Abstract
The authors describe a new modified surgical approach to minimize the postoperative recurrence of a syrinx after surgery to treat syringomyelia associated with spinal adhesive arachnoiditis in two cases. Both patients presented with progressive gait disturbance without any remarkable history, and spinal magnetic resonance imaging revealed a syrinx and broad irregular disappearance of the subarachnoid space and/or deformity of the cord. We successfully performed a partial arachnoid dissection and syrinx-far distal subarachnoid shunt for both cases.
Keywords: syringomyelia, arachnoiditis, untethering, shunt
Background and Importance
Syringomyelia associated with spinal arachnoiditis is commonly caused by trauma, infection, and surgery of the spinal cord. Severe cases with extensive adhesive arachnoiditis are very difficult to treat.1 Several authors have proposed an arachnoid dissection to resolve the major pathogenic factors responsible for syrinx initiation and propagation: cord tethering and blockage of the subarachnoid space caused by adhesion.2,3 However, surgical outcomes by broad arachnoid dissection and syrinx-subarachnoid shunts are limited by the risk of surgical damage to the cord and the postoperative recurrence of adhesions.4 Here, we present two cases that were successfully treated with partial arachnoid dissection and a syrinx-far distal subarachnoid shunt. Patients gave their consent for the publication of their medical information and images. A syrinx-far distal subarachnoid shunt, as opposed to a syrinx-subarachnoidal shunt, indicates that the distal end of the tube is placed at the region beyond the arachnoiditis.
Clinical Presentation
Case 1
A 52-year-old male patient had presented with progressive gait disturbance and had recognized losing the sensation of urination for a few months, without any remarkable history. In addition, he had no history of spinal epidural anesthesia. He had presented with no other sensory disturbances and no lower back pain. The Medical Research Council (MRC) power grade of both his lower limbs was 4 at the time of his hospital visit. Spinal magnetic resonance imaging (MRI) revealed a syrinx and irregular disappearance of the subarachnoid space extending from the C7 to T7 levels (Fig. 1).
Figure 1.
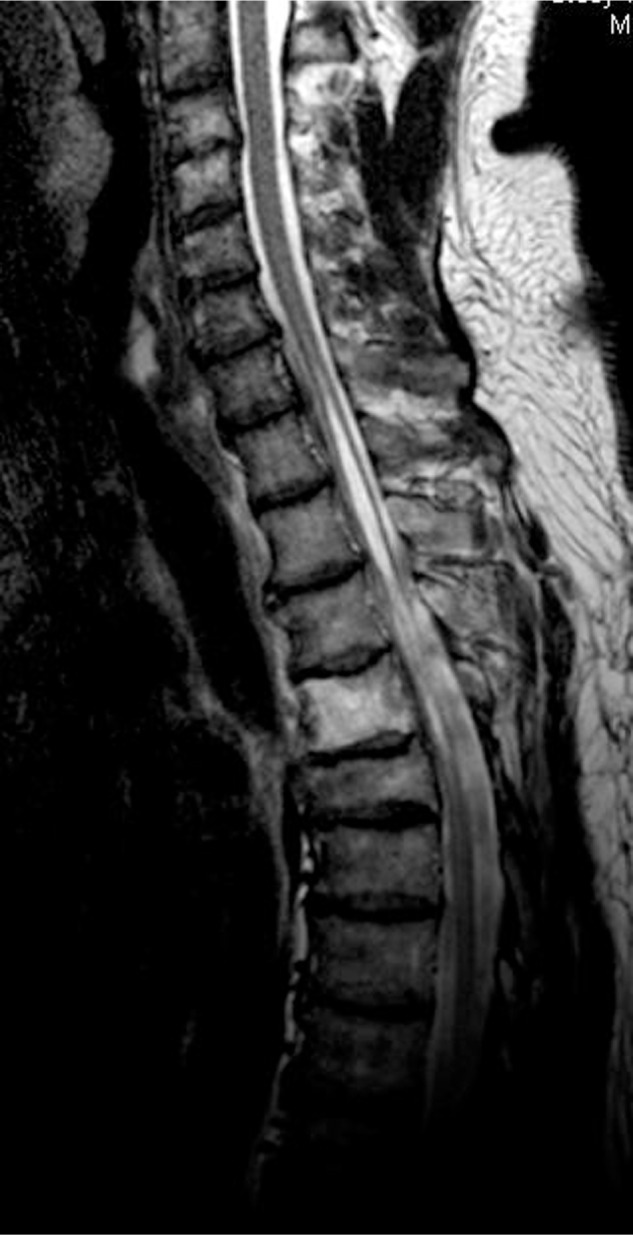
Preoperative MR T2-weighted image, demonstrating both the syrinx and the irregular disappearance of the subarachnoid space extending from the C7 to T7 levels.
