Abstract
Objective
Chromosome 22q11.2 deletion syndrome (22q11DS) confers 25% risk for psychosis and is an invaluable window for understanding the neurobiological substrate of psychosis risk. The Structured Interview for Prodromal Syndromes (SIPS) is well validated in non-deleted populations for detecting clinical risk but has been only recently applied to 22q11DS. We assessed the largest 22q11DS cohort to date and report on SIPS implementation and symptoms elicited.
Method
The SIPS, including its 19 subscales, was administered to 157 individuals with 22q11DS aged 8 to 25. Youth and caregiver interviews were conducted and rated separately, then compared for agreement. Implementation of the SIPS in 22q11DS was challenging due to the prevalence of developmental delay and comorbid conditions. However, by explaining questions and eliciting examples, we were able to help youths and caregivers understand and respond appropriately. Consensus ratings were formulated and analyzed with item-wise and factor analysis.
Results
Subthreshold symptoms were common, with 85% of individuals endorsing one or more. The most commonly rated items were ideational richness (47%) and trouble with focus and attention (44%). Factor analysis revealed a three-factor solution with positive, negative, and disorganized components. Youth-caregiver comparisons suggested that youths report greater symptoms of perceptual abnormalities, suspiciousness, trouble with emotional expression, and bizarre thinking. Caregivers reported more impaired tolerance to normal stress, poor hygiene, and inattention.
Conclusion
The SIPS was adapted for 22q11DS through comprehensive and semi-structured administration methods, yielding a high prevalence of subthreshold psychotic symptoms. The significance and predictive validity of these symptoms require future longitudinal analysis.
Keywords: 22q11.2 Deletion Syndrome, Psychosis, Schizophrenia, Prodromal, Structured Interview for Prodromal Syndromes
Introduction
Chromosome 22q11.2 deletion syndrome (22q11DS) is associated with markedly elevated risk for schizophrenia and is increasingly recognized as a unique window into understanding psychosis risk.1–4 Genetically, 22q11DS arises from a hemizygous deletion of 1.5–3 megabases on chromosome 22 in approximately 1:4000 live births.5,6 The associated phenotype is variable and can include neuropsychiatric and physical features, with cardiac, palate, endocrine, and immunologic abnormalities.5,7–9 Psychiatric disorders are common, affecting three quarters of individuals with 22q11DS; there is increased risk for autism, attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, and, most notably, psychosis.10–15 Approximately a third of individuals with 22q11DS develop psychotic disorders by adulthood, representing a 25-fold increase in psychosis risk over the general population, and 10-fold over other developmentally delayed populations.2,3,16 Therefore, 22q11DS is a unique opportunity to investigate the pathogenesis of psychosis-spectrum disorders.
Early identification of psychosis-proneness has become a focus of ongoing research.3,17–23 Subthreshold symptoms of psychosis are not uncommon in the general population and are more prevalent in children (17.5%) than in adolescents (7.2%) or adults (5%).24,25 Criteria have been developed to define an “at-risk mental state” or “prodrome” for individuals with significant symptomatic burden but who do not meet criteria for schizophrenia spectrum disorders.18,26 The Structured Interview for Prodromal Syndromes (SIPS) is a well-validated instrument evaluating subthreshold psychotic symptoms.27,28 Interrater reliability is excellent, and criteria for the clinical high-risk state predict approximately one fifth convert to psychosis at 1 year and one third at 3 years in non-deleted populations.18,27–30 The SIPS provides 19 subscales comprising the Scale of Prodromal Symptoms (SOPS) that are theoretically grouped into positive, negative, disorganized and general domains, with gradation of severity optimally centered around subthreshold levels.27
The identification of individuals with 22q11DS at clinical risk for psychosis can contribute to elucidating the pathogenesis of psychosis because it can link a specific genetic mechanism to brain and behavior phenotypes. It is therefore essential to assess subthreshold psychotic symptoms in 22q11DS and relate them to neurocognition, neuroimaging, and genomics. Few studies, relatively limited in sample size, have applied the SIPS to investigate subthreshold symptoms of psychosis in 22q11DS.19–23 They report that subthreshold symptoms are common in 22q11DS but vary widely across subscales, with 2–85% reaching subthreshold levels depending on the scale applied.19–21,23 To our knowledge, no prior report has presented the methodology of adapting the SIPS to a developmentally delayed population with medical comorbidity, for which it was not designed.
In this study, we thoroughly investigate subthreshold symptoms of psychosis in the largest sample to date of young individuals with 22q11DS. Our aims are to: [1] Confront the challenges in applying the SIPS to 22q11DS; [2] Characterize subthreshold psychotic symptoms in 22q11DS and assess the effects of age, sex and reading proficiency; [3] Evaluate the factor structure of the SIPS and compare it to the theorized positive, negative, disorganized, and general domains; [4] Compare youth and caregiver reports to detect a possible informant effect on symptom reporting.
