Abstract
Astrocytes actively participate in the central nervous system (CNS) response to injury, including in multiple sclerosis (MS). Astrocytes can play both beneficial and detrimental roles in response to neuroinflammation, however in extreme cases astrogliosis can result in the formation of a glial scar, which can impede the regeneration of injured neurons. While astrocytes do not express voltage-gated sodium channel Nav1.5 in nonpathological human brain, they exhibit robust upregulation of Nav1.5 within acute and chronic MS lesions. Recent work indicates that Nav1.5 contributes to the pathways that regulate glial scar formation in vitro via modulation of intracellular Ca2+. However, the temporal dynamics of astrocytic Nav1.5 channel expression in response to neuroinflammatory pathologies have not been investigated. We examined astrocytes from mice with monophasic and chronicrelapsing experimental autoimmune encephalomyelitis (EAE) by immunohistochemistry to determine whether Nav1.5 is expressed in these cells, and whether the expression correlates with severity of disease and/or phases of relapse and remission. Our results demonstrate that Nav1.5 is upregulated in astrocytes in situ in a temporal manner that correlates with disease severity in both monophasic and chronic-relapsing EAE. Furthermore, in chronic-relapsing EAE, Nav1.5 expression is upregulated during relapses and subsequently attenuated during periods of remission. These observations are consistent with the suggestion that Nav1.5 can play a role in the response of astrocytes to inflammatory pathologies in the CNS and suggest Nav1.5 may be a potential therapeutic target to modulate reactive astrogliosis in vivo.
Keywords: Astrogliosis, experimental autoimmune encephalomyelitis, multiple sclerosis, sodium channels
Introduction
Though astrocytes have traditionally been considered to be electrically inexcitable, studies since the 1990s have demonstrated that these cells express voltage-gated sodium channels (VGSCs) [1], including the isotype Nav1.5 [2,3]. These studies suggest that expression of VGSCs in rodent astrocytes is a dynamic process, changing with response to injury, age, and exposure to the extracellular milieu [4-6]. Notably, Black et al. demonstrated the presence of Nav1.5 in human scarring astrocytes in situ within acute and chronic multiple sclerosis (MS) lesions, and surrounding cerebrovascular accidents and central nervous system (CNS) tumors, suggesting a commonality of upregulated astrocytic Nav1.5 following CNS tissue injury [7].
Despite the well-characterized expression of VGSCs in both rodent and human astrocytes, the functional role of these VGSCs has remained elusive. Astrocytes serve multiple functions in the CNS, including ionic homeostasis and neuronal support. Sontheimer et al. suggested that VGSCs provide a route for [Na+]i influx necessary for Na+/K+-ATPase function within astrocytes and thus participate in K+ homeostasis in the CNS [8]. Astrocytes are also important contributors to the response of the CNS to neuroinflammatory pathologies, including MS. One such response is reactive astrogliosis, which can exhibit both beneficial and detrimental effects to the CNS [9]. Severe forms of astrogliosis involve formation of a scar that is long-lasting and can inhibit the regeneration of injured neurons [10]. Recent work has shown that Nav1.5 plays an important role in an in vitro model of glial injury by triggering reverse mode operation of the Na+-Ca2+ exchanger (NCX) [3]. These results suggest that Nav1.5 and NCX are potential targets for modulation of astrogliosis after injury via their effect on [Ca2+]i.
Given the dynamic expression of VGSCs in rodent astrocytes [4-6], the upregulation of Nav1.5 in human scarring astrocytes [7], and the functional role of Nav1.5 in glial scar formation in vitro [3], we examined the temporal dynamics of Nav1.5 expression in scarring astrocytes in neuroinflammatory pathologies, with respect to disease severity and periods of relapse/remission. Here, we investigate the expression of astrocytic Nav1.5 in two mouse models of MS, monophasic experimental autoimmune encephalomyelitis (EAE) and chronic-relapsing EAE (CR EAE). We show that Nav1.5 upregulation correlates to disease severity and that Nav1.5 expression in astrocytes is modulated in parallel with periods of disease and remission.
