Abstract
In this prospective phase II clinical trial, multiple myeloma (MM) patients were randomized to receive a second (tandem) autologous stem cell transplantation (ASCT) based on whether they achieved a partial response or worse (≤PR) following initial ASCT (ASCT1). Patients who achieved a very good partial response or better (≥VGPR) had salvage ASCT at relapse. Seventy-five patients received conditioning therapy and ASCT1. A total of 44 patients (59%) achieved ≥VGPR, whereas 31 patients entered ≤PR and were offered tandem ASCT. In all, 20 patients agreed to tandem ASCT. Demographic and clinical characteristics were similar between the two cohorts except for median lactate dehydrogenase (LDH) (P = 0.0141) and percentage of marrow plasma cells before ASCT1 (P = 0.0047), both lower in the ≥VGPR group. Intent to treat analysis showed that patients who achieved ≥VGPR to ASCT1 had a trend toward improved progression-free survival (PFS) (37 vs. 26 months, P = 0.078) and superior overall survival (OS) (not reached vs. 50 months, P = 0.0073). Patients with ≤PR who declined tandem transplantation had shortened PFS (20 vs. 28 months, P = 0.05) but similar OS (53 vs. 57.5 months, P = 0.29) compared to those who received it. Thus, a favorable clinical response to ASCT1 identifies a low-risk group with superior long-term prognosis despite similar PFS.
Keywords: multiple myeloma, autologous stem cell transplantation, tandem, survival
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) has remained a mainstay of treatment for over two decades after several prospective randomized trials demonstrated a benefit when compared to conventional chemotherapy (CCT) alone. When compared to CCT, ASCT resulted in higher complete response rates, increased progression free survival (PFS), and in some studies, prolonged overall survival (OS) because of an improvement in the duration of remission.1–3 Consequently, ASCT has become part of the standard of care for most MM patients.
In an effort to further improve outcomes, early non-randomized studies from the Arkansas group showed that tandem ASCT resulted in a higher response rate, prolonged event free survival (EFS), and improved OS when compared to standard therapy.4 Other studies corroborated an improvement in OS associated with tandem ASCT and presented data suggesting that patients who achieve less than a very good partial response (<VGPR) are most likely to benefit from tandem ASCT.5 In contrast, an Italian study found that tandem ASCT improved complete remission (CR) rate, near CR (nCR) rate, and relapse free survival (RFS); however, this group did not find a survival benefit with tandem ASCT.6 Subsequently, a number of other prospective and retrospective studies have attempted to answer this question with mixed results.7 Meta-analyses have added to the controversy by failing to consistently find improved survival after tandem ASCT.8–10
To address this equipoise of single versus tandem ASCT for patients with MM, we conducted the study presented here to better define the role of ASCT in MM. Based on prior work showing the greatest improvement in patients who achieve suboptimal responses after first ASCT (ASCT1),5,6 we hypothesize that patients who achieve ≥VGPR after ASCT1 may have favorable outcome without the need to have a tandem ASCT. Thus, we designed a trial that dictated second ASCT based on response to ASCT1. In specific, patients with a good response after ASCT1 (defined as achieving ≥VGPR) were offered observation or maintenance therapy per their treating physician, whereas those achieving poor response (defined as ≤PR) were offered tandem ASCT.
Subjects and Methods
Inclusion and exclusion criteria
This study was approved by the University of Florida Institutional Review Board, and all patients provided written informed consent to participate. Adults ≥18 years of age with recently diagnosed active symptomatic MM of all stages were eligible to participate. All patients had a Karnofsky performance status of ≥70% at the time of first ASCT (ASCT1). Response to induction therapy was not part of the exclusion criteria for the study; however, patients were offered additional treatment if ≥PR was not achieved after a single course of induction therapy and eventually moved on to ASCT1 regardless of whether ≥PR was achieved. Patients with progressive disease were excluded.
