Abstract
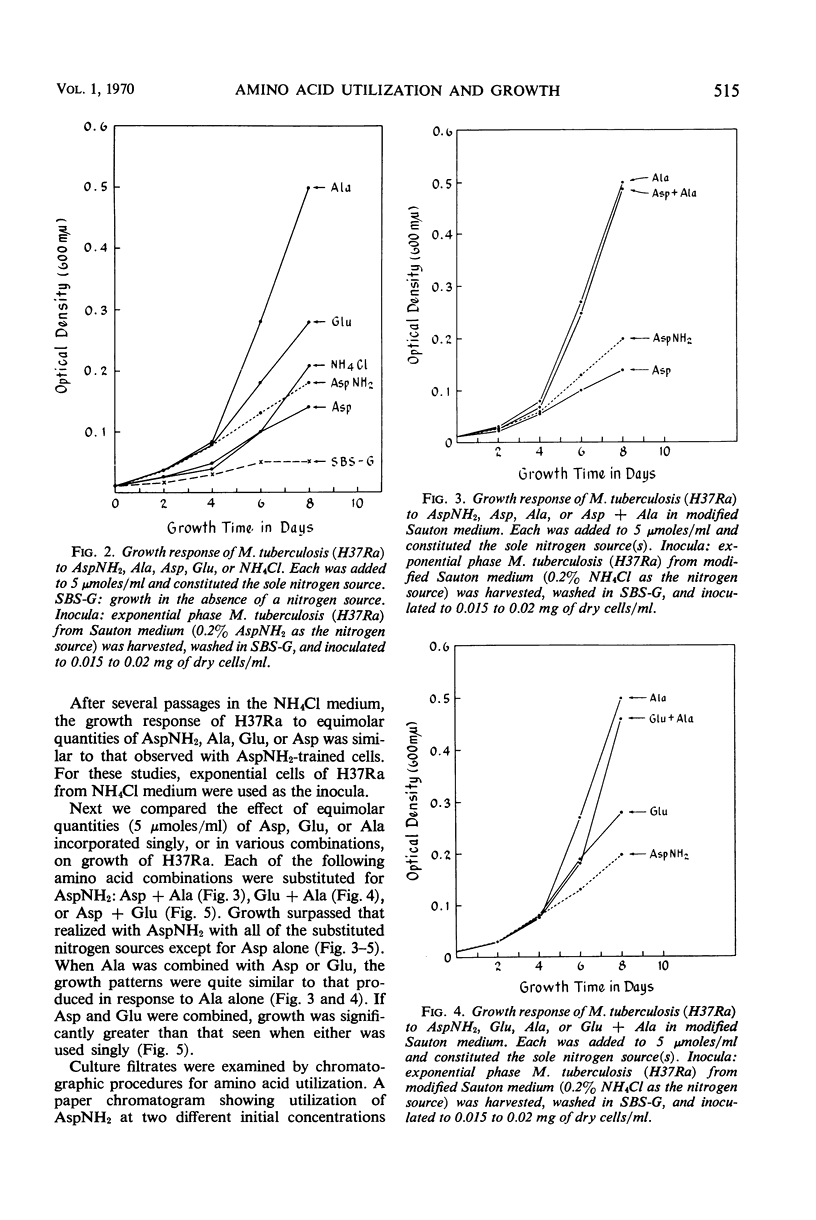
Marked differences were observed in the response of actively growing cells of the saprophyte, Mycobacterium smegmatis 607, and the avirulent human strain, M. tuberculosis (H37Ra), to several different nitrogen sources in aerated (rotary) cultures. The growth-promoting effect and utilization of equimolar concentrations (5 μmoles/ml) of l-alanine, l-aspartic acid, monosodium glutamate, or ammonium chloride were compared with that of l-asparagine, the normal nitrogen source, in Sauton synthetic liquid medium. The saprophyte grew equally well with each nitrogen source. However, marked differences were seen with H37Ra. Based on the rate of growth and cell yield, the relative growth-promoting effect of the amino acids for H37Ra is: alanine ≫ glutamate > asparagine > aspartic. Utilization of alanine, glutamate, and aspartic correlated well with growth. In contrast, utilization of asparagine during early growth of H37Ra was severalfold greater than that of either alanine or glutamate. Extracellular amino acids accumulated during the metabolism of asparagine but not during the utilization of the other nitrogen sources. Balanced metabolism of asparagine does not take place during aerated growth of H37Ra in asparagine media. During the metabolism of l-asparagine by M. tuberculosis (H37Ra) in aerated liquid cultures, metabolic controls may be exerted which impede protein synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEGGS W. H., LICHSTEIN H. C. REPRESSION OF TRYPTOPHANASE SYNTHESIS IN ESCHERICHIA COLI. J Bacteriol. 1965 Apr;89:996–1004. doi: 10.1128/jb.89.4.996-1004.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J. A., Segal W. Kinetics of Utilization of Organic Compounds in the Growth of Mycobacterium tuberculosis. J Bacteriol. 1965 Jul;90(1):157–163. doi: 10.1128/jb.90.1.157-163.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL RIO-ESTRADA C., PATINO H. Nicotinic acid biosynthesis by Mycobacterium tuberculosis. J Bacteriol. 1962 Oct;84:871–872. doi: 10.1128/jb.84.4.871-872.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG B., DUNN M. S. Effect of glutamic acid derivatives on growth and inhibition of Mycobacteria. Am Rev Tuberc. 1957 Apr;75(4):688–691. doi: 10.1164/artpd.1957.75.4.688. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Enzyme systems in the mycobacteria. VII. Purification, properties and mechanism of action of the alanine dehydrogenase. Biochim Biophys Acta. 1959 Aug;34:527–539. doi: 10.1016/0006-3002(59)90305-1. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., GROSSOWICZ N. Hydrolysis of amides by extracts from Mycobacteria. Biochem J. 1957 Apr;65(4):716–720. doi: 10.1042/bj0650716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H. N., Ramakrishnan R., Vaidyanathan C. S. L-asparaginases from Mycobacterium tuberculosis strains H37Rv and H37Ra. Arch Biochem Biophys. 1968 Jul;126(1):165–174. doi: 10.1016/0003-9861(68)90570-5. [DOI] [PubMed] [Google Scholar]