Method
Sample
We evaluated a cohort of 157 youths with 22q11DS, aged 8 to 25 years old (Table 1). Participants were recruited primarily through the genetics clinic “22q and You Center” at the Children’s Hospital of Philadelphia, in addition to social networks. All participants had a molecularly confirmed deletion of the 22q11.2 region. Exclusion criteria included inability to provide assent or informed consent as well as moderate to severe intellectual disability based on clinical evaluation and IQ testing when available (estimated IQ<70). Individuals with significant intellectual disability were excluded because they were likely to have little insight into psychiatric phenomena, and findings would have limited generalizability to the general population.
Table 1.
Sample Characteristics
| Variable | Measures |
|---|---|
| N | 157 |
| Mean Age (yrs ± SD) | 15.2 ± 4.8 |
| Sex (%) | |
| Male | 91 (58) |
| Female | 66 (42) |
| Reading Proficiency (±SD) | 90.1 ± 13.5 |
| Race (%) | |
| Caucasian | 138 (88) |
| African American | 10 (6) |
| Other/Mixed | 9 (6) |
| Education (yrs ± SD) | |
| Proband | 7.8 ± 4.0 |
| Mother | 14.7 ± 2.6 |
| Psychosis Spectrum (%) | 97 (62) |
Note: Reading proficiency is estimated with the Wide Range Achievement Test 4 reading segment; scores are standardized by age to mean (100, SD = 15). Yrs = years.
Study procedures were conducted while the participants were medically stable and ambulatory. No changes were made in the participants’ medical and behavioral treatment. Six participants had used antipsychotic medication within six months of the assessment. The Institutional Review Boards of the University of Pennsylvania and the Children’s Hospital of Philadelphia approved all procedures. Informed consent/assent was obtained from each participant and accompanying parent. A recent publication describes overall psychopathology and treatment for 112 of the participants.10
Administration of the SIPS
The SIPS was administered by Bachelor’s- and Master’s-level interviewers who underwent formal training conducted by a doctoral-level clinical psychology faculty member (MEC) with extensive experience and training in the semi-structured interview assessment and diagnosis of psychotic and sub-psychotic symptoms. The training protocol consisted of a structured program of lectures, supervised practice sessions, and mock interviews. Interviewers then administered the SIPS under direct supervision at least five times and until competency and consistency were established by scoring ≥85% on a standardized 60-item rating scale assessing proficiency in administration and scoring (e.g. establishing the presence/severity/temporal relationship of all symptoms, differentiating among ambiguous symptoms, resolving contradictions, clarifying non-definite answers, avoiding leading questions, soliciting appropriate examples, and establishing good rapport).
The SIPS was modified to produce a parent version by substituting third-person pronouns and inserting the term “your child.” Both versions were computerized. After each positive response on the SIPS, the interviewer followed up to determine severity, duration, context, chronology, and related distress or impairment in order to make ratings on the SOPS. Responses and follow-up questions were transcribed during the course of the interview to allow review. As previously described,10 participants were also assessed for ADHD, mood, and anxiety disorders using components of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS).31 Medical, family, social, and treatment histories were obtained for all participants.
Whenever possible, separate interviews were conducted for probands and their collateral informants – usually the mother. The collateral version was administered independently to all parents of children under 18 years as well as parents of adult participants if feasible. Younger probands (ages 8–10) received clinical evaluations where specific probes asked for subjective and common symptoms (including delusional and bizarre ideas, suspiciousness, grandiosity, perceptual abnormalities, disorganized speech, social anhedonia, and avolition). Information was collected covering all SIPS items along with areas of suspected significance revealed by the collateral interview. Probands aged 11 and older received the full SIPS. Whenever discrepancies emerged, both informants were probed for clarification. SOPS scores were based on both proband and collateral interviews for 76 participants, proband interview alone for 22 participants, and collateral interview alone for 53. For six participants, due to participant preference or time constraints, the interview was conducted with the proband and collateral together.
SOPS Scoring and Consensus Diagnosis
The 19 items on the SOPS were rated according to standardized anchors on a 7-point scale: 0=absent, 1=questionably present, 2=mild, 3=moderate, 4=moderately severe, 5=severe (but not psychotic), 6=severe and psychotic/extreme.27 Scoring was completed separately for the proband and collateral at the time of the interview. Following the completed assessment, information from proband and collateral interviews was integrated into a narrative case summary and given preliminary combined ratings based on all reliably reported symptoms. For younger participants who did not receive the full proband SIPS, scoring was based on the collateral interview as well as a clinical interview with the proband conducted by established clinical investigators. Only symptoms occurring in the last six months were considered for scoring. These ratings were presented and finalized by consensus from at least two doctoral-level clinicians with expertise in psychosis and child psychopathology; ratings were established based on standardized SOPS anchors.27 The consensus conference was a key procedure for maintaining accuracy and consistency in SIPS scoring and diagnosis.
