Abstract
Introduction
The only treatment for celiac disease (CD) is life-long adherence to a gluten-free diet (GFD). Noncompliance is associated with signs and symptoms of celiac disease, yet long-term adherence rates are poor. It is not known how the burden of the GFD compares to other medical treatments, and there are limited data on the socio-economic factors influencing treatment adherence. In this study we compared treatment burden and health state in CD compared with other chronic illnesses and evaluated the relationship between treatment burden and adherence.
Methods
A survey was mailed to participants with: CD, gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), hypertension (HTN), diabetes mellitus (DM), congestive heart failure (CHF), and end stage renal disease on dialysis (ESRD). Surveys included demographic information and visual analog scales measuring treatment burden, importance of treatment, disease-specific and overall health status.
Results
We collected surveys from 341 celiac and 368 non-celiac participants. Celiac participants reported high treatment burden, greater than participants with GERD or HTN and comparable to ESRD. Conversely, patients with CD reported the highest health state of all groups. Factors associated with high treatment burden in CD included poor adherence, concern regarding food cost, eating outside the home, higher income, lack of college education and time limitations in preparing food. Poor adherence in CD was associated with increased symptoms, income, and low perceived importance of treatment.
Discussion
Participants with CD have high treatment burden but also excellent overall health status in comparison with other chronic medical conditions. The significant burden of dietary therapy for celiac disease argues for the need for safe adjuvant treatment as well as interventions designed to lower the perceived burden of the GFD.
Keywords: celiac disease, treatment burden, gluten free diet, adherence
Introduction
Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by gluten-containing foods. The prevalence is estimated to be 1:70 to 1:300 , and the only treatment is life-long adherence to a gluten-free diet (GFD) (1–5). Prior studies estimate adherence to the GFD to be as low as 36–45% (6–8). CD differs from many other chronic diseases because dietary changes are the only current therapy for disease management. Poor dietary compliance is the leading cause of ongoing symptoms in participants with CD (9). This is especially concerning considering the numerous potential complications of untreated CD, including reduction in bone mineral density, malignancy and increased mortality rate (3, 10, 11). Adherence to GFD has been associated with improvements in quality of life(12–15), bone mineral density (16–21), fatigue (22), infertility (23–26), adverse pregnancy outcomes (27–29) and risk lymphoproliferative malignancy (30, 31).
There are multiple potential factors accounting for low dietary adherence in CD, including limited availability and higher cost of gluten free foods (32–34), reduced enjoyment of food (35), and social isolation when dining out (35, 36). While there are many unique features of the GFD which may reduce adherence, it is generally accepted that adherence is better with well-circumscribed treatments, such as medication administration, than with health behaviors including dietary advice (37). Currently, treatment of CD is limited exclusively to lifestyle modification, which may contribute to poor adherence and elevated treatment burden in comparison to many other chronic illnesses; however this hypothesis has not been rigorously evaluated. Close follow-up with a physician, dietician, and support group are routinely recommended in order to monitor and improve dietary adherence (38, 39), although the evidence for these interventions are limited.
In order to improve quality of life and treatment adherence in CD, a robust understanding of the burden of the GFD and the factors that influence adherence is necessary. However relevant data on this topic are limited (40) and in most studies have lacked a validated measure of adherence. The objective of this study was to compare the treatment burden in CD with that of other chronic illnesses and identify factors associated with treatment burden and GFD adherence as measured by a validated survey tool (41).
Methods
Study Subjects
The study population consisted of participants evaluated at The Celiac Center at Beth Israel Deaconess Medical Center or who visited primary care clinics associated with Beth Israel Deaconess Medical Center in Boston, MA. This study was approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Survey Development and Measures
The survey was designed to assess socioeconomic and demographic information, measures evaluating treatment burden and adherence and specific questions relating health and treatment barriers. Visual Analog Scales (VAS) have been used to study burden of disease across a multitude of disorders, including gastroesophageal reflux disease, Crohn’s disease, irritable bowel syndrome, diabetes, and congestive heart failure (42–46). We adapted these existing surveys for the current study. Participants were asked to rate four domains on a scale of 0–100: 1) Difficulty in following treatment 2) Perceived importance of following treatment 3) Disease-specific health and 4) Overall health. (Appendix 1). The questionnaire was first assessed for comprehensiveness and clarity by patients with and without CD before being sent to participants. For difficulty in following treatment, a score of zero indicated that treatment is “very easy” and one hundred that treatment is “very difficult”. For importance of following treatment, zero indicated “not important” and one hundred indicated “very important.” For disease-specific health and overall health, zero indicated “worst imaginable” and one hundred indicated “best imaginable” health states. In addition to the VAS scales, participants with CD also completed the Celiac Dietary Adherence Test (CDAT), a validated measure of adherence to the GFD (41) and the Celiac Symptoms Index (CSI), to measure CD symptoms (47).