Treatment protocol
Our study did not specify the induction therapy to be given. The study patients were referred to us for transplant consideration after receiving induction therapy by their local oncologist. If needed, patients received second and third chemotherapy regimens to achieve better control of the disease, preferably achieve partial response. Thus, patients were treated with multi-drug induction chemotherapy before stem cell collection to reduce the plasma cell volume, alleviate symptoms, and prevent end-organ damage. After induction therapy, all patients underwent disease reassessment before ASCT1. All patients underwent peripheral blood stem cell (PBSC) collection using granulocyte colony stimulating factor (G-CSF) with the aim of collecting an optimal dose of 10 × 106 CD34+ cells/kg of ideal body weight for two transplants with minimal target dose of 4 × 106 CD34+ cells/kg. Patients with chemotherapy-sensitive disease were conditioned with our standard regimen at the time that includes busulfan 0.75 mg/kg PO every six hours on days −8 to −5, cyclophosphamide 60 mg/kg IV on days −3 and −2, and etoposide 10 mg/kg IV on days −4 to −2. Etoposide was omitted if patients were ≥65 years old. Our published experience with this regimen showed no major differences compared to high-dose melphalan conditioning regimen.11 A standardized target dose of autologous PBSC (5 × 106 CD34+/kg) was administered on day 0. G-CSF 5 μg/kg was administered subcutaneously starting on day +6 post-ASCT and until engraftment achieving absolute neutrophil count of ≥1500/mm3.
Disease response was evaluated at days +60–100 after ASCT using quantitative immunoglobulins, serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP), immunofixation, and skeletal survey. Bone marrow aspiration and biopsy were performed only to confirm CR, nCR, and VGPR. The uniform response criteria of the International Myeloma Working Group12 were used to assess treatment response, except that we counted the nCR into the VGPR group, which was defined by as one response category that required bone marrow biopsy to show <5% plasma cells as well. Patients who achieved ≥VGPR were offered maintenance therapy, whereas patients who achieved ≤PR were offered a second transplant (ASCT2) within four months from ASCT1. Maintenance was not specified by the study and was left for the treating physician and patient choice.
Patients with ≤PR who underwent ASCT2 received continuous intravenous cyclophosphamide 6 g/m2 infused over 96 hours (days −6 to −3) and low-dose total body irradiation (TBI) at 150 cGy twice daily for two days (days −2 and −1). In patients who had received prior irradiation, the TBI was replaced by melphalan 140 mg/m2 on day −2.13 The remaining frozen autologous PBSC dose was administered on day 0.
All patients were regularly followed up every two to three months in the outpatient clinic by our transplant team. Median follow-up was calculated from first transplant to the last available patient encounter.
Statistical methods
Our original plan was to enroll 30 patients in each arm to allow us to detect 20% difference in response rate (CR/VGPR/PR) between the two groups with a 95% confidence interval of 42–78% using intent to treat analysis. Because we had high percentage of patients refusing to stay in the tandem group, we had to extend the study further to allow more patients to complete the tandem transplant treatment plan. Indeed, because of the significant number of patients intended to undergo tandem transplant refused to do so, the main statistical analysis was done on the basis of intent to treat. Wilcoxon rank sum testing was used to compare differences in patient characteristics between two patient cohorts such as age at diagnosis, time from diagnosis to treatment, beta-2 microglobulin (β2M), and lactate dehydrogenase (LDH) levels in addition to assessments of bone marrow cellularity and plasma cell percentages at diagnosis and at ASCT1. Chi-squared testing was used to evaluate differences in race, gender, disease stage (stage ≤II vs. III), prior radiotherapy, marrow cellularity, and plasma cell percentage. Contingency table and Fisher’s exact test were used to compare response status at ASCT1 (≤PR vs. ≥VGPR/CR), and cytogenetics/fluorescence in situ hybridization (FISH) at diagnosis and at ASCT1 (standard risk vs. high risk). The LIFETEST procedure was used to compare PFS and OS between patients intended for single versus tandem autologous transplantation.