MATERIALS AND METHODS
Induction of EAE
Experiments were carried out in accordance with NIH guidelines for the care and use of laboratory animals; all animal protocols were approved by the IACUC of VA Connecticut Healthcare System, West Haven, CT. C57BL/6 (Harlan, Indianapolis, IN) and Biozzi (Harlan Sera-Lab Ltd, Loughborough, UK) mice 6–10 weeks of age were injected subcutaneously in the flank with 200 μl of an emulsion of 300 μg of rat myelin-oligodendrocyte glycoprotein (MOG) 35–55 peptide (W. M. Keck Biotechnology Resource Center, Yale University) in incomplete Freund’s adjuvant (IFA; Sigma, St Louis, MO) supplemented with 500 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI), as described previously [11]. The MOG injection, with mycobacterium supplemented IFA, was repeated in the contralateral flank 1 week later. The mice also received an injection of 250 ng pertussis toxin (Sigma) in 200 μl phosphate-buffered saline (PBS) intraperitoneally (i.p.) immediately after the first immunization with MOG and then again 48 h later. In agreement with previous descriptions, the C57BL/6 mice developed a monophasic clinical course, while the Biozzi mice exhibited a chronic-relapsing (CR) clinical phenotype. Control animals received the same injections, with the omission of MOG. A total of 18 C57BL/6 and 20 Biozzi mice were injected.
Clinical Assessment
Immunized mice were observed daily and scored on a 0 to 6 clinical scale with increasing clinical score reflecting clinical worsening as follows: 1—flaccid tail; 2— abnormal righting reflex and/or abnormal gait in the absence of weakness; 3—partial hindlimb paralysis; 4—complete hindlimb paralysis; 5—moribund; and 6—death[12]. We applied the scale in 0.5 increments. To characterize the clinical course of CR EAE, the initial episode was defined as a clinical score of ≥ 2.0 for two or more consecutive days. Subsequent relapses were identified by clinical scoring increases of at least 1.0 and defined remissions were identified by clinical scoring decreases of at least 1.0. To estimate overall clinical burden of disease in CR EAE, mean clinical score was determined by averaging clinical scores for all of the sick days of each animal.
Tissue Collection
Mice were sacrificed at varying relapse-remission phases and lengths of disease (range 9-59 days) from date of induction of EAE. Mice were anesthetized with ketamine/xylazine (80/5 mg/kg i.p.) and perfused through the heart with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer. Spinal cords and brains were carefully excised, cryoprotected with 30% sucrose in PBS and frozen.
Immunocytochemistry
Twelve μm transverse sections of L1-L2 spinal cords and sagittal sections of the cortices (bregma 0-1 mm, M2) were cut and incubated with primary antibodies [mouse anti-glial fibrillary acidic protein (GFAP), 1:1000, Covance, Princeton, NJ, catalog #SMI-22R; rabbit anti-Nav1.5, 1:100, Alomone, Jerusalem, Israel, catalog #ASC-013] overnight at 4° C on a rotating shaker. Sections were rinsed 3 times with phosphate-buffered saline (PBS) and incubated with secondary antibodies [donkey anti-mouse immunoglobulin G-Alexa Fluor 488, 1:1000, Invitrogen, Grand Island, NY, cat #P-11065; donkey anti-rabbit immunoglobulin G Cy3, 1:500, Jackson, West Grove, PA, cat #711-165-152] overnight. Slides were rinsed with PBS and coverslips were mounted with Aqua Poly mount (Polysciences, Warrington, PA). Control experiments were performed with the omission of the primary antibodies and only background labeling was observed.
Data Acquisition and Analysis
Multiple images of control, C57BL/6, and Biozzi tissues were accrued with a Nikon C1si confocal microscope (Nikon USA, Melville, NY) operating under identical gain settings with frame lambda (sequential) mode and saturation indicator activated to prevent possible bleed-through between channels. For quantitative analysis of Nav1.5, NIS Elements software was utilized. For C57BL/6 animals (monophasic EAE), high magnification images of astrocytes in the anterolateral white matter in the L1-L2 region of the spinal cord were selected for analysis. Regions of interest for mean RGB intensity analysis were created by manually outlining individual astrocytes based on GFAP staining. Multiple high magnification images for each animal were taken, quantified, and averaged. For description of astrocytic expression of Nav1.5 in the motor cortex, high magnification images of astrocytes in the parasagittal motor cortex (bregma 0-1 mm, M2) were obtained.