Celiac Cohort
To be eligible for participation in the celiac cohort, individuals had to be > 18 years of age with biopsy-confirmed CD for more than three months, have a valid United States home address and have cognitive ability and English proficiency suitable for independent completion of the surveys.
Non-Celiac Cohorts
To be eligible for participation, individuals had to have cognitive ability and English proficiency suitable for independent completion of the surveys, be > 18 years of age and have a valid United States address. Participants were chosen on the basis of one of seven chronic illnesses including hypertension (HTN), diabetes mellitus (DM), congestive heart failure (CHF), end stage renal disease requiring dialysis (ESRD), gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). Diagnoses were preliminarily identified through ICD9 codes and confirmed in all cases through independent review of the medical record. The presence of multiple illnesses did not preclude inclusion. In these scenarios participants were included only in the illness diagnosis first used for identification.
Statistical Analysis
Univariate statistics were used to evaluate cohort means and standard deviations. Missing data was handled by cohort response means. Student t-statistic and one-way analysis of variance compared means between two groups and across three or more groups, respectively. Post-hoc scheffe multiple comparison test was used to evaluate the differences between each pair of means. Stepwise linear regression analysis controlling for age and gender was used to determine predictors of treatment burden, GFD adherence, and perceived importance of treatment for the celiac cohort. A p value of <0.05 was considered statistically significant. All analyses were performed using Stata (StataCorp LP; College Station, Texas).
Results
Characteristics of Study Participants
Of 773 surveys mailed to CD participants, 341 (45%) responded. Mean age at diagnosis was 42.98 years (95% CI 41.35, 44.61) and mean age at time of the survey was 51.14 years (95% CI 49.53, 52.75). Participants had followed a GFD for a mean of 85.49 months (95% CI 77.63, 93.46). Of the 1288 surveys mailed out to non-celiac disease participants, 368 (29%) responded. Baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics of Celiac and non-Celiac cohorts
| CD | HTN | DM | CHF | ESRD | GERD | IBD | IBS | |
|---|---|---|---|---|---|---|---|---|
| Number of participants | 341 | 75 | 69 | 41 | 18 | 61 | 67 | 37 |
| Mean age, years (SE) | 51.14 (0.81) | 63.27 (1.37) | 64.03 (1.31) | 70.58 (2.07) | 56.61 (4.27) | 57.84 (1.62) | 46.13 (1.90) | 50.59 (0.81) |
| % Female, (SE) | 76.26 (0.02) | 45.95 (0.06) | 38.24 (0.06) | 53.66 (0.08) | 44.44 (0.12) | 40.00 (0.06) | 46.97 (0.06) | 52.78 (0.36) |
| % Caucasian, (SE) | 96.77 (0.01) | 80.00 (0.05) | 70.59 (0.06) | 68.29 (0.07) | 61.11 (0.12) | 77.96 (0.05) | 93.73 (0.03) | 88.89 (0.05) |
| % Income* < $75,000, (SE) | 36.50 (0.03) | 57.33 (0.06) | 69.56 (0.06) | 87.80 (0.05) | 88.89 (0.08) | 40.98 (0.06) | 41.79 (0.06) | 41.67 (0.08) |
| % No college education (SE) | 26.71 (0.02) | 48.00 (0.06) | 56.52 (0.06) | 56.10 (0.08) | 66.67 (0.11) | 42.62 (0.06) | 28.36 (0.06) | 30.56 (0.08) |
| Mean specialist visits/year (SE) | 0.75 (0.03) | 0.48 (0.12) | 1.65 (0.21) | 2.34 (0.23) | 4.39 (0.24) | 1.26 (0.21) | 2.51 (0.17) | 1.82 (0.24) |
| Mean PCP visits/year (SE) | 2.19 (0.08) | 2.54 (0.16) | 3.13 (0.18) | 3.61 (0.24) | 3.28 (0.36) | 2.60 (0.16) | 2.18 (0.18) | 2.85 (0.28) |
CD, Celiac disease; HTN, Hypertension; DM, Diabetes Mellitus; CHF, Congestive heart failure; ESRD, End-stage renal disease; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome
SE, standard error
Income in US dollars
Celiac responders were younger than those with HTN (p<0.001), GERD (p=0.001), DM (p<0.001), and CHF (p<0.001) and older than those with IBD (p=0.008). There was no difference in age between the celiac cohort and those with ESRD (p=0.112) or IBS (p=0.903). Celiac responders were more likely to be female compared to all other cohorts (HTN, p<0.001; GERD, p<0.001; ESRD, p=0.006; DM, p<0.001; CHF, p=0.003; IBD, p<0.001; IBS, p=0.004) and were more likely to identify as Caucasian compared to those with HTN (p<0.001), GERD (p<0.001), ESRD (p<0.001), DM (p<0.001), CHF (p<0.001), but not with IBD (p=0.052) or IBS (p=0.092). Celiac responders were more likely to report education of college or greater than those with HTN (p<0.001), GERD (p=0.015), ESRD (p=0.001), DM (p<0.001), CHF (p<0.001), but not with IBD (p=0.817) or IBS (p=0.647). Celiac responders were less likely to report income less than $75,000 compared to those with HTN (p<0.001), ESRD (p=0.001), DM (p<0.001), CHF (p<0.001), but not compared to GERD (p=0.055), IBD (p=0.455), or IBS (p=0.579).