Determination of participants’ status as “psychosis-prone,” “psychotic,” and “psychosis spectrum” also occurred during consensus case conference. Twelve participants (8%) were fully psychotic, meeting DSM-IV-TR criteria for schizophrenia (n=7), psychosis not otherwise specified (NOS; n=3), and delusional disorder (n=2).32 Following the Criteria of Prodromal Syndromes, 30 participants (19%) were considered to have Attenuated Positive Symptom (APS) syndrome, displaying ≥1 “positive” symptom that occurred at least weekly, was scored 3–5, and had worsened significantly or appeared in the past year.27 In addition, we established criteria for “psychosis-proneness” to include individuals with significant positive subthreshold symptoms but without recent worsening, as well as those with significant negative and disorganized symptoms. Participants were considered “psychosis-prone” if they did not meet criteria for a lifetime DSM-IV-TR psychotic disorder or mood disorder with psychotic features and met at least one of the following criteria: [1] One or more clinically significant “positive” symptom rated 3–5 on the SOPS (unusual thought content, suspiciousness/persecutory ideas, grandiose ideas, perceptual abnormalities, disorganized communication); [2] Two or more clinically significant “negative” or “disorganized” symptoms rated 3–6 on the SOPS (social anhedonia, avolition, expression of emotions, experience of emotions and self, ideational richness, occupational functioning, odd behavior or appearance, bizarre thinking, impaired attention, impairment in personal hygiene). “Negative” and “disorganized” symptoms were included at a higher threshold because they have been predictive of conversion to psychosis in the general population but may be less specific in 22q11DS due to intellectual disability and comorbidity.29,33 Eighty-five of the 157 participants (54%) were considered psychosis-prone. The majority of individuals with psychosis-proneness (82%) displayed positive symptomatology. All individuals who were psychosis-prone or psychotic were considered a part of the “psychosis-spectrum.”
Challenges to Administering the SIPS in 22q11DS
We anticipated and met several challenges in applying the SIPS to 22q11DS. These were largely related to the increased prevalence of intellectual disability and medical as well as psychiatric comorbidity.
Ambiguous symptoms were often reported in response to SIPS items. In these cases, medical comorbidities and other psychiatric disorders like anxiety and ADHD seemed more probable triggers for the elicited symptom than underlying psychotic phenomena. For example, an individual’s health concerns may potentially be a product of somatic false beliefs or medical problems and excess anxiety. The comprehensiveness of our assessments provided the most useful resolutions to these dilemmas. Clarifying information was obtained by referencing medical history, comparing collateral and proband reports, eliciting or rejecting other psychosis-spectrum and non-psychotic symptoms, and specifying details for the symptom in questions (e.g. severity, onset, frequency, conviction). All available information was presented at the consensus case conference, where the SOPS rating was finalized.
Some subscales on the SIPS are more enmeshed with non-psychotic comorbidities. For example, D3, “trouble with focus and attention,” overlaps significantly with ADHD, and N5, “ideational richness,” overlaps with intellectual disability. In these cases, we considered that these symptoms nevertheless might represent features of psychosis-proneness. Therefore, we rated these SOPS items based on their presence and without regard to other comorbidities. For example, participants reporting multiple substantial symptoms of inattention would receive a diagnosis of ADHD as well as a clinically significant rating on D3, regardless of whether a clinician might believe that the trouble with focus and attention is “better explained” by ADHD than psychosis-proneness.
Finally, some of the items on the SIPS are complex in their wording or meaning. These presented difficulty for participants with more concrete thinking. We found that participants were more likely to understand the questions if the interviewer paused after each concept to divide complex questions into a series of simpler ones. Clarifying follow-ups were also extremely useful, such as asking for specific examples and for degree of conviction. In our administrations of the SIPS, useful follow-up questions were compiled for each item and listed alongside the standard prompt to facilitate consistency among the interviewers.
Data Analysis
Reading proficiency was calculated for each participant using the Wide Range Achievement Test 4 reading segment.34,35 Measurements are standardized with respect to age against a large population sample aged 5 – 94 years old (mean=100; SD=15; test re-test reliability coefficients=0.78 – 0.90).35
Data for individual subscales were analyzed with STATA SE version 12.0 (Statacorp LP; College Station, TX). To determine the relationship of SOPS symptomatology to age, sex, and reading proficiency, ordinal logistic regression was performed by covarying the three predictors against each SOPS subscale. There were no significant two-way interactions among age, sex, and reading proficiency for any of the subscales. A Bonferroni correction was used for these comparisons, and significance threshold was set at α=0.017.
For the 76 participants assessed with independent proband and collateral interviews, “informant divergence” was defined as the difference between the SOPS ratings based on the proband interview alone versus the collateral interview (informant divergence = proband-based score – collateral-based score). Informant divergence is greater than zero when the proband-based score is higher, less than zero when the collateral-based score is higher, and equal to zero when they agree. The statistical significance of deviations away from 0 was calculated using one-sample T-tests. Significance threshold was two-tailed with the probability of a Type I error set to 5%.
Exploratory factor analyses were performed with Mplus (Version 7.11).36 Because the distributions of some variables departed from normality, robust maximum likelihood (MLR) estimation was used. In one case (the 4-factor EFA model), the MLR procedure did not converge due to a Heywood case, so Bayesian estimation was used instead.37 Composite scores were calculated using the item-grouping indicated by each of the factors and taking the mean of component subscale scores. Participants were considered to have a subthreshold rating for a factor if they reached a subthreshold level for any of the component subscales.
