellular pore loop sequences.
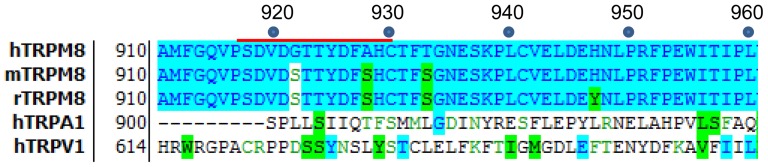
Alignment of the third extracellular loop sequences of the human, rat and mouse TRPM8 channel and the human TRPA1 and TRPV1 channels. The red line indicates the epitope sequence that ACC-049 was generated against.
Cell-based assays
Complementary DNAs of recombinant human, rat and mouse TRPM8 were stably transfected into Tetracycline-Regulated Expression cell line, T-REx-Chinese hamster ovary cell line from Invitrogen, Inc. (Carlsbad, CA). Twenty four hours before the assay, cells were seeded in Cytostar-T 96-well plates at a density of 3.0×104 per well, and TRPM8 channel expression was induced with 0.5 µg/ml tetracycline. On the day of the assay, culture media were removed, and cells were incubated with TRPM8 antibody (ACC-049), antagonist M8-B, or control IgG for 30 min before addition of 45Ca2+. Stock solutions of ACC-049, M8-B, and control IgG were diluted to the desired final concentrations in the assay buffer (F-12 Ham's containing 15 mM HEPES and 0.1 mg/ml BSA) keeping the final concentration of DMSO lower than 0.5% for M8-B. The activity of TRPM8 was monitored as function of cellular uptake of radioactive calcium. The final concentration of 45Ca2+in the 45Ca2+ uptake assay was 10 µCi/ml. Icilin (1 µM) or menthol (100 µM) induced 45Ca2+ uptake was used for evaluating blockade of chemically induced TRPM8 channel activation by ACC-049, antagonist M8-B and control IgG, as reported previously [25]. For cold activation of TRPM8, cold assay buffer at 10°C was used. ACC-049 was assayed in cells stably expressing human, rat, or mouse TRPM8 channels. The other commercial antibodies listed in Table 1 were assayed at a single concentration in 30 µl in icilin induced activation of human TRPM8 channels. The TRPM8 antagonist M8-B was used as a positive control and control IgG was used as a negative control in all assays. TRPM8 activation was calculated as percent of control (POC) with agonist (cold, icilin or menthol) induced radioactive calcium uptake set as 100 percent and assay buffer only plus 45Ca2+ set as zero percent with exception of cold activation, where blockade with M8-B at 1 µM was set as zero percent. Evaluation of antagonism at heat activation of TRPV1 [26], and noxious cold temperature activation of TRPA1 [24] was conducted as reported previously.
Dorsal root ganglion (DRG) neuron preparation
Six male Sprague Dawley rats weighing 150–200 g were obtained from Harlan (Hayward, CA). Rats were cared for in accordance to the Guide for the Care and Use of Laboratory Animals, 8th Edition [27] and research protocols were approved by Amgen's Institutional Animal Care and Use Committee. Rats were group housed in facilities internationally accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AALAC). Rats were kept in non-sterile ventilated micro-isolator housing on corn cob bedding. Rats had ad libitum access to pelleted feed (Harlan Teklad 2020X, Indianapolis, IN) and water (on site reverse osmosis-purified) via automatic watering system or water bottle. Rats were maintained on a 12∶12 hour light-dark cycle in rooms at (70±5°F, 50±20% RH) and had access to enrichment opportunities. On the experimental day, rats were sacrificed by an overdose of Fatal-Plus Solution and approximately 20 DRGs per animal were removed. DRG neurons were isolated from the ganglia using the Papain Dissociation System kit according to the manufacturer's instructions. Pelleted neurons were then immediately resuspended in Neurobasal medium with 1X B-27 Supplement and 1X Glutamax, containing 10 µg/ml of Insulin. Neurons were then seeded in poly-D-lysine coated Cytostar-T 96 well plates at a density of 2.0×104 per well and incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 hours. On the day of the assay, culture media were removed and neurons were subjected to the 45Ca2+ uptake assay as described above.
Statistical Analysis
GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) software was used to plot the data and to calculate the 50% inhibitory concentrations (IC50) of compounds and antibodies using a sigmoidal dose-response equation.
Results
We have evaluated the ability of polyclonal antibody ACC-049 that was generated against the pore loop of TRPM8 to act as an antagonist in a functional cell based 45Ca2+ uptake assay using icilin, menthol, or cold buffer as agonists in cultured Chinese hamster ovary (CHO) cells constitutively expressing human, rat or mouse TRPM8 channels, as well as in dissociated rat dorsal root ganglion neurons endogenously expressing rat TRPM8 channels.