Celiac responders were less likely to visit specialists compared to all other diagnoses (GERD, p=0.006; ESRD, p<0.001; DM, p<0.001; CHF, p<0.001; IBD, p<0.001; and IBS, p<0.001), except for those with HTN (p=0.105). Celiac responders were also less likely to visit their primary care physician compared to participants with ESRD (p=0.002), DM (p<0.001), CHF (p<0.001), GERD (p=0.050), and IBS (p-value=0.011), but not when compared to HTN (p=0.055), IBD (p=0.948).
Treatment Burden
The mean reported burden of following a GFD was 44.90 points on the VAS (0 very easy and 100 very difficult). In univariate regression analysis, patient factors significantly associated with increased treatment burden were poor adherence to GFD (p<0.001), increased severity of current celiac symptoms (p=0.001), time limitations for the research, purchase and preparation of foods (p=0.001), difficulty with eating outside the home (p=0.005), concern with cost of food (p=0.001), lack of college education (p=0.004), and hospitalizations within the last year (p=0.006). In the multivariate linear regression model controlling for age and gender, time limitations for the research, purchase and preparation of foods (p=0.010), difficulty with eating outside the home (p=0.023), concern with food cost (p=0.012), lack of college education (p=0.010), and poor adherence (p<0.001) remained significantly associated with increased treatment burden. Income greater than >$200,000 (p=0.017) was also significant in multivariate analysis, but increased severity of current celiac symptoms and hospitalizations were not. (Table 2)
Table 2.
Celiac patient factors associated with high GFD treatment burden
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β coefficient | 95% CI | β coefficient | 95% CI | |
| Time limitations | 14.21 | 6.09, 22.33 | 10.41 | 2.51, 18.31 |
| Eating outside the home | 12.64 | 3.82, 21.46 | 10.13 | 1.44, 18.82 |
| Concern regarding food cost | 11.41 | 4.63, 18.18 | 8.91 | 1.94, 15.88 |
| No college education | 10.78 | 3.48, 18.09 | 9.73 | 2.33, 17.14 |
| Hospitalization in the past year | 9.01 | 2.62, 15.39 | NS | NS |
| Poor GFD adherence | 2.42 | 1.54, 3.31 | 1.77 | 0.82, 2.72 |
| Increased celiac symptoms | 0.61 | 0.27, 0.96 | NS | NS |
| Income* >$200,000 | NS | NS | 11.51 | 2.08, 20.94 |
NS, not significant
Income in US dollars
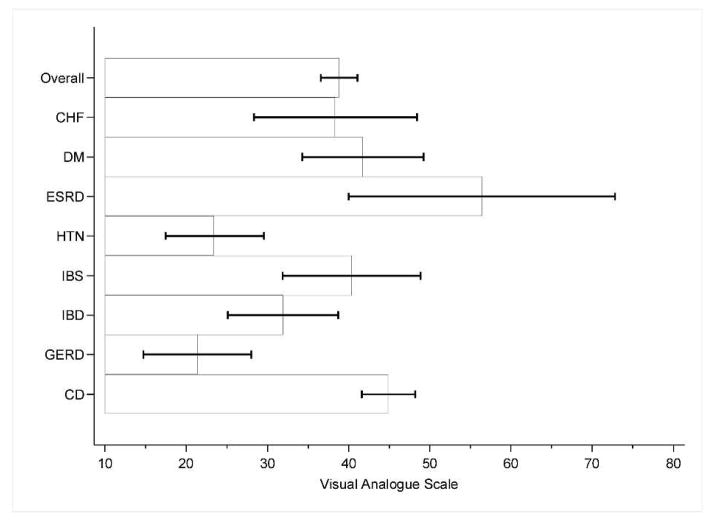
Treatment burden, perceived treatment importance, disease-specific health, and overall health were compared between all medical conditions assessed. Figures 1–4 graphically depict these results. The reported treatment burden for CD of 44.90 was higher than all other groups in aggregate (mean 33.01, p<0.001). CD also had the highest reported treatment burden of all conditions assessed with the exception of ESRD, though this only reached statistical significance for HTN (mean 23.50, p<0.001) and GERD (mean score 21.34, p<0.001). Treatment burden was highest overall for ESRD (mean 56.41), followed by CD, DM (mean 41.74), IBS (mean 40.38), CHF (mean 38.46) and IBD (mean 31.91).