For Biozzi animals (CR EAE), low magnification images of the L1-L2 region of the spinal cord were analyzed. To quantify astrocytic Nav1.5 levels, thresholds were set at the same value for all images by creating a binary layer based on visible GFAP staining in control tissues. Subsequently, regions of interest were manually defined in anterolateral white matter and the RGB mean intensity of the region of interest was extracted within the binary layer. Multiple low magnification images for each animal were taken, quantified, and averaged. One of the twenty Biozzi EAE animals was excluded secondary to poor tissue quality due to severity of disease. Images were composed and processed to enhance contrast in the figures in Adobe Photoshop, with identical settings for the different conditions.
Statistical Methods
Clinical scores for C57/B6 animals (monophasic EAE) were represented by clinical score on the day of euthanasia. Clinical scores for Biozzi animals (CR EAE) were computed by averaging the clinical scores for each day of disease, to estimate mean overall disease severity. To assess correlation between Nav1.5 immunolabelling and animal clinical status, correlation coefficients (Spearman for normally distributed data; Pearson for nonparametric data) and two-tailed p-values were calculated with GraphPad Prism (GraphPad Software, La Jolla, CA). Data were normalized to percent fluorescence compared to control animals.
RESULTS
Astrocytes in control human brain do not display sodium channel Nav1.5 immunolabeling above background levels, but exhibit robust Nav1.5 immunofluorescence within acute and chronic multiple sclerosis (MS) lesions.11 In order to examine the temporal pattern of Nav1.5 expression in astrocytes and the relationship of the degree of Nav1.5 expression to disease severity in a murine model of MS, we examined tissue from mice with acute monophasic and chronic-relapsing experimental autoimmune encephalomyelitis (EAE).
Spinal cord and motor cortex astrocytes in mice with monophasic EAE exhibit upregulated Nav1.5 expression
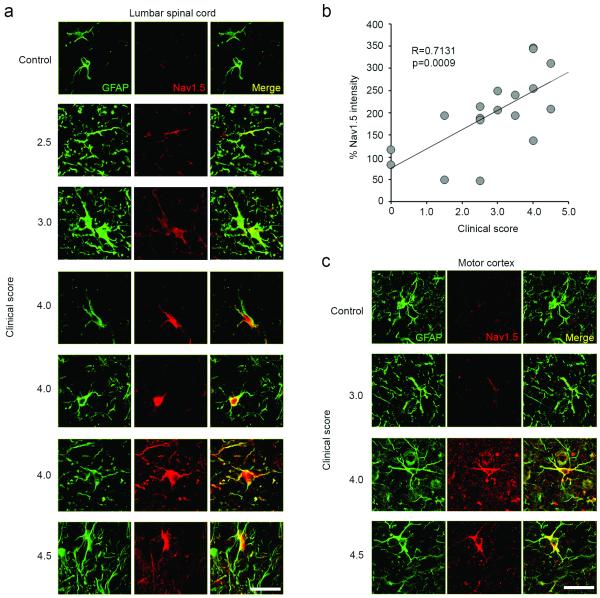
Astrocytes within the spinal cord anterolateral white matter of control C57BL/6 mice did not display Nav1.5 immunolabeling above background levels (Fig. 1a). In contrast, the progression of clinical severity in C57BL/6 mice with monophasic EAE was associated with increasing levels of Nav1.5 immunofluorescence (Fig. 1b). Nav1.5 labeling was detectable at low levels above background in astrocytes from mice with EAE with a clinical score of 2.5 (abnormal righting reflex/mild weakness in hindlimbs), while robust Nav1.5 reactivity was generally exhibited by astrocytes in mice with clinical scores greater than 4.0 (complete hindlimb paralysis).
Figure 1.