Results
Subthreshold Symptoms of Psychosis and Relationship to Age, Sex, and Reading Proficiency
Of 157 participants, 134 (85%) reported at least one symptom at a subthreshold level of severity. Descriptive statistics for each of the 19 SOPS subscales are detailed in Table 2, along with the percentage of participants who endorsed that item at a clinically significant level (≥3). The most prevalent subthreshold symptom was ideational richness, clinically significant in 47% of participants. This was followed by trouble with focus and attention (44%), avolition (38%), and impaired tolerance for normal stress (38%). The least prevalent were motor disturbance (6%) and experience of emotions and self (6%). The subgroup of children aged 8–11 years old are described separately in Supplemental Table S1 (available online).
Table 2.
Subthreshold Symptoms of Psychosis in Chromosome 22q11.2 Deletion Syndrome (22q11DS) and Relationship to Age, Sex, and Reading Proficiency (RP)
| Subscale | Description | Mean | Median | Range | Subthreshold/ Threshold (%) |
Relationship to Age/Sex/RP |
|---|---|---|---|---|---|---|
| P1 | Unusual Thought Content/Delusional Ideas | 1.6 | 1 | 0 – 6 | 27% | - |
| P2 | Suspiciousness/Persecutory Ideas | 1.5 | 1 | 0 – 6 | 27% | - |
| P3 | Grandiosity | 0.7 | 0 | 0 – 6 | 10% | - |
| P4 | Perceptual Abnormalities/Hallucinations | 1.6 | 1 | 0 – 6 | 33% | - |
| P5 | Disorganized Communication | 1.4 | 1 | 0 – 5 | 22% | - |
| N1 | Social Anhedonia | 1.5 | 1 | 0 – 6 | 24% | - |
| N2 | Avolition | 1.9 | 2 | 0 – 5 | 38% | - |
| N3 | Expression of Emotions | 1.2 | 1 | 0 – 6 | 21% | ↑Age (p=0.000) |
| N4 | Experience of Emotions and Self | 0.4 | 0 | 0 – 5 | 6% | - |
| N5 | Ideational Richness | 2.2 | 2 | 0 – 5 | 47% | ↓RP (p=0.002) |
| N6 | Occupational Functioning | 1.6 | 1 | 0 – 6 | 32% | - |
| D1 | Odd Behavior or Appearance | 1.1 | 0 | 0 – 6 | 18% | - |
| D2 | Bizarre Thinking | 0.7 | 0 | 0 – 6 | 12% | - |
| D3 | Trouble with Focus and Attention | 2.2 | 2 | 0 – 5 | 44% | - |
| D4 | Personal Hygiene | 0.9 | 0 | 0 – 5 | 15% | - |
| G1 | Sleep Disturbances | 1.5 | 2 | 0 – 5 | 31% | ↑Age (p=0.006) |
| G2 | Dysphoric Mood | 1.5 | 1 | 0 – 6 | 24% | ↑Age (p=0.003) |
| G3 | Motor Disturbances | 0.6 | 0 | 0 – 5 | 6% | - |
| G4 | Impaired Tolerance to Normal Stress | 1.8 | 2 | 0 – 6 | 38% | - |
| Factor1 | Positive | 1.1 | 0.7 | 0 – 5 | 52% | - |
| Factor2 | Negative | 1.6 | 1.6 | 0 – 4.6 | 75% | - |
| Factor3 | Disorganized | 1.3 | 1.3 | 0 – 4.5 | 54% | ↓RP (p=0.011) |
Note: Descriptive statistics for consensus Scale of Prodromal Symptoms (SOPS) subscale scores are shown with percentage of participants who scored in the subthreshold or psychotic range (rating ≥3). Composite factor scores are also described with percentages representing proportion of participants reaching a subthreshold level in any subscale of which the factor is comprised. Statistically significant relationships to age, sex, and reading proficiency are designated with arrows. ↑ = Positive relationship between increasing subscale severity and predictor variable; ↓ = Negative relationship.
Several subscales were associated with age or reading proficiency. Older participants experienced more trouble with expression of emotion (p<.001), sleep disturbance (p=.006), and dysphoric mood (p=.003). Lower reading proficiency was correlated with more trouble with ideational richness (p=.002). There were no subscales for which more than one association was significant (Table 2).
Factor Structure of the SOPS
Unidimensional, two-, three-, and four-factor exploratory solutions were computed. The best fit was found with the three-factor solution, which yields one factor dominated by positive symptoms, another by negative symptoms, and the third by disorganized symptoms. Structure and loadings of the three-factor model are detailed in Table 3. All models, factor loadings, correlations, and fit statistics are given in Supplemental Table S2 (available online). The skews were consistently in the positive direction, and correlation coefficients were not affected. The subscale for motor disturbance did not load well onto any factor, but is nominally grouped with Factor 3. The four-factor solution was largely consistent with the three-factor, additionally separating out the affect-related subscales (N3 and N4). Therefore, while the three-factor model suggests positive, negative, and disorganized domains, the four-factor model further specifies that the negative domain may be additionally divided into a dysfunctional affect and an amotivation component.
Table 3.