Case 2
A 73-year-old male patient presented with progressive gait disturbance and numbness in both legs for 7 years, with no other remarkable history including spinal epidural anesthesia. He had presented with no other sensory disturbances and no lower back pain. The MRC power grade of both lower limbs was 4 at the time of his hospital visit. Spinal MRI revealed a deformity of the spinal cord, a syrinx at the level of T12, and the partial disappearance of the subarachnoid space extending from the T8 to L 1 levels (Fig. 2).
Figure 2.
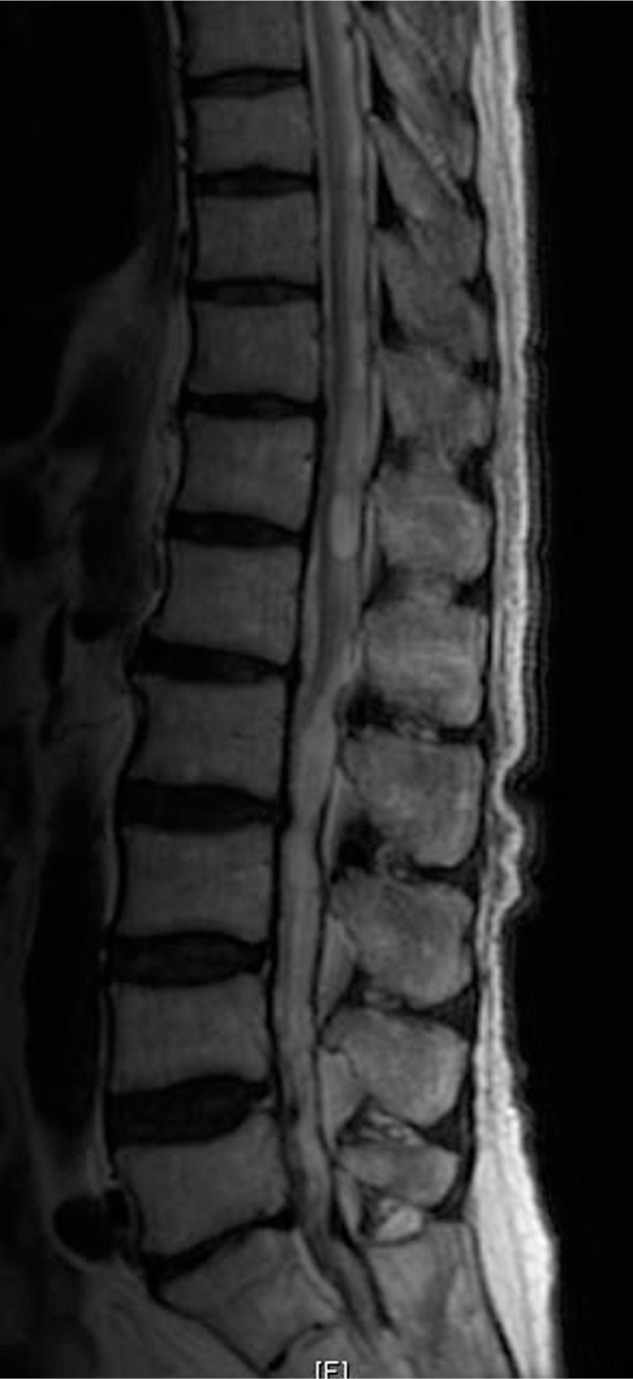
Preoperative MR T2-weighted image, demonstrating the deformity of the cord, the syrinx, and the partial disappearance of the subarachnoid space extending from the T8 to L 1 levels.