Astrocytes in monophasic EAE upregulate Nav1.5 in the spinal cord and brain, in correlation with increasing disease severity. (a) GFAP-positive astrocytes (green) from the anterolateral white matter of the L1-L2 spinal cord of mice with monophasic EAE exhibit prominent Nav1.5 immunolabelling (red), which is upregulated in correlation with increasing clinical score (panels arranged top to bottom in order of increasing severity of disease). Control animal exhibits little astrocytic Nav1.5 immunolabeling (top panel). Merged images of GFAP and Nav1.5 are yellow. Scale bars 25 μm. (b) Increasing clinical score is positively correlated with percent of astrocytic Nav1.5 upregulation as compared to control animals. Data represented as mean % intensity as compared to control animals (c) GFAP-positive astrocytes (green) from the motor cortex of mice with monophasic EAE exhibit prominent Nav1.5 immunolabelling (red), which is upregulated in correlation with increasing clinical score (panels arranged top to bottom in order of increasing severity of disease). Control animal exhibits little astrocytic Nav1.5 immunolabeling (top panel). Merged images of GFAP and Nav1.5 are yellow. Scale bar 25 μm.
As shown in Fig. 1b, quantification of the Nav1.5 immunofluorescence in astrocytes within spinal cord anterolateral white matter of mice with monophasic EAE yielded a positive correlation between mean astrocytic Nav1.5 signal and clinical score (R=0.7131, p=0009, n=18 animals), consistent with increased expression of Nav1.5 within astrocytes as mice with monophasic EAE exhibited increasing disease severity.
The enhanced Nav1.5 immunofluorescence in astrocytes with increasing clinical score was also manifest in astrocytes within different regions of the CNS. For example, astrocytes within motor cortex of mice with monophasic EAE exhibited increased Nav1.5 immunolabeling with increasing disease severity, as observed in Fig. 1c.
Spinal cord astrocytes in mice with chronic-relapsing EAE display upregulated Nav1.5 expression
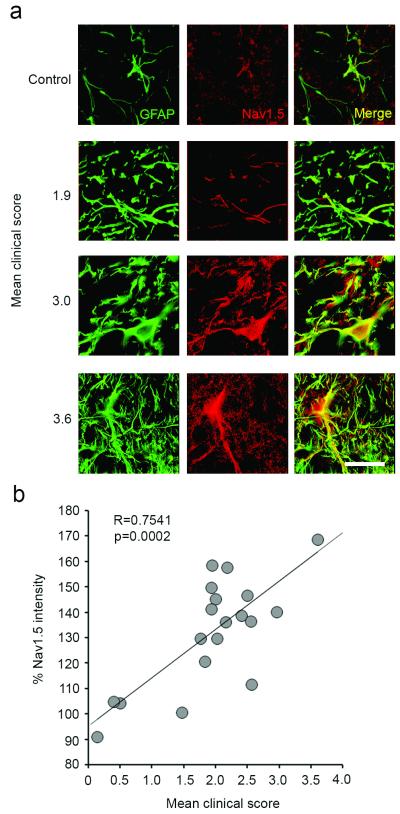
To assess the disease severity of Biozzi mice with chronic-relapsing EAE (CR EAE), totals of the clinical score at each day following the onset of the disease (clinical score >1.0) to euthanasia were calculated and then divided by the number of days in the disease state, to yield a mean clinical score, reflective of overall disease burden. As exemplified in Fig. 2a, astrocytes within spinal cord anterolateral white matter exhibited enhanced Nav1.5 immunofluorescence with increasing mean clinical score. Quantification of the mean astrocytic Nav1.5 signal intensity yielded a positive correlation (R=0.7541, p=0.0002, n=19) with increasing mean clinical score (Fig. 2b).
Figure 2.
Astrocytes in chronic-relapsing EAE upregulate Nav1.5 in the anterolateral spinal cord, in correlation with increasing disease severity. (a) GFAP-positive astrocytes (green) from the L1-L2 spinal cord of mice with chronic-relapsing EAE exhibit prominent Nav1.5 immunolabelling (red), which is upregulated in correlation with increasing mean clinical score (panels arranged top to bottom in order of increasing severity of disease). Control animal exhibits little astrocytic Nav1.5 immunolabeling (top panel). Merged images of GFAP and Nav1.5 are yellow. Scale bar 25 μm. (b) Increasing mean clinical score is positively correlated with percent of astrocytic Nav1.5 upregulation as compared to control animals. Data represented as mean % intensity as compared to control animals.