Three-Factor Structure of the Structured Interview for Prodromal Syndromes (SIPS) in Chromosome 22q11.2 Deletion Syndrome (22q11DS)
| Subscale | Description | Factor 1 (Positive) |
Factor 2 (Negative) |
Factor 3 (Disorganized) |
|---|---|---|---|---|
| P1 | Unusual Thought Content/Delusional Ideas | 0.83 | ||
| P4 | Perceptual Abnormalities/Hallucinations | 0.81 | ||
| D2 | Bizarre Thinking | 0.66 | 0.31 | |
| P2 | Suspiciousness/Persecutory Ideas | 0.55 | ||
| P3 | Grandiosity | 0.43 | 0.33 | |
| N4 | Experience of Emotions and Self | 0.39 | ||
| N2 | Avolition | 0.81 | ||
| N6 | Occupational Functioning | 0.78 | ||
| G4 | Impaired Tolerance to Normal Stress | 0.74 | ||
| N1 | Social Anhedonia | 0.62 | ||
| D4 | Personal Hygiene | 0.62 | ||
| G2 | Dysphoric Mood | 0.62 | ||
| G1 | Sleep Disturbances | 0.56 | ||
| D3 | Trouble with Focus and Attention | 0.49 | ||
| N3 | Expression of Emotion | 0.30 | 0.39 | |
| D1 | Odd Behavior or Appearance | 0.51 | ||
| P5 | Disorganized Communication | 0.47 | ||
| N5 | Ideational Richness | 0.34 | ||
| G3 | Motor Disturbance | 0.33 |
Note: Subscales are ordered by factor, then by loading. Loadings < 0.30 are not included.
Descriptive statistics for the three factors as well as their relationship to age, reading proficiency, and sex are listed in Table 2. Seventy-five percent of participants exhibited at least one Factor 2 (negative) symptom while 54% exhibited a Factor 3 (disorganized) symptom and 52% a Factor 1 (positive) symptom. The severity of Factor 3 (disorganized) was correlated with decreased reading proficiency (p=.011). Factor 1 explained 35% of the variance, while Factor 2 explained 51%, and Factor 3 14%.
Divergence Between Proband and Collateral Reports
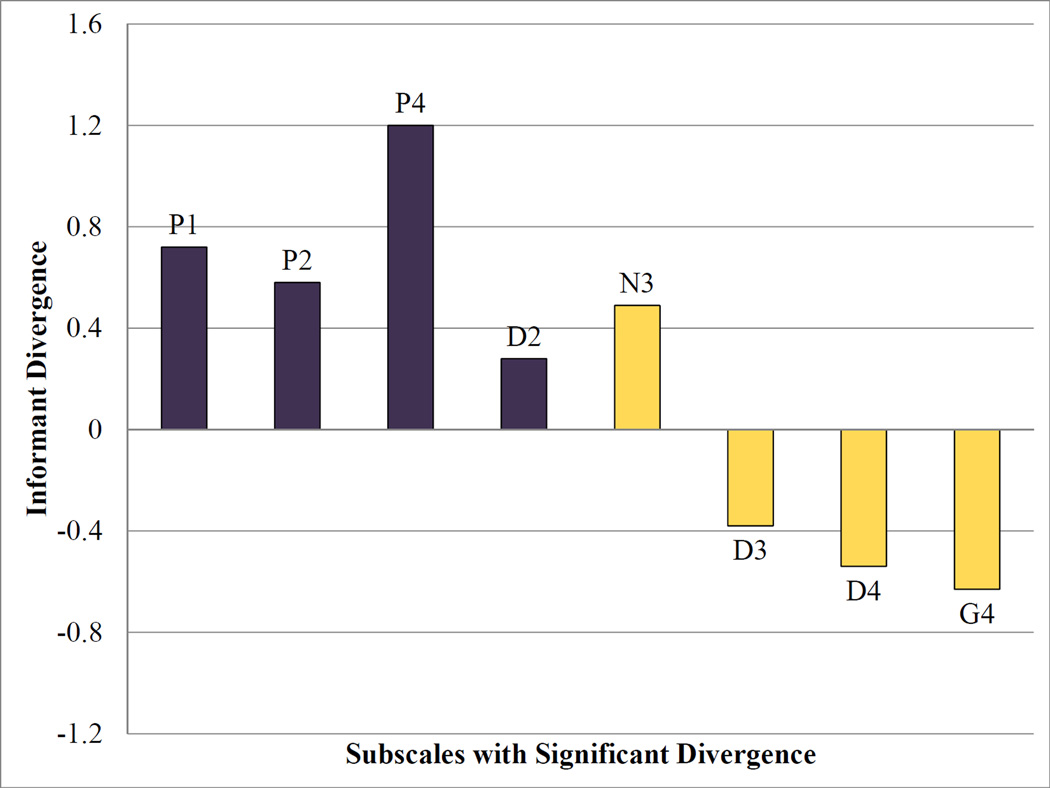
Systematic divergences between proband and collateral reports were present for the 76 participants assessed with independent proband and caregiver interviews. Statistically significant comparisons are displayed in Figure 1. The youth-perspective led to higher SOPS ratings for unusual thought content/delusional ideas, suspiciousness/persecutory ideas, perceptual abnormalities/hallucinations, expression of emotion, and bizarre thinking. On the other hand, the caregiver-perspective was associated with higher ratings on trouble with focus and attention, personal hygiene, and impaired tolerance to normal stress. All subscales more highly endorsed by collateral informants fell under Factor 2 (negative), while all subscales more highly endorsed by probands fell under Factor 1 (positive), with the exception of expression of emotion. Thus, parents were often not aware of the probands’ internal experiences, while probands did not deny their dysfunctional behavior, although they underestimated its severity.