ACC-049 acts as a selective antagonist of TRPM8
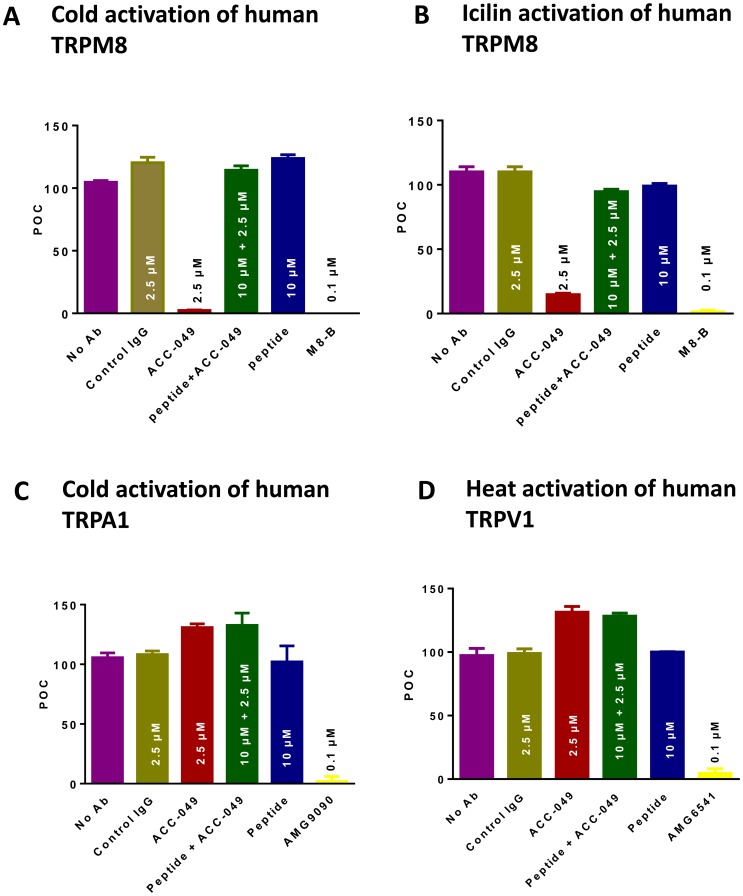
In a single concentration (2.5 µM) evaluation, ACC-049 specifically inhibited activation of human TRPM8 by cold temperature (Fig. 2A) or synthetic agonist icilin (Fig. 2B), but had no effects on noxious cold temperature activation of human TRPA1 (Fig. 2C) or heat activation of human TRPV1 (Fig. 2D). ACC-049 blocked cold activation of human TRPM8 nearly completely (Fig. 2A) and icilin activation by more than 80% (Fig. 2B). The same concentration (2.5 µM) of control IgG had no effect on TRPM8, TRPA1 or TRPV1 activation (Fig. 2A–D). Pre-absorption with an excess amount (10 µM) of the peptide that ACC-049 was generated against abolished the antagonism of cold (Fig. 2A) and icilin (Fig. 2B) activation of human TRPM8 channels, whereas no effects were observed on TRPA1 (Fig. 2C) or TRPV1 (Fig. 2D) channel activation. The peptide alone had no effects on TRPA1, TRPM8, or TRPV1 activation (Fig. 2A–D). The positive control compounds, M8-B (Fig. 2A, B), AMG9090 (Fig. 2C), and AMG6451 (Fig. 2D), completely inhibited activation of human TRPM8, human TRPV1 and human TRPA1, respectively.
Figure 2. ACC-049 is a selective inhibitor of TRPM8.
Specificity of ACC-049 (2.5 µM) for blocking human TRPM8 activation induced by the specific natural agonist cold (A) or synthetic agonist icilin (D). No effect of ACC-049 on noxious cold induced human TRPA1 (B) or heat induced TRPV1 activation (C). Small molecule antagonists AMG9090 and AMG6541 are the positive control for TRPA1 (B) or TRPV1 (C) blockage, respectively. Note the near complete blockade of TRPM8 activation by ACC-049 at 2.5 µM, similar to that by the positive small molecule antagonist control M8-B (A). Neither control IgG, nor peptide-absorbed ACC-049, or peptide alone blocked activation of any of the channels tested (A–D). Values are means of triplicate measures in a single experiment and expressed as percent of control (POC). Agonist induced 45Ca2+ uptake in the absence of antibodies (no Ab) was considered as 100 percent and wells with small molecule antagonists plus 45Ca2+ were set as zero percent.