Surgical approach
Because the symptoms were progressive, laminectomies and partial arachnoid dissections of the dorsal surface of the cord, from the C7 to T7 (case 1) or from T8 to L (case 2), were performed along with a syrinx-far distal subarachnoid shunt (for both cases). We decided on the range of laminectomy and arachnoid dissection of the spinal cord to restore the blockage of cerebrospinal fluid (CSF) flow causing the syrinx. In both the cases, on opening the dura mater, the arachnoid membrane was found to be thick and adhered to the dura mater and spinal cord irregularly (Fig. 3). The adhesions were carefully dissected along the plane between the dura mater and arachnoid membrane under an operating microscope, and the spinal cord was released. We avoided manipulating the spinal cord as much as possible, and dissected only the arachnoid membrane. We were able to confirm this without retraction of the spinal cord. The adhesion was recognized to be nearly circumferential. We performed the dissection only on the dorsal surface of the cord to avoid retraction of the spinal cord. Shunt placement was performed between the syrinx and the far distal subarachnoid space without arachnoiditis to avoid the possibility of obstruction (Figs. 4–6). We took 6 hours in case 1, and 7 hours in case 2, for the surgery. In both the cases, we could see the syrinx through the pia mater at the posterior median sulcus, so we cut the pia mater and placed a 6-cm shunt tube for syringomyelia (Create Medic Co., Ltd., Yokohama, Japan). Because of arachnoid dissection-related CSF leakage, syrinx pressure was unremarkable, and was thus not recorded. We did, however, note a spout of CSF at the arachnoid dissection, particularly at the proximal region of the syrinx.
Figure 3.
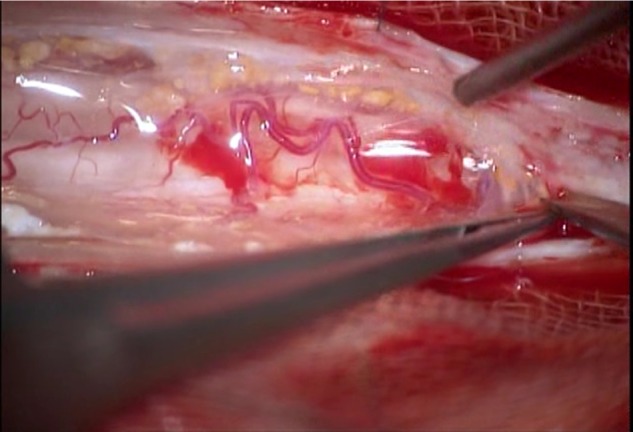
Intraoperative view. There are many arachnoid adhesions to the spinal cord and yellowish connective tissues in the subarachnoid space.
Figure 4.