Figure 1. Proband and collateral informant divergence.
Note: Informant divergence for each Scale of Prodromal Symptoms (SOPS) item was calculated by subtracting the collateral-based rating from the proband-based rating. Significant effects are shown above p<0.05. Informant effect >0 if the proband-based score is higher, <0 if the collateral-based score is higher. Purple bars=Factor 1 (positive); yellow bars=Factor 2 (negative). D2=bizarre thinking; D3=trouble with focus and attention; D4=personal hygiene; G4=impaired tolerance to normal stress; N3=expression of emotion; P1=unusual thought content/delusional ideas; P2=suspiciousness/persecutory thinking; P4=perceptual abnormalities/hallucinations.
Overall, youth-caregiver agreement on SOPS ratings was moderate. Agreement on psychosis status was 72%, with 17% appearing psychosis-spectrum only from the youth perspective, and 11% appearing psychosis-spectrum only from the caregiver-perspective. There are no significant interactions between informant effect and participant age, sex, or reading proficiency.
Discussion
We examined subthreshold psychotic phenomena in 157 individuals with 22q11DS. This is the largest sample reported for an application of the SIPS in 22q11DS. Several main conclusions can be drawn.
The SIPS was created to evaluate risk for psychosis in individuals seeking clinical help. The SIPS is being applied to a population, those with 22q11DS, who have a higher frequency of medical and psychiatric comorbidities and reduced intellectual functioning.6–8,38 Our administration of the SIPS in the 22q11DS population coped with several challenges, including ambiguous elicited symptoms and overly concrete understanding of the interview questions by some participants. These challenges were effectively addressed through semi-structured interviewing with probing follow-up questions and a comprehensive assessment that included medical, psychiatric, social, and family history. Previous studies using the SIPS in 22q11DS have been less detailed in their description of administration methods.19–23 However, we anticipate that others may encounter similar challenges and could benefit from our experience.
The most commonly rated abnormal SOPS subscales were ideational richness, trouble with focus and attention, avolition, and impaired tolerance to normal stress. These results are highly concordant with previous studies,21–23 and we can conclude that these are the areas in which individuals with 22q11DS experience the greatest difficulty. Regarding positive psychotic symptomatology, we found that perceptual abnormalities/hallucinations, unusual thought content/delusional ideas, and suspiciousness/persecutory ideas were the most prevalent. This is largely in agreement with previous studies that found subthreshold perceptual abnormalities in 25–32% and unusual thought content in 21–25%.21,23 Thus, we provide additional evidence that while subthreshold positive symptoms are less common than generalized features of psychopathology, they are nevertheless highly prevalent in 22q11DS. In contrast, fewer of the participants met criteria for threshold psychotic disorders (8%). This lower rate is predominantly due to the young age of our sample and is consistent with the rate of psychotic disorder in similarly aged cohorts of 22q11DS.3,11,20
A few correlations with age and reading proficiency were significant. Older participants were more likely to experience dysfunctional expression of emotions, sleep disturbance, and dysphoric mood. These trends may reflect the peak in mood disorders during the transition to adulthood, previously described in other 22q11DS cohorts.3,10,11 Deficits in ideational richness were more pronounced for individuals with lower reading proficiency. Thus, though individuals with 22q11DS are relatively less impaired in verbal than spatial tasks,39 poor reading proficiency may nevertheless relate to deficit in ideational richness.
Three factors emerged from analyzing the structure of the SOPS: positive, negative and disorganized symptoms. These are consistent with traditional conceptualizations of the symptom structure of schizophrenia.32 The factors are also rational in their subscale compositions, except motor disturbance, which does not load well conceptually or statistically with any of the subscales. Other factor analyses of psychosis items in 22q11DS have also yielded three-factor solutions, but resulted in a “general” rather than “disorganized” third factor.3,21 Our findings may deviate from others due to differences in sample size, sample population, and assessment methods. Also, based on factor loadings reported in previous studies, factor analyses of the SOPS appear especially vulnerable to statistical anomalies (such as Heywood cases) that hinder interpretation of the results. Notably, the four-factor structure further suggests that negative symptoms may be subdivided into amotivation and functional deficits versus disordered affect. Similar findings have been reported both in patients with 22q11DS and non-deleted patients with schizophrenia.21,40
It is also noteworthy that although the SOPS was rationally designed with “positive,” “negative,” and “disorganized” groups of symptoms, the original subscale categories deviate somewhat from the factor structure described here and in other factor analyses of the SOPS both in the general population and in 22q11DS.21,41,42 The structure described here may provide an empirically derived SOPS categorization that can be used to enhance understanding of the relationships among subthreshold symptoms of psychosis, risk potential and neurobiological correlates.