Results
Patient characteristics
Patients were enrolled in the study between April 2001 and February 2008. Seventy-five patients received induction therapy for MM and then received high-dose conditioning chemotherapy with busulfan, cyclophosphamide, and etoposide followed by ASCT. All 75 patients were included in the intention to treat analyses.
In all, 44 patients (58.7%) achieved ≥VGPR and 31 (41.3%) achieved ≤PR. There was no difference in age, race, sex, or Durie–Salmon stage between the two groups (Table 1). Time from diagnosis to ASCT1 and use of prior radiotherapy were also similar between the ≥VGPR (single ASCT) and ≤PR groups (tandem). β2M levels at diagnosis were similar between the two groups (median 3.77 vs. 3.2, P = 0.4332). Median LDH immediately before ASCT1 (LDH at diagnosis was not available) was significantly lower in the single transplant group compared to the tandem group (187 vs. 334, P = 0.0141). There were significantly more patients who achieved VGPR/CR status before ASCT1 in the single ASCT group (P = 0.0432).
Table 1.
Patient characteristics.
| ≥VGPR/SINGLE ASCT | ≤PR/TANDEM ASCT | P-VALUE | |
|---|---|---|---|
| Number of patients | 44 | 31 | |
|
| |||
| Age at diagnosis | 0.96 | ||
| Median (range) | 56 (27–70) | 56 (36–71) | |
|
| |||
| Race | 0.76 | ||
| White | 34 | 23 | |
| Other | 10 | 8 | |
|
| |||
| Sex | 0.29 | ||
| Male | 23 | 20 | |
| Female | 21 | 11 | |
|
| |||
| Time from diagnosis to transplant, months median (range) | 7.0 (5–104) | 9.0 (5–149) | 0.053 |
|
| |||
| Prior Radiotherapy | 0.80 | ||
| Yes | 13 | 10 | |
| No | 31 | 21 | |
|
| |||
| Durie-salmon stage | 0.29 | ||
| 1A | 2 | 1 | |
| 2A | 14 | 8 | |
| 2B | 2 | 0 | |
| 3A | 16 | 17 | |
| 3B | 10 | 5 | |
|
| |||
| Response status at ASCT1 | 0.0432 | ||
| PR | 30 | 28 | |
| VGPR/CR | 9/4 | 2/1 | |
| SD | 1 | 0 | |
|
| |||
| Number of induction regimens prior to ASCT1, (%) | 0.37 | ||
| 1 | 30 (68.2) | 18 (58.1) | |
| 2 | 10 (22.7) | 12 (38.7) | |
| 3 | 4 (9.1) | 1 (3.2) | |
|
| |||
| LDH at ASCT1*, median (range) | |||
| 187 (115–693) | 334 (119–739) | 0.0141 | |
|
| |||
| B-2 microglobulin at diagnosis, Median (range) | 3.37 (1.4–26.3) | 3.77 (1.1–24.8) | 0.43 |
Note:
LDH at diagnosis was not available for majority of patients.
Abbreviations: VGPR, very good partial response; PR, partial response; MR, minimal response; ASCT, autologous stem cell transplant.
There were no statistically significant differences in bone marrow cellularity between the two groups at the time of diagnosis or ASCT1 (Table 2). Percentage of plasma cells at diagnosis was similar between the two groups (Table 2). The percentage of plasma cells at the time of ASCT1 was significantly lower in the patients achieving ≥VGPR compared to those achieving ≤PR (median 7.5 vs. 3%, P = 0.0047). Cytogenetic ± FISH results at the time of diagnosis were known in only 35 patients, whereas it was available in most patients at the time of ASCT1. No significant differences were found between the two patient cohorts (Table 2). Overall at ASCT1, eight patients (18%) had chromosomal abnormalities (including deletion 13/13q and complex abnormalities) in the single transplant group versus five patients (16%) in the tandem group.
Table 2.