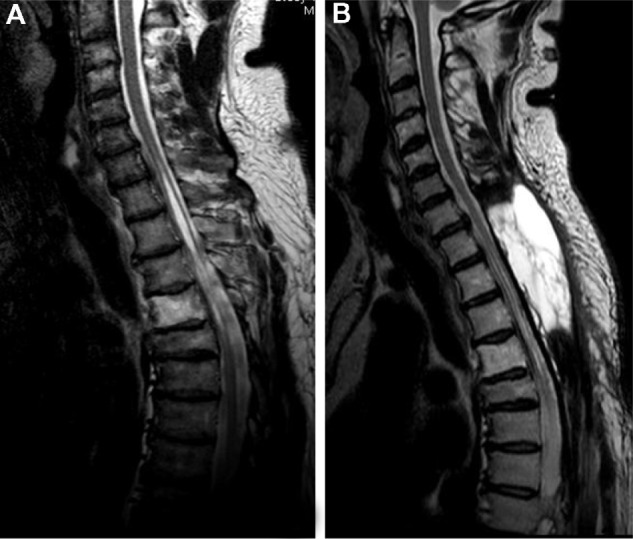
MRI in case 1. Preoperative (A) and postoperative (B) T2-weighted images demonstrating shrinkage of the syrinx. Note also the development of a large pseudomeningocele (in image B) 1 year after the surgery.
Figure 6.
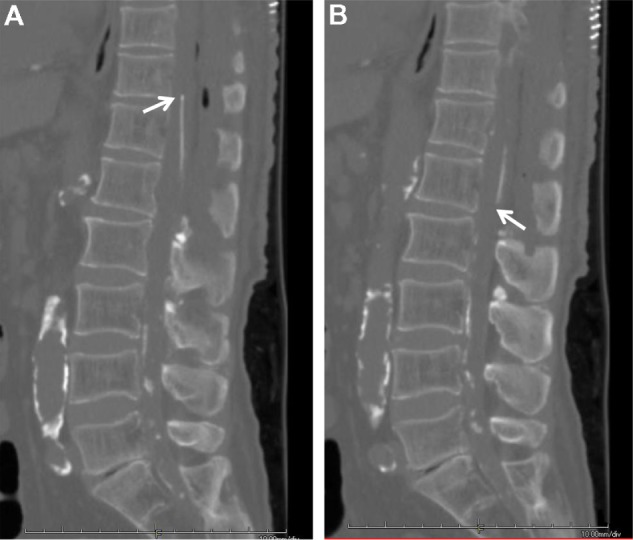
Postoperative CT showing the proximal tip of the shunt tube (A) and the distal tip of the tube (B).
Postoperatively, we identified transient motor dysfunction of MRC grade 3 in both lower limbs of both patients; this resolved to preoperative levels in a few days. Both patients underwent 4 weeks of rehabilitation following the surgery. The gait disturbance had gradually improved at a 1-year postoperative follow-up. An MRI taken at this follow-up revealed a large pseudomeningocele without symptoms in case 1 (see Fig. 4b).
Discussion
The association between spinal arachnoiditis and syringomyelia was first reported by Vulpian in 1861 and by Charcot and Joffroy in 1869. Some authors believed that the occlusion of blood vessels supplying the spinal cord by the arachnoid scarring was the underlying pathophysiological process for intramedullary cavitation.5 Williams implicated craniospinal pressure dissociation, secondary to obstruction of the subarachnoid space, as the factor responsible for cyst formation and extension.6 In 2004, Chang and Nakagawa7 went on to explain that blockage of the spinal subarachnoid CSF pathway produces a relative increase of the pressure inside the spinal cord, distal to the blockage point. Repetitive formation of this pressure gradient then induces CSF leakage into the spinal parenchyma, leading to the formation of syringomyelia. Milhorat considered this type of syringomyelia to be of the non-communicating type, because no connection between the cyst and the fourth ventricle could be demonstrated.8
The surgical treatment of syringomyelia associated with spinal adhesive arachnoiditis has been directed toward the drainage of the syrinx by myelotomy, or by shunting, with good short-term results.2,9 However, recent studies have revealed an unsatisfactory long-term prognosis with high rates of syrinx recurrence.10
Hence, several approaches for arachnoid dissection and decompression of the subarachnoid space have been reported.2 However, surgery to restore the free flow of CSF sometimes proves unsuccessful. On other occasions, adhesive arachnoiditis may be too extensive for any realistic attempts to be made. At times, it is also impossible to identify a focal point of obstruction to the CSF flow. Thus, when normal CSF circulation cannot be re-established, direct drainage of syringomyelia cavities, using syringo-subarachnoid, syringo-pleural, and syringo-peritoneal shunts with arachnoid dissection is a well-defined and accepted treatment modality.11,12 There is a possibility of closure of the shunt tube in the case of syringo-subarachnoid shunts around the arachnoiditis, and broad arachnoid dissection has the risk of surgical damage to the cord.4,13
Here, we reported two cases of syringomyelia associated with spinally adhesive arachnoiditis treated with a partial arachnoid dissection and syrinx-far distal subarachnoid shunt. The arachnoid dissection was based on the nature of the pathology, and we added the syrinx-far distal subarachnoid shunt. We placed the distal end of the shunt at the syrinx-far distal subarachnoid space, without arachnoiditis, to avoid shunt obstruction as much as possible.