- KIRCHHEIMER F., WHITTAKER C. K. Asparaginase of Mycobacteria. Am Rev Tuberc. 1954 Nov;70(5):920–921. doi: 10.1164/art.1954.70.5.920. [DOI] [PubMed] [Google Scholar]
- KONNO K., KURZMANN R., BIRD K. T. The metabolism of nicotinic acid in Mycobacteria: a method for differentiating tubercle bacilli of human origin from other Mycobacteria. Am Rev Tuberc. 1957 Apr;75(4):529–537. doi: 10.1164/artpd.1957.75.4.529. [DOI] [PubMed] [Google Scholar]
- Kilburn J. O., Stottmeier K. D., Kubica G. P. Asparatic acid as a precursor for niacin synthesis by tubercle bacilli grown on 7H-10 agar medium. Am J Clin Pathol. 1968 Nov;50(5):582–586. doi: 10.1093/ajcp/50.5.582. [DOI] [PubMed] [Google Scholar]
- Konno K., Oizumi K., Shimizu Y., Tamagawa S., Oka S. Niacin metabolism in mycobacteria. Mechanism of excess niacin production by human tubercle bacilli. Am Rev Respir Dis. 1966 Jan;93(1):41–46. doi: 10.1164/arrd.1966.93.1.41. [DOI] [PubMed] [Google Scholar]
- LENERT T. F., STASKO I., HOBBY G. L. The cultivation of the bacille-Calmette-Guerin strain of M. tuberculosis (BCG). Am Rev Tuberc. 1958 Dec;78(6):934–938. doi: 10.1164/artpd.1958.78.6.934. [DOI] [PubMed] [Google Scholar]
- LYON R. H., LICHSTEIN H. C., HALL W. H. Influence of age on metabolic activity of Mycobacterium tuberculosis. Proc Soc Exp Biol Med. 1958 Oct;99(1):79–81. doi: 10.3181/00379727-99-24253. [DOI] [PubMed] [Google Scholar]
- Lyon R. H., Hall W. H., Costas-Martinez C. Upte and distribution of labeled carbon from 14C-asparagine by Mycobacterium tuberculosis. J Bacteriol. 1969 Apr;98(1):317–318. doi: 10.1128/jb.98.1.317-318.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MARSHAK A. Differences in response of a virulent strain of the tubercle bacillus and its avirulent variant to metabolites and their genetic significance. J Bacteriol. 1951 Jan;61(1):1–16. doi: 10.1128/jb.61.1.1-16.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLEBROOK G., COHN M. L. Bacteriology of tuberculosis: laboratory methods. Am J Public Health Nations Health. 1958 Jul;48(7):844–853. doi: 10.2105/ajph.48.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTT J. L. Asparaginase from mycobacteria. J Bacteriol. 1960 Sep;80:355–361. doi: 10.1128/jb.80.3.355-361.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULETTA G., DEFRANCESCHI A. Studies on the amino-acid metabolism of Mycobacterium tuberculosis. I. Preliminary investigation by paper chromatography. Biochim Biophys Acta. 1952 Sep;9(3):271–282. doi: 10.1016/0006-3002(52)90161-3. [DOI] [PubMed] [Google Scholar]
- WEISS D. W. The effects of mechanical agitation on the growth of the H37Ra strain of M. tuberculosis. Am Rev Tuberc. 1959 Jun;79(6):813–815. doi: 10.1164/artpd.1959.79.6.813. [DOI] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. Studies on the metabolism of Mycobacterium tuberculosis. V. The effect of amino acids on the growth of M. tuberculosis var. hominis. J Bacteriol. 1954 Jun;67(6):734–737. doi: 10.1128/jb.67.6.734-737.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]