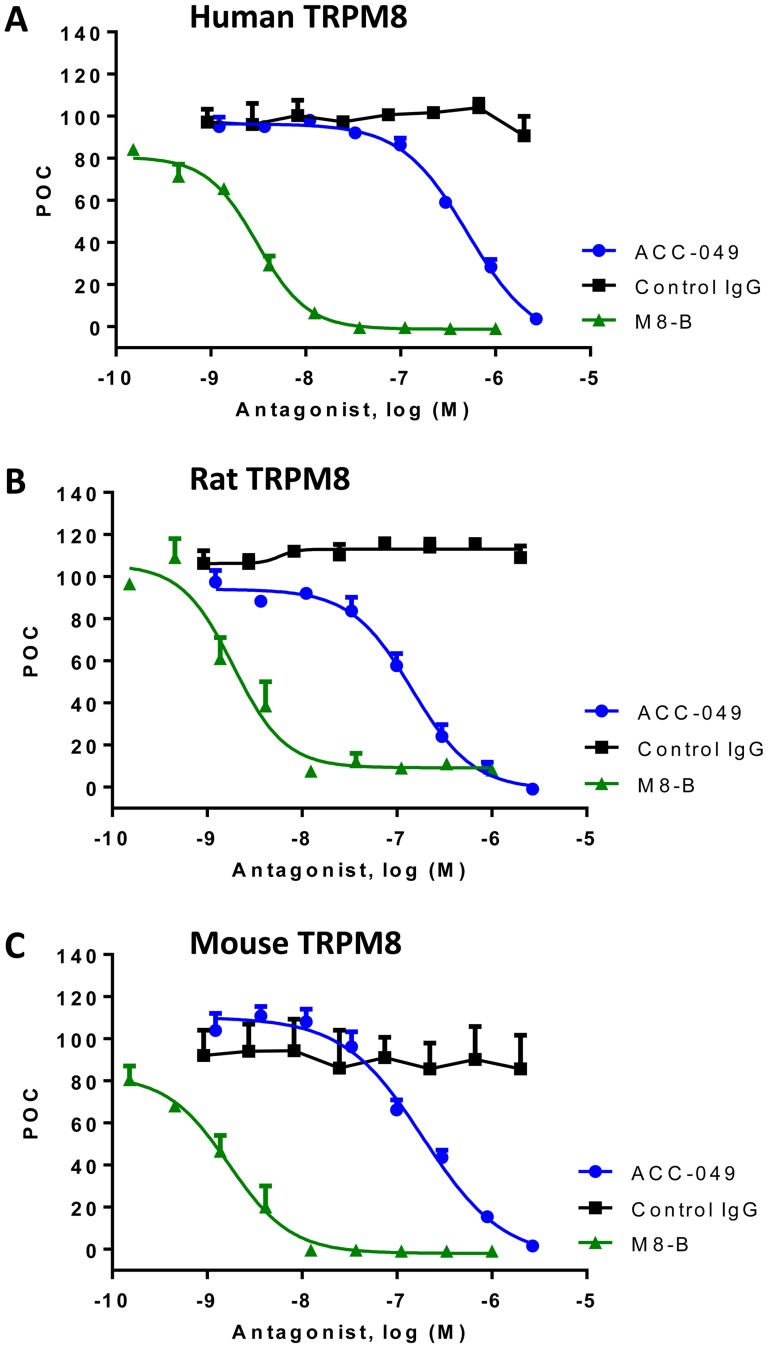
ACC-049 blocks cold activation of human and rodent TRPM8 channels in a concentration dependent manner
In order to determine the potency of ACC-049 at cold temperature (10°C) activation of human and rodent TRPM8, various concentrations of ACC-049 were incubated with CHO cells expressing human, rat or mouse TRPM8 channels prior to measuring cold-induced 45Ca2+ uptake. ACC-049 showed a concentration dependent inhibition of TRPM8 activation by cold (Fig. 3 and Table 2). ACC-049 inhibited cold activation of human TRPM8 channels with an IC50 value of 508±1.2 nM, and was slightly more potent at blocking rat and mouse channels with IC50 values of 144±1.2 nM and 188±1.3 nM, respectively (Fig. 3A–C and Table 2). Positive control small molecule antagonist M8-B inhibited human and rodent TRPM8 with IC50 values ranging from 2 to 4 nM, the same range as previously reported [9], while the negative control IgG had no effects (Table 2).
Figure 3. Cold activation of TRPM8.
Concentration dependent antagonism of cold activation (10°C) of the human (A), rat (B), or mouse (C) TRPM8 channels by ACC-049, control IgG and M8-B measured by 45calcium uptake. Note the right shifted concentration response of ACC-049 on human TRPM8 (A) compared to rat (B) or mouse (C) TRPM8, while the small molecule antagonist positive control M8-B exhibited comparable responses on TRPM8 channels of all species tested (A–C). Values are means of triplicate measures in a single experiment and expressed as percent of control (POC). Cold induced 45Ca2+ uptake was considered as 100 percent and wells with M8-B at 1 µM plus 45Ca2+ were set as zero percent.