Comparison of bone marrow cellularity, plasma cells percentage, and cytogenetics at diagnosis and ASCT1, between single and tandem ASCT groups.
| ≥VGPR/SINGLE ASCT | ≤PR/TANDEM ASCT | P-VALUE | |
|---|---|---|---|
| Bone marrow at diagnosis | |||
| Cellularity % | 64 (5–100) | 55 (40–100) | 0.65 |
| Plasma cells % | 40 (2.5–100) | 50 (2.5–90) | 0.94 |
|
| |||
| Bone marrow at ASCT1 | |||
| Cellularity % | 40 (10–82.5) | 47.5 (20–97.5) | 0.36 |
| Plasma cells % | 3 (0–70) | 7.5 (1.5–80) | 0.0047 |
|
| |||
| Cytogenetics at diagnosis, N* | |||
| High-risk** | 5 | 3 | 0.71 |
| Standard-risk | 16 | 11 | |
|
| |||
| Cytogenetics at ASCT1, N | |||
| High-risk | 7 | 3 | 0.76 |
| Standard-risk | 29 | 24 | |
Notes:
Cytogenetics ± FISH were available only in 35 patients.
High risk includes del 13/13q by cytogenetics only, complex chromosomal abnormalities, and hypodiploidy. Other known high-risk abnormalities were not detected in our patients.
Of the 44 patients who achieved a good response of VGPR or better, 20 patients (45.5%) received maintenance therapy consisting of interferon (N = 10),14 prednisone (N = 6), thalidomide (N = 3), or lenalidomide (N = 1). All patients were offered salvage ASCT at relapse.
Of the 31 patients who achieved ≤PR after ASCT1 and were offered tandem transplantation, 20 (64.5%) received second autologous transplant. Reasons for not undergoing the tandem transplant included absence of socioeconomic resources, co-morbidities, or patient refusal. Of the 11 who achieved ≤PR after ASCT1 but did not receive the tandem ASCT, 9 (82%) received maintenance therapy with interferon (N = 6), prednisone (N = 2), or thalidomide (N = 1).
Clinical outcomes
The median follow-up was 50.3 months (range, 3–130.9) in the single ASCT arm and 49.1 months (range, 10–126.9) in the tandem ASCT arm.
Of the 20 patients who underwent tandem transplant, 4 patients achieved CR, 8 had VGPR, and 7 remained in PR and 1 in stable disease.
There were no treatment-related deaths in either group. The median PFS after ASCT1 between the groups was 37 months for the single ASCT versus 26 months for the tandem group (P = 0.078) (Fig. 1). OS was superior in the single ASCT group compared to those in the tandem group (unable to estimate because of limited follow-up vs. 50 months, respectively, P = 0.0073) (Fig. 2). Among the 11 patients who achieved ≤PR and were to undergo tandem transplantation but did not, there was a significant difference in PFS (20 vs. 28 months, P = 0.05) favoring the group that achieved ≥VGPR to ASCT1. Despite the improvement in PFS, there was no difference in OS (53 vs. 57.5 months, respectively, P = 0.29).
Figure 1.
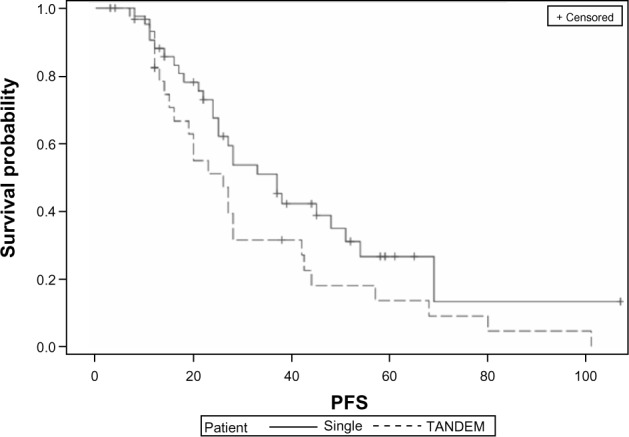
Kaplan–Meier curves showing PFS in single versus tandem ASCT on intent to treat analysis (P = 0.078).
