Abstract
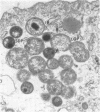
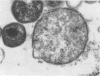
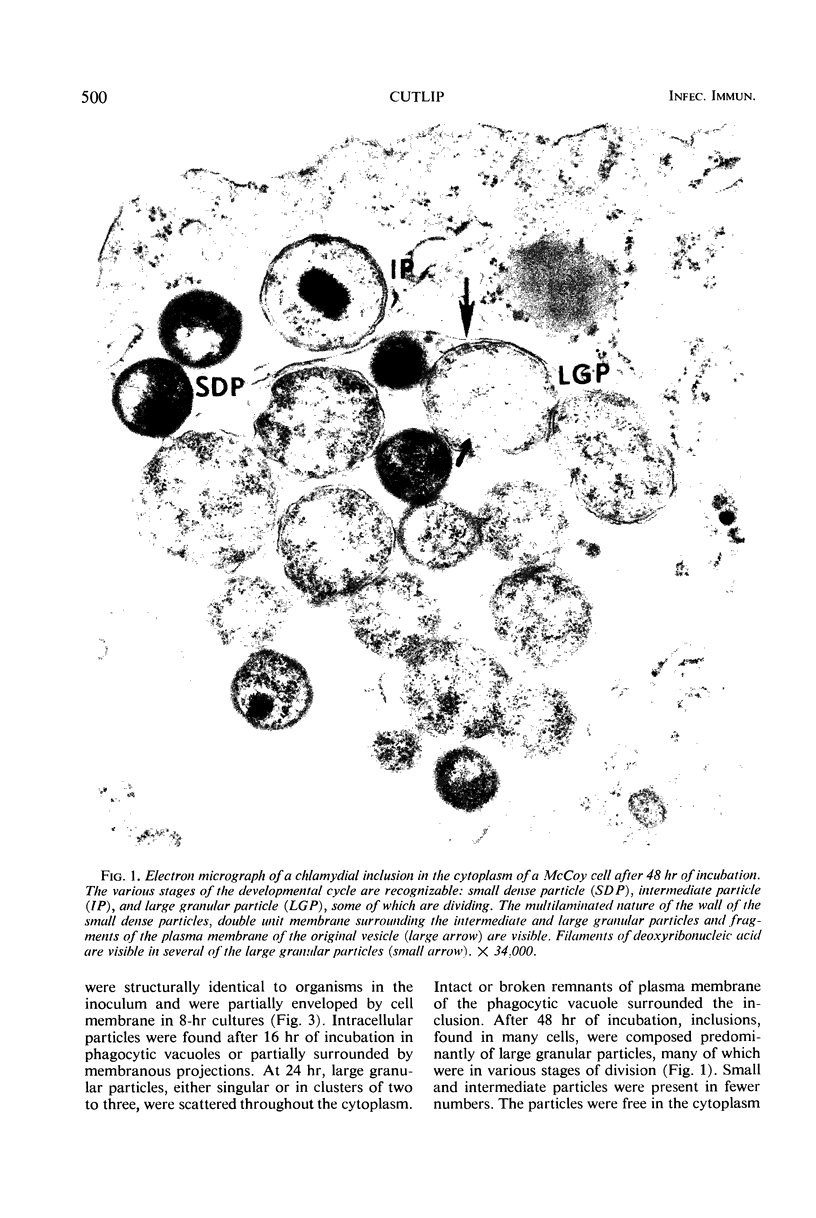
McCoy cell cultures infected with the agent of ovine chlamydial polyarthritis were examined with the electron microscope. The agent was seen as small dense particles (250 to 450 nm) with an eccentric nucleoid and a multilaminated cell wall, as large (800 to 1,200 nm) granular particles surrounded by two unit membranes and as intermediate particles. Replication, which occurred throughout the cytoplasm, was initiated by phagocytosis of a small dense particle and terminated by rupture of the plasma membrane. Upon entering a cell, the small dense particles developed into large granular particles which divided by binary fission. Daughter particles either repeated the division or condensed to form new small dense particles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. A., REED S. E. NATURE AND ORIGIN OF INITIAL BODIES IN LYMPHOGRANULOMA VENEREUM. Nature. 1964 Jan 25;201:371–373. doi: 10.1038/201371a0. [DOI] [PubMed] [Google Scholar]
- ERLANDSON R. A., ALLEN E. G. THE ULTRASTRUCTURE OF MENINGOPNEUMONITIS. Virology. 1964 Mar;22:410–418. doi: 10.1016/0042-6822(64)90031-5. [DOI] [PubMed] [Google Scholar]
- GAYLORD W. H., Jr Intracellular forms of meningopneumonitis virus. J Exp Med. 1954 Dec 1;100(6):575–580. doi: 10.1084/jem.100.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg K. C., Saxena R. C., Bisaria K. K. Bilateral symmetrical sectoral pigmentary lesions of retina. Am J Ophthalmol. 1967 Jan;63(1):165–167. doi: 10.1016/0002-9394(67)90602-2. [DOI] [PubMed] [Google Scholar]
- Higashi N. The mode of reproduction of psittacosis-lymphogranuloma-trachoma (PLT) group viruses. Int Rev Exp Pathol. 1964;3:35–64. [PubMed] [Google Scholar]
- LITWIN J., OFFICER J. E., BROWN A., MOULDER J. W. A comparative study of the growth cycles of different members of the psittacosis group in different host cells. J Infect Dis. 1961 Nov-Dec;109:251–279. doi: 10.1093/infdis/109.3.251. [DOI] [PubMed] [Google Scholar]
- MITSUI Y., KAJIMA M., NISHIMURA A., KONISHI K. Morphology and developmental cycle of the trachoma agent. Morphology of trachoma agent in conjunctiva and chick embryo. Ann N Y Acad Sci. 1962 Mar 5;98:131–144. doi: 10.1111/j.1749-6632.1962.tb30538.x. [DOI] [PubMed] [Google Scholar]
- NASS M. M., NASS S., AFZELIUS B. A. THE GENERAL OCCURENCE OF MITOCHONDRIAL DNA. Exp Cell Res. 1965 Mar;37:516–539. doi: 10.1016/0014-4827(65)90204-1. [DOI] [PubMed] [Google Scholar]
- SHUPE J. L., STORZ J. PATHOLOGIC STUDY OF PSITTACOSIS-LYMPHOGRANULOMA POLYARTHRITIS OF LAMBS. Am J Vet Res. 1964 Jul;25:943–951. [PubMed] [Google Scholar]
- STORZ J., SHUPE J. L., JAMES L. F., SMART R. A. POLYARTHRITIS OF SHEEP IN THE INTERMOUNTAIN REGION CAUSED BY A PSITTACOSIS-LYMPHOGRANULOMA AGENT. Am J Vet Res. 1963 Nov;24:1201–1206. [PubMed] [Google Scholar]