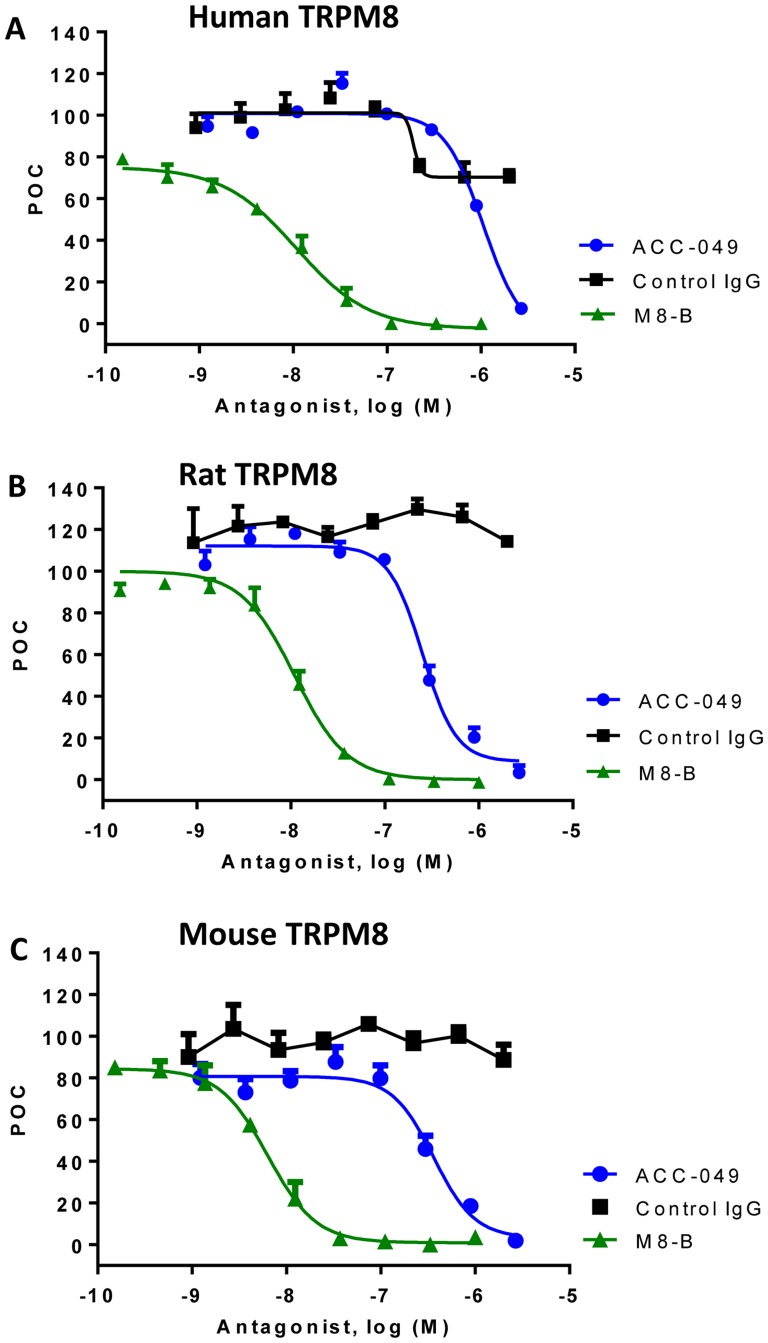
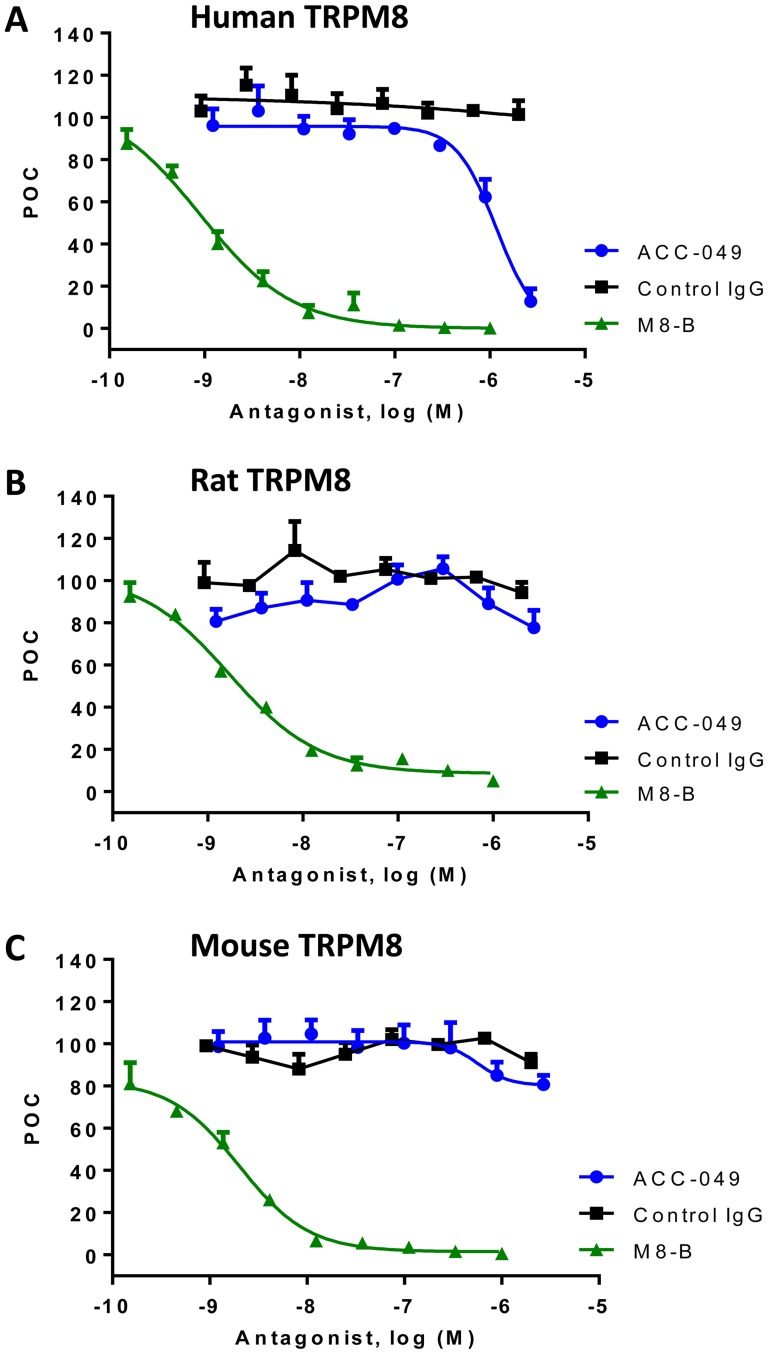
Table 2. IC50 values (nM) of cold, icilin, and menthol induced human, rat, or mouse TRPM8 channel activation by ACC-049.