Figure 2.
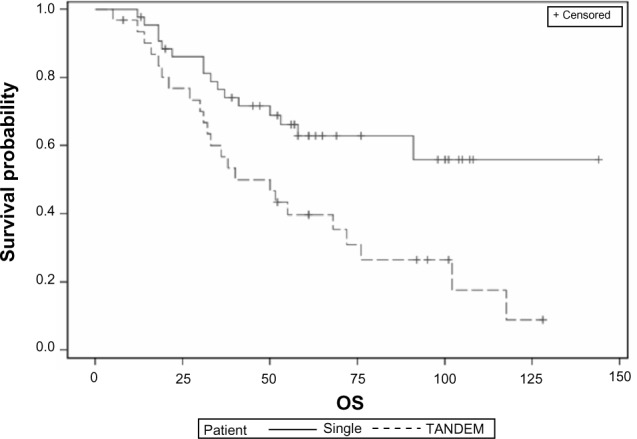
Kaplan–Meier curves showing OS in single versus tandem ASCT on intent to treat analysis (P = 0.0073). Median survival for the single group is not reached at the time of analysis.
As of the last follow-up, 33 patients (75.0%) from the single ASCT group had relapsed with a median time to disease progression of 21.3 months (range, 1.4–50.7 months). Among these patients, 26 (59.0%) were still alive. Six patients (13.6%) had undergone salvage ASCT and six patients (13.6%) had undergone salvage allogeneic stem cell transplantation. Alternatively, 19 patients (61.3%) in the tandem group relapsed with a median time to disease progression of 30.9 months (range, 7–87.7 months). Six of the tandem patients (30%) were alive at the time analysis. Among the patients in the ≤PR group who declined tandem transplantation, zero is alive today. From the tandem ASCT group, two patients (18.2%) underwent a third (salvage) ASCT and four patients (36.4%) underwent salvage allogeneic transplantation.
Discussion
Results from our study show that MM patients who achieve ≥VGPR after ASCT1 have significantly improved OS compared to those patients who achieve ≤PR despite receiving tandem transplantation. These findings are significant in that a favorable response to ASCT1 may identify a low-risk cohort of patients with a superior prognosis who may not require immediate tandem ASCT. Interestingly, this group of patients also had a higher VGPR/CR response rate even before their first ASCT.
Our study shows that the percentage of plasma cells seen on bone marrow aspiration and biopsy preceding ASCT1 is significantly lower in the low-risk single transplant group compared to the high-risk tandem group. Because of low-risk group’s association with a superior OS, plasma cell burden may represent a surrogate marker for patients who will have favorable outcomes following ASCT1. This finding confirms prior studies that have shown that bone marrow plasma cell percentage is an important prognostic marker for transplant.5,6 Furthermore, elevation in LDH before ASCT1 appears to represent a poor prognostic marker consistent with previous reports as well.15,16
Limitations of this study include its relatively small size that may have prevented some of the trends we have presented from reaching statistical significance. The attrition rate of 35.5%, due in part to patient refusal and co-morbidities, is another limitation of our study and underscores the toxicity of this treatment approach. Similar attrition rates have been reported in other published tandem transplant studies.5,6 The fact that our study was done before the availability of the novel drugs, bortezomib and lenalidomide, may be seen as another limitation; however, our study’s main conclusion should still be valid except that the use of these drugs may possibly have resulted in more patients achieving ≥VGPR after ASCT1 and therefore we would have seen less patients needing to undergo tandem transplants. Finally, the fact that our patients are heterogeneous and not highly selected could have wider therapeutic implications because our study design may better reflect the real practice in a tertiary referral center like ours.
Indeed, with the advent of newer biologic therapies, the role of tandem transplantation has become less clear. A future study in which newly diagnosed patients are treated with ASCT and those with ≤PR are randomized to maintenance with a novel agent versus tandem ASCT may further define the role of tandem ASCT in this disease. A current randomized clinical trial sponsored by BMT Clinical Trial Network is designed to address this question.