Informant divergence was detected when comparing proband and collateral endorsements of subthreshold psychotic symptoms. With the exception of expression of emotion, all symptoms more prominent in the proband perspective belonged to Factor 1 (positive) while all subscales more prominent in the collateral perspective belonged to Factor 2 (negative). This may represent a tendency for youths with 22q11DS to report more subjective symptoms of psychosis, while their caregivers are more attuned to behavioral symptoms with functional impairment. The exception is expression of emotion, perhaps because this subscale was based solely on reported symptoms for collateral interviews, whereas direct interviewer observations played a role in upgrading proband ratings. Both youths with 22q11DS and their caregivers may be accustomed to and therefore fail to observe flattened affect.
Overall, agreement between total SIPS ratings based on youth and collateral perspectives was moderate, consistent on psychosis-spectrum diagnosis for 72% of participants. A study looking at SIPS youth-caregiver agreement in a non-deleted population found similar results.43 In our study, a significant proportion of psychosis-spectrum youths (17%) would not have been thus categorized based on caregiver report alone. Anecdotally, youths often acknowledged withholding information from their parents for fear of burdening them. Compatibility between informants’ responses was easily established upon probing. For greatest sensitivity, we suggest that subjective symptoms of psychosis be independently assessed with the proband.
There are several limitations to this study. The SIPS was not designed for younger children and is difficult to administer in this population because of its length and complex content. Due to this consideration, in addition to administering a full collateral SIPS, we elected to probe the youngest participants (<11 years) with a clinical interview rather than administer the SIPS in its original form. This less-structured format may have resulted in greater variability in interview content as well as failure to elicit some symptoms in this subsample. Results for this age group are presented separately in Supplemental Table S1 (available online). Despite our extensive experience with the SIPS and adherence to published anchors and guidelines, we did not undergo formal reliability training or analysis with regard to the Yale criteria. Our definition of psychosis-proneness is also more inclusive than traditional Clinical High Risk criteria. For all of these reasons, our methods may not have the same predictive validity as other investigations using the SIPS in non-deleted help-seeking groups. Additionally, research into subthreshold symptoms of psychosis in children <13 years is relatively scarce, and there is little basis for comparison. Individuals with significant intellectual disability were excluded from the study because our long-term aim is to investigate neurobiological substrates of psychosis in 22q11DS that are informative about psychosis in the general population. Therefore, the generalizability of our findings to individuals with 22q11DS and significant intellectual disability is limited. Participants might over- or under-report symptoms due to guardedness or lack of understanding. These possibilities were reduced but not eliminated by our comprehensive semi-structured assessment methods. Antipsychotic use by six participants may also have affected our results; however, this effect is likely to be dampened by the large sample size. Only by determining eventual conversion to psychotic disorders can we establish the validity of our assessments for predicting psychosis-proneness.
The early identification of psychosis in 22q11DS can contribute to understanding the pathophysiology of psychosis and developing interventions. Despite well-demonstrated predictive validity among non-deleted help-seeking youths, the SIPS is unproven in the high-risk 22q11DS population. This study adds to the literature by investigating subthreshold symptoms of psychosis in 157 individuals with 22q11DS. Assessing subthreshold psychotic symptoms in 22q11DS is challenging due to increased medical and psychiatric comorbidity as well as intellectual disability. However, through comprehensive assessment and by probing participants with detailed follow-up questions, many of the difficulties were resolved. Subthreshold psychotic symptoms were common in 22q11DS; the most prevalent were ideational richness, trouble with focus and attention, avolition, and impaired tolerance to normal stress. Factor structure of the SOPS revealed three components dominated by positive, negative, and disorganized symptoms, with negative symptoms possibly further divided into amotivation and functional impairment versus dysfunctional affect. Significant differences emerged when comparing youth and caregiver perspectives. Longitudinal studies with eventual conversion to psychosis are needed to establish the predictive utility of the instrument.
Supplementary Material
Clinical Guidance.
Subthreshold psychotic symptoms are highly prevalent in 22q11DS.
Separate interviews are recommended for probands and collaterals, as probands tend to better report subjective experiences, while collaterals have more insight into functional and behavioral impairments.
Application of the SIPS to 22q11DS elicited many subthreshold symptoms of psychosis, but increased efficiency and substantiation of its predictive validity are needed before widespread clinical implementation in this and other populations of young and developmentally delayed individuals.
Acknowledgments
This study was funded by grants from the National Institutes of Health (MH087626 and MH087636), the Doris Duke Charitable Foundation Clinical Research Fellowship (SXT), and grants T32 MH MH019112 (JY) and K08 MH079364 (MEC).
Dr. Warren B. Bilker, PhD, served as the statistical expert for this research.