We have followed up these patients after 1 year following surgery. There is a possibility of re-adhesion of the arachnoid by the surgeries. Because this surgical procedure has the potential for risk of infection, CSF leakage, shunt obstruction, and other complications, we have to consider surgery for this condition carefully.
Conclusion
Based on the pathology of syringomyelia associated with spinally adhesive arachnoiditis, a partial arachnoid dissection of the cord and a syrinx-far distal subarachnoid shunt might be a good approach.
Figure 5.
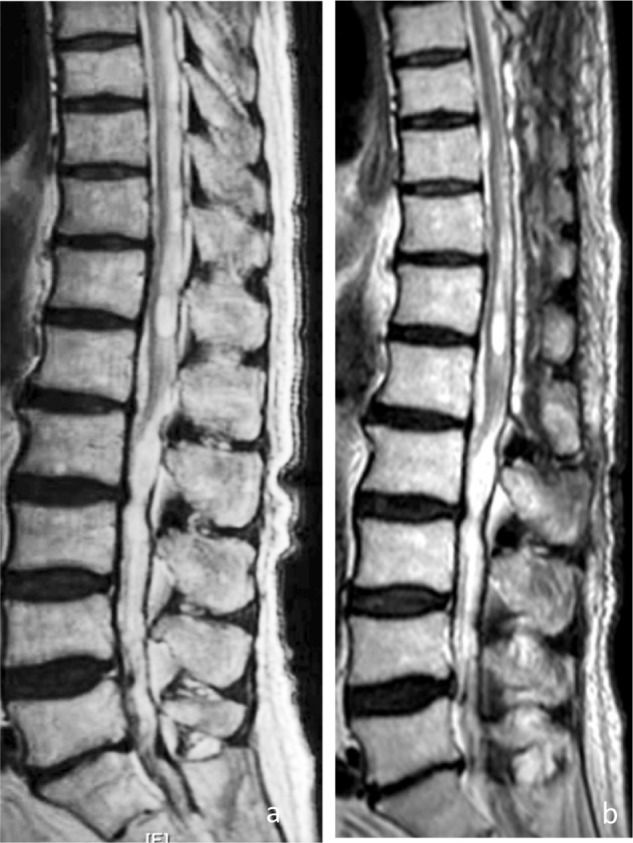
MR images in case 2. Preoperative (A) and postoperative (B) T2-weighted images demonstrating shrinkage of the syrinx 1 year after the surgery.
Figure 7.