While the transplant-related mortality (TRM) in this study was 0%, other studies have reported TRM as high as 4.6% with tandem ASCT that has been corroborated in a meta-analysis.8 Thus, because of the 0% mortality in our study, the different outcomes described here among the two groups are more likely to reflect the intrinsic biology of the disease, hence the title of our manuscript. Thus, our study suggests that achievement of a ≥VGPR following ASCT1 may identify a group of low-risk patients with superior prognosis and may not benefit from upfront tandem ASCT. This biologic factor of response to first ASCT can certainly have clinical implication in determining which patients should receive more treatments including consolidation with tandem ASCT or other combination therapy with the aim of further improving their long-term outcomes.
Footnotes
Author Contributions
Conceived and designed the experiments: JSM, HL, MNC. Analyzed the data: MB, DS, QA, JSM, MNC. Wrote the first draft of the manuscript: MB, JSM. Contributed to the writing of the manuscript: MB, JSM, JRW, CRC. Agree with manuscript results and conclusions: MB, DS, HL, CRC, AD, JWH, LW, MNC, QA, JRW, JSM. Jointly developed the structure and arguments for the paper: JSM, MB. Made critical revisions and approved final version: JSM. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: CRC has received grant funding as a Leukemia & Lymphoma Society Scholar in Clinical Research.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–7. doi: 10.1182/blood-2004-02-0408. [DOI] [PubMed] [Google Scholar]
- 4.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–93. [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 6.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 7.Bergantim R, Trigo F, Guimarães JE. Impact of tandem autologous stem cell transplantation and response to transplant in the outcome of multiple myeloma. Exp Hematol Oncol. 2012;1:35. doi: 10.1186/2162-3619-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Kharfan-Dabaja MA, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101:100–6. doi: 10.1093/jnci/djn439. [DOI] [PubMed] [Google Scholar]
- 9.Kharfan-Dabaja MA, Hamadani M, Reljic T, et al. Comparative efficacy of tandem autologous versus autologous followed by allogeneic hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol. 2013;6:2. doi: 10.1186/1756-8722-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naumann-Winter F, Greb A, Borchmann P, Bohlius J, Engert A, Schnell R. First-line tandem high-dose chemotherapy and autologous stem cell transplantation versus single high-dose chemotherapy and autologous stem cell transplantation in multiple myeloma, a systematic review of controlled studies. Cochrane Database Syst Rev. 2012;10:CD004626. doi: 10.1002/14651858.CD004626.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogle CR, Moreb JS, Leather HL, et al. Busulfan, cyclophosphamide, and etoposide as conditioning for autologous stem cell transplantation in multiple myeloma. Am J Hematol. 2003;73:169–75. doi: 10.1002/ajh.10342. [DOI] [PubMed] [Google Scholar]
- 12.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 13.Byrne M, Wingard JR, Moreb J. Continuous infusion cyclophosphamide and low-dose total body irradiation is a safe and effective conditioning regimen for autologous transplant in multiple myeloma. Transplant Proc. 2013;45:3361–5. doi: 10.1016/j.transproceed.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Salmasinia D, Chang M, Wingard JR, Hou W, Moreb JS. Combination of IFN-α/Gm-CSF as a maintenance therapy for multiple myeloma patients after autologous stem cell transplantation (ASCT): a prospective phase II study. Clin Med Insights Oncol. 2010;4:117–25. doi: 10.4137/CMO.S6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlowski R, Shah N, Bashir Q, et al. Role of serum lactate dehydrogenase (LDH) as a prognostic marker for autologous hematopoietic stem cell transplantation for multiple myeloma; American Society of Hematology Annual Meeting and Exposition; 2012; Atlanta, GA. [Google Scholar]
- 16.Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med. 1991;115:931–5. doi: 10.7326/0003-4819-115-12-931. [DOI] [PubMed] [Google Scholar]