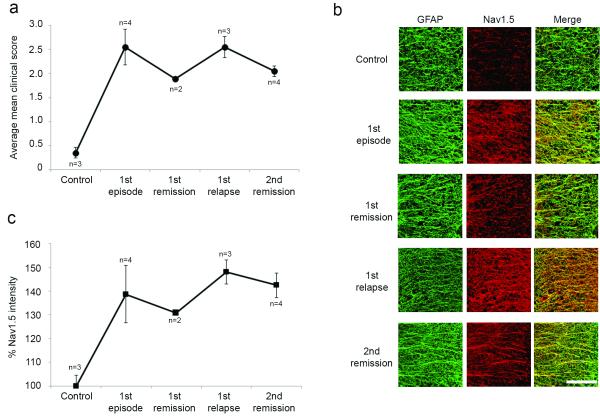
We also determined whether the level of Nav1.5 immunolabeling in astrocytes in Biozzi mice with chronic-relapsing EAE was altered in remitting and relapsing phases of the disease. As shown in Fig. 3a, MOG inoculation of Biozzi mice induced a relapsingremitting form of EAE, with mean clinical scores of ~2.5 in the first episode and first relapse and ~1.8 and ~2.0 in the first and second remissions, respectively. Astrocytes within spinal cord anterolateral white matter displayed Nav1.5 immunofluorescent signals that paralleled the relapsing-remitting clinical course (Fig. 3b). Quantification of Nav1.5 immunofluorescence in astrocytes in the chronic-relapsing phases is provided in Fig. 3c, and was consistent with an upregulation of Nav1.5 in astrocytes in the first episode that was attenuated in the 1st remission, further upregulated in the 1st episode, and again attenuated in the 2nd remission.
Figure 3.
Astrocytic Nav1.5 expression is attenuated during periods of remission in chronic-relapsing EAE. (a) MOG inoculation of Biozzi mice induced a chronic-relapsing form of EAE, with average mean clinical scores of ~2.5 in the first episode and first relapse and ~1.8 and ~2.0 in the first and second remissions, respectively (b) Astrocytes within spinal cord anterolateral white matter displayed Nav1.5 immunofluorescent signals that seemed to parallel the relapsing-remitting clinical course. (c) Quantification of Nav1.5 immunofluorescence in astrocytes in the chronic-relapsing phases is consistent with an upregulation of Nav1.5 in astrocytes in the first episode that is attenuated in the 1st remission, further upregulated in the 1st episode, and again attenuated in the 2nd remission. Data represented as mean % intensity as compared to control animals. Scale bar 500 μm.
DISCUSSION
Previous studies have shown that rodent astrocytes express voltage-gated sodium channels (VGSCs) (for review see Sontheimer et al.) [1], including Nav1.5 sodium channels [2,3]. Black et al. (1998) demonstrated Nav1.5 mRNA and protein in rodent astrocytes in vitro and in situ and Black et al. (2010) showed upregulated expression of Nav1.5 in astrocytes associated with acute and chronic multiple sclerosis (MS) lesions, new and old stroke lesions, and central nervous system (CNS) tumors, including gliomas and a metastatic carcinoma [2,7]. However, the temporal sequence of Nav1.5 expression in activated astrocytes has yet to be established. Here, we examine the expression of astrocytic Nav1.5 in different phases of monophasic and chronic-relapsing (CR) EAE models of MS. We show that Nav1.5 upregulation in astrocytes correlates with disease severity of the mice, as indicated by clinical score. The low level of Nav1.5 expression in astrocytes of control animals suggests that Nav1.5 upregulation is part of the biological response of astrocytes to CNS insult.
The expression of Nav1.5, which is tetrodotoxin (TTX)-resistant [13] in astrocytes appears to be a dynamic process, changing with regard to age of the animal and culture conditions, exposure to extracellular factors, and response to injury [3-6]. MacFarlane and Sontheimer described a shift from TTX-sensitive sodium currents to TTX-resistant sodium currents with properties ascribed to Nav1.5 in rodent astrocytes in an in vitro model of glial injury [4]. Our finding that Nav1.5 expression in astrocytes is modulated in parallel with clinical course of the animal is consistent with the notion that Nav1.5 is regulated temporally in astrocytes in response to neuroinflammation.