| Human TRPM8 (nM) | Human TRPM8 (nM) | Rat TRPM8 (nM) | Rat TRPM8 (nM) | Mouse TRPM8 (nM) | Mouse TRPM8 (nM) | |
| ACC-049 | M8-B | ACC-049 | M8-B | ACC-049 | M8-B | |
| Cold | 508±1.2 | 3±1.1 | 144±1.2 | 2±1.2 | 188±1.3 | 2±1.2 |
| Icilin | 1054±1.3 | 11±1.2 | 252±1.1 | 11±1.1 | 363±1.2 | 6±1.1 |
| Menthol | 1160±1.1 | 1±1.4 | >2000 | 2±1.3 | >2000 | 2±1.2 |
Values are expressed as mean ± SD of one experiment conducted in triplicate wells in a 96 well format. Concentration ranges of ACC-049 and respective agonists are described in the text. IC50 of control IgG in all assays was >2500 nM.
ACC-049 blocks icilin activation of human and rodent TRPM8 channels in a concentration dependent manner
Next, we determined the potency of ACC-049 at icilin activation of human and rodent TRPM8 by preincubation of different concentrations of ACC-049 prior to quantitation of icilin-induced 45Ca2+ uptake. Similar to the blockade of cold activation of TRPM8, ACC-049 inhibited activation of rat and mouse TRPM8 channels by 1 µM icilin more potently than human TRPM8 with respective IC50 values of 252±1 nM and 363±1 nM, as compared to human TRPM8 channels with an IC50 value of 1054±1 nM (Fig. 4A–C and Table 2). Positive control small molecule antagonist M8-B inhibited human and rodent TRPM8 channels with IC50 values ranging from 6 to 11 nM, the same range as previously reported [9], while the negative control IgG had no effects (Table 2).
Figure 4. Icilin activation of TRPM8.
Concentration dependent antagonism of icilin induced activation of the human (A), rat (B) or mouse (C) TRPM8 channels by ACC-049, control IgG and M8-B measured by 45calcium uptake. Note the right shifted concentration response of ACC-049 on human TRPM8 (A) compared to rat (B) or mouse (C) TRPM8, while the small molecule antagonist positive control M8-B exhibited comparable responses on TRPM8 channels of all species tested (A–C). Values are means of triplicate measures in a single experiment and expressed as percent of control (POC). Icilin induced 45Ca2+ uptake was considered as 100 percent and wells with only assay buffer plus 45Ca2+ were set as zero percent.
Species differences in ACC-049 inhibition of TRPM8 by menthol activation
ACC-049 showed concentration dependent antagonism of human but not rodent TRPM8 activation by menthol revealing species differences. IC50 value at TRPM8 activation by 100 µM menthol was 1160±1 nM at human TRPM8 whereas no inhibition was observed up to 2 µM concentration of ACC-049 in CHO cells expressing either rat or mouse TRPM8 (Fig. 5A-C and Table 2). The positive control M8-B inhibited TRPM8 activation with similar potency (IC50 values of 1 to 2 nM) at all three species and the negative control IgG had no effects (Fig. 3–5 and Table 2).
Figure 5. Menthol activation of TRPM8.
Concentration dependent antagonism of menthol induced activation of the human (A), rat (B) or mouse (C) TRPM8 channels by ACC-049, control IgG and M8-B measured by 45calcium uptake. Human TRPM8 channel activation was blocked by ACC-049 in a concentration dependent manner (A), but there was no antagonistic effect of ACC-049 on either rat (B) or mouse (C) TRPM8 channels activated by menthol. The small molecule antagonist positive control M8-B exhibited comparable responses on TRPM8 channels of all species tested (A–C). Values are means of triplicate measures and expressed as percent of control (POC). Menthol induced 45Ca2+ uptake was considered as 100 percent and wells with only assay buffer plus 45Ca2+ were set as zero percent.