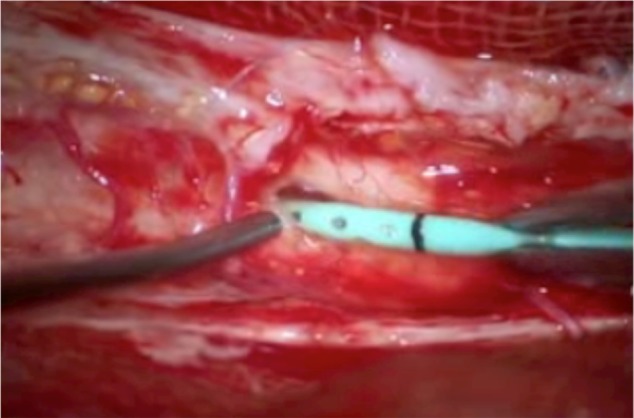
Intraoperative view. The syrinx was recognized through the pia mater and the shunt tube was inserted into the syrinx after cutting the pia mater.
Footnotes
Author Contributions
Conceived and designed the experiments: KI, Y-IO, KN, TM, TO. Analyzed the data: KI, Y-IO, KN. Wrote the first draft of the manuscript: KI. Contributed to the writing of the manuscript: KI, TY, Y-IO, KN, TM, TO. Agree with manuscript results and conclusions: KI, TY, Y-IO, KN, TM, TO. Jointly developed the structure and arguments for the paper: KI, TY, Y-IO, KN, TM, TO. Made critical revisions and approved final version: KI, TY. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Klekamp J, Iaconetta G, Batzdorf U, Samii M. Syringomyelia associated with foramen magnum arachnoiditis. J Neurosurg. 2002;97(3 suppl):317–22. doi: 10.3171/spi.2002.97.3.0317. [DOI] [PubMed] [Google Scholar]
- 2.Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86(2):233–40. doi: 10.3171/jns.1997.86.2.0233. [DOI] [PubMed] [Google Scholar]
- 3.Tobimatsu Y, Nihei R, Kimura T, et al. A quantitative analysis of cerebrospinal fluid flow in post-traumatic syringomyelia. Paraplegia. 1995;33(4):203–7. doi: 10.1038/sc.1995.45. [DOI] [PubMed] [Google Scholar]
- 4.Guyer DW, Wiltse LL, Eskay ML, Guyer BH. The long-range prognosis of arachnoiditis. Spine. 1989;14(12):1332–41. doi: 10.1097/00007632-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990;53(2):106–13. doi: 10.1136/jnnp.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams B. The distending force in the production of communicating syringomyelia. Lancet. 1969;2(7613):189–93. doi: 10.1016/s0140-6736(69)91427-5. [DOI] [PubMed] [Google Scholar]
- 7.Chang HS, Nakagawa H. Theoretical analysis of the pathophysiology of syringomyelia associated with adhesive arachnoiditis. J Neurol Neurosurg Psychiatry. 2004;75(5):754–7. doi: 10.1136/jnnp.2003.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milhorat TH, Capocelli AL, Jr, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82(5):802–12. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 9.Alvisi C, Cerisoli M. Long-term results of the surgical treatment of syringohydromyelia. Acta Neurochir (Wien) 1984;71(1–2):133–40. doi: 10.1007/BF01401158. [DOI] [PubMed] [Google Scholar]
- 10.Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82(1):1–10. doi: 10.3171/jns.1995.82.1.0001. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro NM, Wilson CB, Gutin PH, Edwards MS. Surgical treatment of syringomyelia. Favorable results with syringoperitoneal shunting. J Neurosurg. 1984;61(3):531–8. doi: 10.3171/jns.1984.61.3.0531. [DOI] [PubMed] [Google Scholar]
- 12.Phillips TW, Kindt GW. Syringoperitoneal shunt for syringomyelia: a preliminary report. Surg Neurol. 1981;16(6):462–6. doi: 10.1016/0090-3019(81)90248-2. [DOI] [PubMed] [Google Scholar]
- 13.Jenik F, Tekle-Haimanot R, Hamory BH. Non-traumatic adhesive arachnoiditis as a cause of spinal cord syndromes. Investigation of 507 patients. Paraplegia. 1981;19(3):140–54. doi: 10.1038/sc.1981.31. [DOI] [PubMed] [Google Scholar]