Astrocytes perform multiple functions in the CNS, and the functional roles of VGSCs in astrocytes is incompletely understood, although studies have provided certain insight (for review of noncanonical roles of VGSCs in astrocytes and other non-excitable cell types, see Black and Waxman) [14]. It has been shown that VGSCs in astrocytes are able to be localized to the plasma membrane, where they are capable of producing sodium currents [15]. A standing Na+ influx in astrocytes is critical for Na+/K+-ATPase activity and Sontheimer et al. proposed that VGSCs channels provide a pathway for Na+ to enter the cell to maintain [Na+]i at levels necessary for Na+/K+-ATPase activity [8]. This action, in turn, supports the regulation of ionic homeostasis in the CNS, with particular regards to K+ fluxes. Inlese et al. demonstrated through 23Na MRI that sodium concentrations are elevated in acute and chronic MS lesions when compared to normal appearing white matter (NAWM) [16], providing a possible clinical correlate to the suggestion of Black et al. (2010) that upregulation of Nav1.5 may provide a compensatory mechanism to support ionic homeostasis mediated by Na+/K+-ATPase activity in areas of CNS damage [7].
In addition to other functions in the CNS, astrocytes are prominent participants in the response to inflammatory insults through the process of reactive astrogliosis, which occurs following injury in many CNS pathologies, including MS. Although activated astrocytes can exert beneficial and detrimental actions following CNS injury [9], recent work has linked proinflammatory effects of activated astrocytes as critical contributors to the inflammatory response in EAE [17]. In addition, severe forms of astrogliosis are associated with the formation of a glial scar, which is often persistent and can impede neuronal regeneration after injury [10].
Accumulating evidence indicates a role of [Na+]i transients in contributing to astroglial excitability and cellular homeostasis, with a prominent mechanism involving the linkage of transmembrane movements of Na+ and Ca2+ through reverse mode of the Na+/Ca2+ exchanger (NCX) [18]. The reversal potential of NCX in astrocytes is set at levels close to the resting membrane potential [19], therefore NCX can quickly switch into reverse mode in response to small [Na+]i increases and/or depolarization [18,20], as would be seen with VGSC activation, causing an increase in [Ca2+]i.
Recent work has shown that Nav1.5 plays an important role in an in vitro model of glial injury via reverse action of NCX [3]. Astrocyte wound closure after mechanical injury is attenuated by pharmacological treatment with TTX and KB-R7943 (at a dose that selectively blocks reverse Na+/Ca2+ exchange), and by knockdown of Nav1.5 mRNA. The robust [Ca2+]i response seen in astrocytes after mechanical injury, which participates in the pathways leading to astrogliosis [21] is also attenuated by TTX, KB-R7943, and Nav1.5 knockdown [3]. The upregulation of Nav1.5 in astrocytes in situ in animals with monophasic and CR EAE in correlation with increasing disease severity is consistent with these in vitro results. Together, these findings support the suggestion that Nav1.5 may be a potential target for modulation of astrogliosis after injury via effects on [Ca2+]i.
There is a growing body of evidence detailing the favorable effect of sodium channel blockade in animal models of neurological diseases, including EAE. Several sodium channel blockers have been studied, including phenytoin [22,23], lamotrigine [24], carbamazepine [23], safinamide and flecainide [25]. It is noteworthy that flecainide, a Class Ic cardiac antiarrhythmic, has strong state-dependent effects on Nav1.5 [26]. The present findings, along with others detailed here, raise the possibility that attenuation of reactive astrogliosis could contribute to the improved outcomes seen with sodium channel blockade. This study lends further insight to the suggestion that Nav1.5 can play a role in the response of astrocytes to neuroinflammatory pathologies, identifying this sodium channel as an attractive potential therapeutic target for modulating reactive astrogliosis in vivo.
Acknowledgements
The authors thank Pam Zwinger for excellent technical assistance.