Antagonism of poly-and monoclonal antibodies against the third extracellular loop
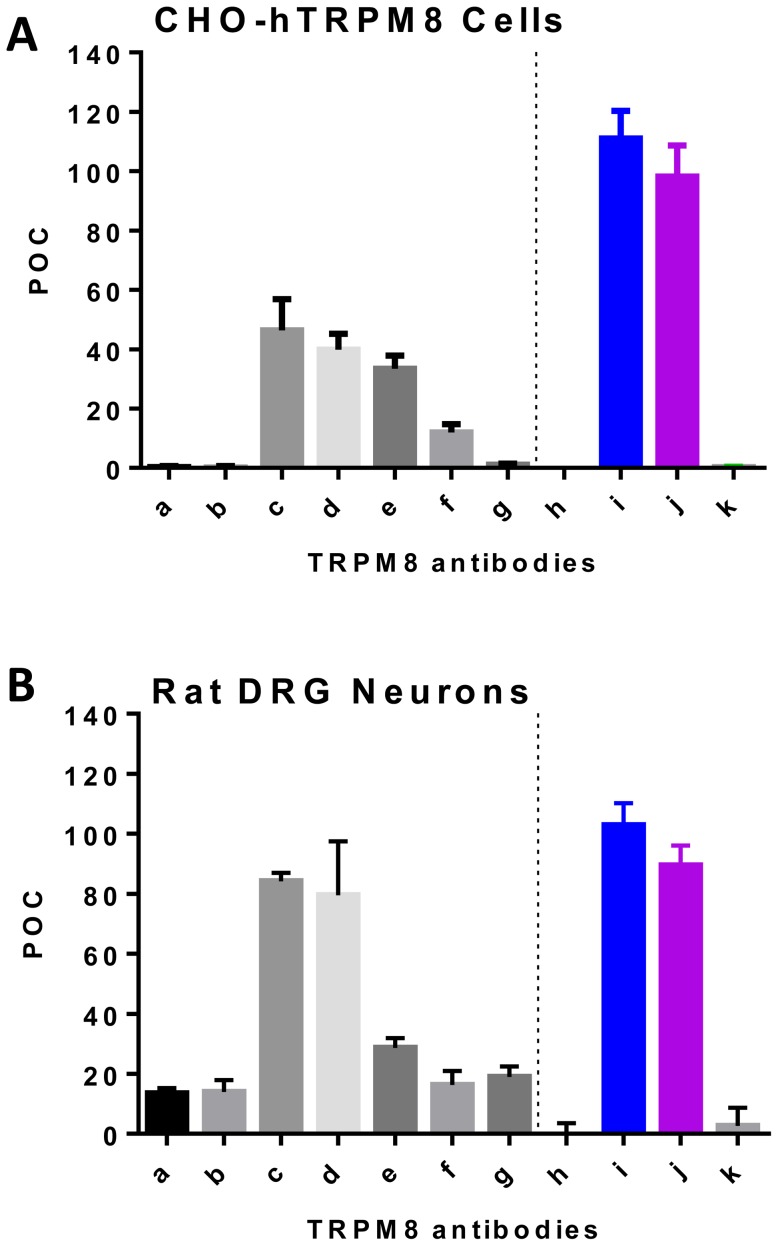
To further confirm that the blocking capabilities of ACC-049 were due to its binding to the extracellular pore loop, we tested additional six commercially available antibodies (Table 1) that were also generated against the third extracellular loop (the pore region) of human TRPM8 at a single concentration/volume in an icilin-induced 45Ca2+ uptake assay. All antibodies, polyclonal and monoclonal, blocked TRPM8 activation by 60% to 100% inhibition (Fig. 6). These data can only be interpreted in a qualitative manner because the concentrations of the antibody stocks were not provided by the manufacturers. Therefore, the differences seen in potency could be simply due to the differences of antibody concentration in the 30 µl of antibody solution used for the assay. At this single concentration, both poly- and monoclonal antibodies significantly blocked TRPM8 activation in this assay. Two polyclonal antibodies, ACC-049 (Alomone) and BML-SA664 (Enzo Life Sciences) as well as one monoclonal antibody, MBS609041 (MyBiosource) blocked icilin-induced 45Ca2+ uptake fully, as did the positive control antagonist M8-B. Other monoclonal (CABT 37242RH from Creative Diagnostics; LS-B6668 from Lifespan Biosciences) and polyclonal (OST00133W from Thermo Scientific; ABIN351226 from Antibodies online) antibodies blocked icilin-induced 45Ca2+ uptake partially. The peptide corresponding to the epitope in ACC-049 had no effects on its own in the icilin-induced 45Ca2+ uptake assay.
Figure 6. Icilin activation of TRPM8 in CHO cells and rat DRG neurons.
Antagonism of icilin induced activation of human TRPM8 recombinantly expressed by CHO cells (A) and rat DRG neurons (B) by additional poly- and monoclonal antibodies generated against the third extracellular pore loop. a. Alomone ACC-049. b. MyBiosource MBS609041. c. Creative Diagnostics CABT37242RH. d. Thermo Scientific OST00133W. e. Antibodies Online ABIN351226. f. Lifespan Biosciences LS-B6668. g. Enzo Lifesciences BML-SA664. h. M8-B. i. 1 µM icilin. j. 1 µM icilin + peptide (SDVD GTTYDFAHC). k. Buffer. A. Note the complete block of TRPM8 channel activation by ACC-049 (a), MyBiosource (b) and Enzo Lifesciences (g) antibodies at the single concentration tested. Small molecule positive control M8-B also completely blocked TRPM8 channel activation (h). B. Five out of seven antibodies tested (a, b, e, f, g) block icilin activation of rat DRG neurons by 70–80%, two antibodies (c, d) are ineffective. Values are means of triplicate measures in a single experiment and expressed as percent of control (POC). 45Ca2+ uptake of CHO-TRPM8 cells activated with 1 µM icilin and antigen peptide (j) was considered as 100 percent and wells with only assay buffer plus 45Ca2+ were set as zero percent.