The authors thank the participants and their families, as well as Emily Wilkins, BA, Catherine Conroy, MSE, Omar Abbas, BA, Amy Cassidy, MA, and Adam Savitt, BA, of the Department of Neuropsychiatry at the University of Pennsylvania. At the Children’s Hospital of Philadelphia, the authors thank Alice Bailey, BA, of the “22q and You” Center, Colleen Franconi, BS, of the Department of Human Genetics, and Karin Borgmann-Winter, MD, of the Department of Child and Adolescent Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: the authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 Deletion Syndrome as a Window into Complex Neuropsychiatric Disorders Over the Lifespan. Biol Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider M, Van der Linden M, Glaser B, et al. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res. 2012;196:277–284. doi: 10.1016/j.psychres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Gothelf D, Schneider M, Green T, et al. Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry. 2013;52:1192–1203.e3. doi: 10.1016/j.jaac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Schreiner MJ, Lazaro MT, Jalbrzikowski M, Bearden CE. Converging levels of analysis on a genomic hotspot for psychosis: insights from 22q11.2 deletion syndrome. Neuropharmacology. 2013;68:157–173. doi: 10.1016/j.neuropharm.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald-McGinn DM, Kirschner R, Goldmuntz E, et al. The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- 6.McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, et al. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genet Med. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gur RE, Yi JJ, McDonald-McGinn DM, et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. [doi:10.1038/mp.2013.189];Mol Psychiatry. doi: 10.1038/mp.2013.189. [Published Online January 21, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi JJ, Tang SX, McDonald-McGinn DM, et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;165:137–147. doi: 10.1002/ajmg.b.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett AS, McDonald-McGinn DM, Devriendt K, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159:332–339. e1. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green T, Gothelf D, Glaser B, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 12.Antshel KM, Fremont W, Roizen NJ, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45:596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- 13.Vorstman JA, Morcus ME, Duijff SN, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 14.Fabbro A, Rizzi E, Schneider M, Debbane M, Eliez S. Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS) Eur Child Adolesc Psychiatry. 2012;21:379–385. doi: 10.1007/s00787-012-0273-x. [DOI] [PubMed] [Google Scholar]
- 15.Gothelf D, Feinstein C, Thompson T, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 16.Morgan VA, Leonard H, Bourke J, Jablensky A. Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry. 2008;193:364–372. doi: 10.1192/bjp.bp.107.044461. [DOI] [PubMed] [Google Scholar]
- 17.Cannon, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esterberg ML, Ousley OY, Cubells JF, Walker EF. Prodromal and autistic symptoms in schizotypal personality disorder and 22q11.2 deletion syndrome. J Abnorm Psychol. 2013;122:238–249. doi: 10.1037/a0028373. [DOI] [PubMed] [Google Scholar]
- 20.Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2010;49:333–344. [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider M, Van der Linden M, Glaser B, et al. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res. 2012;196:277–284. doi: 10.1016/j.psychres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophr Res. 2011;129:20–28. doi: 10.1016/j.schres.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res. 2010;118:118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42:1857–1863. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 25.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- 26.Schimmelmann BG, Walger P, Schultze-Lutter F. The significance of at-risk symptoms for psychosis in children and adolescents. Can J Psychiatry. 2013;58:32–40. doi: 10.1177/070674371305800107. [DOI] [PubMed] [Google Scholar]
- 27.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 28.Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 29.Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidman LJ, Giuliano AJ, Meyer EC, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. Washington DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 33.Tarbox SI, Addington J, Cadenhead KS, et al. Premorbid functional development and conversion to psychosis in clinical high-risk youths. Dev Psychopathol. 2013;25:1171–1186. doi: 10.1017/S0954579413000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg PC, Calkins ME, Richard J, et al. Computerized neurocognitive profile in young people with 22q11.2 deletion syndrome compared to youths with schizophrenia and at-risk for psychosis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:87–93. doi: 10.1002/ajmg.b.32005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT4) Psychological Assessment Resources, Lutz. 2006 [Google Scholar]
- 36.Muthén LK, Muthén BO. Mplus Users Guide. Los Angeles, CA: Muthén and Muthén; 2012. [Google Scholar]
- 37.Muthén B, Asparouhov T. Bayesian structural equation modeling: a more flexible representation of substantive theory. Psychol Methods. 2012;17:313–335. doi: 10.1037/a0026802. [DOI] [PubMed] [Google Scholar]
- 38.Bearden CE, Woodin MF, Wang PP, et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- 39.Antshel KM, Fremont W, Kates WR. The neurocognitive phenotype in velo-cardio-facial syndrome: a developmental perspective. Dev Disabil Res Rev. 2008;14:43–51. doi: 10.1002/ddrr.7. [DOI] [PubMed] [Google Scholar]
- 40.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins KA, McGlashan TH, Quinlan D, et al. Factorial structure of the Scale of Prodromal Symptoms. Schizophr Res. 2004;68:339–347. doi: 10.1016/S0920-9964(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 42.Comparelli A, Savoja V, Kotzalidis GD, et al. Factor-structure of the Italian version of the Scale Of Prodromal Symptoms (SOPS): a comparison with the English version. Epidemiol Psychiatr Sci. 2011;20:45–54. doi: 10.1017/s2045796011000114. [DOI] [PubMed] [Google Scholar]
- 43.Golembo-Smith S, Bachman P, Senturk D, et al. Youth-caregiver Agreement on Clinical High-risk Symptoms of Psychosis. J Abnorm Child Psychol. 2014;42:649–658. doi: 10.1007/s10802-013-9809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.