Funding
This work was supported by grants from the Rehabilitation Research Service and Medical Research Service, Department of Veterans Affairs (S.G.W.). The Center for Neuroscience and Regeneration Research is a collaboration of the Paralyzed Veterans of America with Yale University. L.W.P. was supported in part by the Medical Scientist Training Grant (NGM007205) from the National Institute of General Medical Studies, National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- [1].Sontheimer H, Black JA, Waxman SG. Voltage-gated Na+ channels in glia: properties and possible functions. Trends Neurosci. 1996;19:325–331. doi: 10.1016/0166-2236(96)10039-4. [DOI] [PubMed] [Google Scholar]
- [2].Black JA, Dib-Hajj S, Cohen S, Hinson AW, Waxman SG. Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia. 1998;23:200–208. [PubMed] [Google Scholar]
- [3].Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na(+)/Ca(2+) exchange. Glia. 2014;62:1162–1175. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].MacFarlane SN, Sontheimer H. Spinal cord astrocytes display a switch from TTX-sensitive to TTX-resistant sodium currents after injury-induced gliosis in vitro. J Neurophysiol. 1998;79:2222–2226. doi: 10.1152/jn.1998.79.4.2222. [DOI] [PubMed] [Google Scholar]
- [5].Sontheimer H, Minturn JE, Black JA, Ransom BR, Waxman SG. Two types of Na(+)-currents in cultured rat optic nerve astrocytes: changes with time in culture and with age of culture derivation. Journal of neuroscience research. 1991;30:275–287. doi: 10.1002/jnr.490300202. [DOI] [PubMed] [Google Scholar]
- [6].Thio CL, Sontheimer H. Differential modulation of TTX-sensitive and TTX-resistant Na+ channels in spinal cord astrocytes following activation of protein kinase C. J Neurosci. 1993;13:4889–4897. doi: 10.1523/JNEUROSCI.13-11-04889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Black JA, Newcombe J, Waxman SG. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain. 2010;133:835–846. doi: 10.1093/brain/awq003. [DOI] [PubMed] [Google Scholar]
- [8].Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J Neurosci. 1994;14:2464–2475. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- [11].Black JA, Liu S, Hains BC, Saab CY, Waxman SG. Long-term protection of central axons with phenytoin in monophasic and chronic-relapsing EAE. Brain. 2006;129:3196–3208. doi: 10.1093/brain/awl216. [DOI] [PubMed] [Google Scholar]
- [12].Matthaei I, Polman CH, de Groot CJ, Dijkstra CD, Koetsier JC, Sminia T. Observer agreement in the assessment of clinical signs in experimental allergic encephalomyelitis. J Neuroimmunol. 1989;23:25–28. doi: 10.1016/0165-5728(89)90068-4. [DOI] [PubMed] [Google Scholar]
- [13].Rogart RB, Cribbs LL, Muglia LK, Kephart DD, Kaiser MW. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989;86:8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Black JA, Waxman SG. Noncanonical roles of voltage-gated sodium channels. Neuron. 2013;80:280–291. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- [15].Barres BA, Chun LL, Corey DP. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989;2:1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- [16].Inglese M, Madelin G, Oesingmann N, Babb JS, Wu W, Stoeckel B, et al. Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 tesla. Brain. 2010;133:847–857. doi: 10.1093/brain/awp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–467. doi: 10.1002/glia.22616. [DOI] [PubMed] [Google Scholar]
- [18].Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- [19].Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012:4. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, et al. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- [21].Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH, et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- [22].Lo AC, Saab CY, Black JA, Waxman SG. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J Neurophysiol. 2003;90:3566–3571. doi: 10.1152/jn.00434.2003. [DOI] [PubMed] [Google Scholar]
- [23].Black JA, Liu S, Carrithers M, Carrithers LM, Waxman SG. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann Neurol. 2007;62:21–33. doi: 10.1002/ana.21172. [DOI] [PubMed] [Google Scholar]
- [24].Bechtold DA, Miller SJ, Dawson AC, Sun Y, Kapoor R, Berry D, et al. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J Neurol. 2006;253:1542–1551. doi: 10.1007/s00415-006-0204-1. [DOI] [PubMed] [Google Scholar]
- [25].Morsali D, Bechtold D, Lee W, Chauhdry S, Palchaudhuri U, Hassoon P, et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain. 2013;136:1067–1082. doi: 10.1093/brain/awt041. [DOI] [PubMed] [Google Scholar]
- [26].Ramos E, O’Leary ME. State-dependent trapping of flecainide in the cardiac sodium channel. J Physiol. 2004;560:37–49. doi: 10.1113/jphysiol.2004.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]