In order to test the ability of antibodies to block native TRPM8 channels, we repeated the icilin-induced 45Ca2+ uptake assay with the same concentration/volume of the antibodies using freshly isolated rat DRG neurons. ACC-049 (Alomone), MBS609041 (MyBiosource), LS-B6668 (Lifespan Biosciences),BML-SA664 (Enzo Life Sciences), and ABIN351226 (Antibodies online) blocked icilin-induced 45Ca2+ uptake by 70 - 80% and CABT 37242RH (Creative Diagnostics) and OST00133W (Thermo Scientific) did not show significant block of icilin-induced 45Ca2+ uptake in this assay. Buffer alone or the peptide corresponding to the epitope recognized by ACC-049 did not affect the magnitude of icilin-induced on 45Ca2+ uptake. The positive control antagonist M8-B showed full inhibition of icilin-induced 45Ca2+ uptake in rat DRG neurons.
Discussion
In order to assess if antibodies could block TRPM8 channels, we have first characterized a commercially available rabbit polyclonal antibody (ACC-049) as a TRPM8 selective antagonist using agonist-induced cell-based 45Ca2+ uptake assays. This antibody acted as a full antagonist of TRPM8 activation by cold and icilin but showed species-specific differences at menthol activation.
ACC-049 blocked activation of human TRPM8 expressed in CHO cells by all three agonists tested, i.e., cold, icilin, and menthol. In contrast, only cold and icilin, but not menthol-induced activation was blocked by ACC-049 in rat and mouse TRPM8 expressing CHO cells. Interestingly, ACC-049 was approximately 2 to 4 fold more potent on rodent than human TRPM8 in blocking cold or icilin-induced activation. These differences in human versus rodent TRPM8 antagonism by ACC-049 could arise from species differences in antibody binding and/or species specific conformational changes of TRPM8 induced by the different agonists. The antigenic peptide sequence of ACC-049 differs between human and rodents (Figure 1). ACC-049 was generated against an extracellular epitope (amino acids 917-929) close to the pore region of human TRPM8. The human peptide sequence contains a glycine in position 921 and an alanine in position 927, whereas the rodent channel contains serine residues in these positions. Different critical residues/regions within the TRPM8 channel have been described for cold, menthol and icilin (for review, see [28]). Cold activation depends on the C-terminus [29], the icilin binding site is situated within the S2-S3 linker [30], [31], and the residues important for activation by menthol are found at multiple sites, e.g., within the C-terminus, the TRP box and the S2, S4 and S4-S5 linker region of human TRPM8 [32], [33]. The specific residue differences in human versus rodent TRPM8 may account for differential pharmacology of ACC-049 at icilin versus menthol activation.
Our finding that ACC-049 blocked both human and rodent TRPM8 activation by different ligands, which bind to different regions of the channel, suggests that high affinity binding of this antibody to the pore loop locks the channel in a closed conformation. Furthermore, we showed that not only ACC-049, but also other poly- and monoclonal antibodies directed against the same pore loop blocked icilin activation of the human TRPM8 channels recombinantly expressed in CHO cells and TRPM8 channels endogenously expressed in rat DRG neurons. This suggests that there are no pharmacological differences between endogenous or recombinant TRPM8 channels for inhibition by small molecule or antibody antagonists.
These data confirm previously published findings that targeting the third extracellular loop that forms the pore in several ion channels represents a promising strategy to generate antagonist antibodies [21], [22]. Several tool antagonist antibodies to ion channels have been described including polyclonal antibodies to potassium channels, e.g. Kv1.2 and Kv3.1 [34], TRP channels, e.g. TRPC1, TRPC5, TRPV1 and TRPM3 [20], [22], [35] and CaV1.2 channels [36], as well as monoclonal antibodies to hERG [37] and TRPA1 channels [38].
While the affinity of ACC-049 and the other commercial antibodies tested in our study is not sufficient for a therapeutic consideration, our data are encouraging for two reasons. First, we observed full blockade of the agonist-induced TRPM8 activation by ACC-049, albeit at high concentrations of the antibody. In the published studies mentioned above, only partial blockade of the respective ion channels had been achieved with antibodies. For therapeutic purposes, full channel blockade may be required and our data suggest that it is possible to block channel activation fully with antibodies. Second, in single concentration experiment, we identified three monoclonal antibodies that achieved 60% to 100% blockade of icilin-induced activation of the human TRPM8 channel. Only two monoclonal antagonist antibodies, one to hERG potassium channel [37], and another to TRPA1 channel [38], have been published to date, whereas two other monoclonal antibodies against the pore loop of TRPV1 did not block channel activation [20]. Our data support the view that it is feasible to generate monoclonal antagonist antibodies that show full block of channel activation by different mechanisms. Although challenges remain, the continued research with tool antagonist antibodies, and the availability of existing and emerging technologies to improve antibody performance may enable the development of monoclonal ion channel antagonist antibody therapeutics in the future.
Acknowledgments
We like to thank Mary Dovlatyan for help with ordering the antibodies and compiling Table 1, Nuria Tamayo for help with references, and Ken Wild for critical reading of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58. [DOI] [PubMed] [Google Scholar]
- 2. McCoy DD, Knowlton WM, McKemy DD (2011) Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol 300: R1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715. [DOI] [PubMed] [Google Scholar]
- 4. Dhaka A, Earley TJ, Watson J, Patapoutian A (2008) Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28: 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, et al. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208. [DOI] [PubMed] [Google Scholar]
- 6. Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, et al. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54: 379–386. [DOI] [PubMed] [Google Scholar]
- 7. Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, et al. (2007) TRPM8 is required for cold sensation in mice. Neuron 54: 371–378. [DOI] [PubMed] [Google Scholar]
- 8. Tajino K, Hosokawa H, Maegawa S, Matsumura K, Dhaka A, et al. (2011) Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLoS One 6: e17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, et al. (2012) Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32: 2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gavva NR, Davis C, Lehto SG, Rao S, Wang W, et al. (2012) Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, et al. (2009) Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 296: H1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Earley S, Brayden JE (2010) Transient receptor potential channels and vascular function. Clin Sci (Lond) 119: 19–36. [DOI] [PubMed] [Google Scholar]
- 13. Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, et al. (2010) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150: 439–450. [DOI] [PubMed] [Google Scholar]
- 14. Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, et al. (2006) Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balayssac D, Ferrier J, Descoeur J, Ling B, Pezet D, et al. (2011) Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf 10: 407–417. [DOI] [PubMed] [Google Scholar]
- 16. Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, et al. (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 3: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, et al. (2013) A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 33: 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel R, Goncalves L, Newman R, Jiang FL, Goldby A, et al. (2014) Novel TRPM8 Antagonist Attenuates Cold Hypersensitivity after Peripheral Nerve Injury in Rats. J Pharmacol Exp Ther 349: 47–55. [DOI] [PubMed] [Google Scholar]
- 19. Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, et al. (2008) AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol 295: F803–810. [DOI] [PubMed] [Google Scholar]
- 20. Klionsky L, Tamir R, Holzinger B, Bi X, Talvenheimo J, et al. (2006) A polyclonal antibody to the prepore loop of transient receptor potential vanilloid type 1 blocks channel activation. J Pharmacol Exp Ther 319: 192–198. [DOI] [PubMed] [Google Scholar]
- 21. Naylor DJ (2009) Extracellular Ion Channel Inhibitor Antibodies. The Open Drug Discovery Journal 1: 36–42. [Google Scholar]
- 22. Xu SZ, Zeng F, Lei M, Li J, Gao B, et al. (2005) Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol 23: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 23.Gore VK, Ma VV, Norman MH, Ognyanov VI, Xi N (2006) Preparation of imidazolylpyridinylpiperazines as vanilloid receptor ligands for treatment of pain. US Pat Appl Publ US 20060084640 A20060084641 20020060420.
- 24. Klionsky L, Tamir R, Gao B, Wang W, Immke DC, et al. (2007) Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain 3: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gavva NR, Tamir R, Klionsky L, Norman MH, Louis JC, et al. (2005) Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol 68: 1524–1533. [DOI] [PubMed] [Google Scholar]
- 26. Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, et al. (2005) AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313: 474–484. [DOI] [PubMed] [Google Scholar]
- 27.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies and National Research Council (2011) Guide for the care and use of laboratory animals. National Academies Press 8th edition.
- 28. Latorre R, Brauchi S, Madrid R, Orio P (2011) A cool channel in cold transduction. Physiology (Bethesda) 26: 273–285. [DOI] [PubMed] [Google Scholar]
- 29. Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R (2006) A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26: 4835–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chuang HH, Neuhausser WM, Julius D (2004) The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 43: 859–869. [DOI] [PubMed] [Google Scholar]
- 31. Pedretti A, Marconi C, Bettinelli I, Vistoli G (2009) Comparative modeling of the quaternary structure for the human TRPM8 channel and analysis of its binding features. Biochim Biophys Acta 1788: 973–982. [DOI] [PubMed] [Google Scholar]
- 32. Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, et al. (2006) High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 9: 493–500. [DOI] [PubMed] [Google Scholar]
- 33. Voets T, Owsianik G, Janssens A, Talavera K, Nilius B (2007) TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3: 174–182. [DOI] [PubMed] [Google Scholar]
- 34. Zhou BY, Ma W, Huang XY (1998) Specific antibodies to the external vestibule of voltage-gated potassium channels block current. J Gen Physiol 111: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ (2008) Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol 155: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watschinger K, Horak SB, Schulze K, Obermair GJ, Wild C, et al. (2008) Functional properties and modulation of extracellular epitope-tagged Ca(V)2.1 voltage-gated calcium channels. Channels (Austin) 2: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomez-Varela D, Zwick-Wallasch E, Knotgen H, Sanchez A, Hettmann T, et al. (2007) Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res 67: 7343–7349. [DOI] [PubMed] [Google Scholar]
- 38. Lee KJ, Wang W, Padaki R, Bi V, Plewa CA, et al. (2014) Mouse monoclonal antibodies to transient receptor potential ankyrin 1 act as antagonists of multiple modes of channel activation. J Pharmacol Exp Ther 350: 223–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.