Figure 1.
Treatment burden of celiac disease in comparison with non-celiac chronic illnesses
*CD= Celiac disease, HTN= Hypertension, DM=Diabetes Mellitus, CHF=Congestive heart failure, ESRD=End-stage renal disease, GERD=gastroesophageal reflux disease, IBD=inflammatory bowel disease, IBS=irritable bowel syndrome
**VAS: score of zero=very easy, score of one hundred=very difficult
***Mean Scores: CD 44.9 (SD 30.9), GERD 21.3 (SD 25.3), HTN 23.5 (SD 25.7), IBD 31.9 (SD 27.7), CHF 38.5 (SD 31), IBS 40.4 (SD 24.4), DM 41.7 (SD 30.4), ESRD 56.4 (SD 31.9)
****Based on results of post-hoc multiple comparisons, treatment burden was statistically significant for the following groups: CD vs GERD (p value < 0.001), CD vs HTN (p value < 0.001), GERD vs ESRD (p value 0.01), GERD vs DM (p value 0.04). Error bars represent 95% confidence intervals.
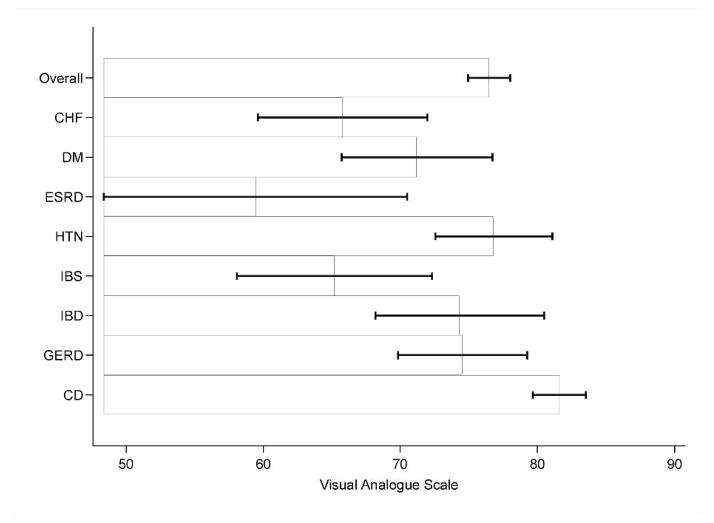
Figure 4.
Perceived overall health state of celiac disease in comparison with non-celiac chronic illnesses
*CD= Celiac disease, HTN= Hypertension, DM=Diabetes Mellitus, CHF=Congestive heart failure, ESRD=End-stage renal disease, GERD=gastroesophageal reflux disease, IBD=inflammatory bowel disease, IBS=irritable bowel syndrome
**VAS: score of zero=best imaginable health, score of one hundred=worst imaginable health
***Mean Scores: CD 78 (SD 16.7), GERD 71.6 (SD 21.6) ,HTN 72.4 (SD 20.9), IBD 76.7 (SD 19.1), CHF 57.8 (SD 21.2), IBS 70.5 (SD 18.1), DM 69.9 (SD 23), ESRD 55.4 (SD 17.8)
****Based on results of post-hoc multiple comparisons, overall health was statistically significant for the following groups: CD vs ESRD (p value 0.001), CD vs CHF (p value < 0.001), IBD vs ESRD (p value 0.014), IBD vs CHF (p value 0.001), HTN vs CHF (p value 0.029). Error bars represent 95% confidence intervals.
Adherence to GFD
The mean CDAT score in the CD cohort was 11.93 (95% CI 11.55, 12.31). In univariate linear regression, poor adherence was associated with income < $200,000 (p=0.047), unemployed status (p=0.050), increased severity of current celiac symptoms (p<0.001), lower perceived importance of treatment (p<0.001), and greater treatment burden (p<0.001). In the multivariate model, income<$200,000 (p=0.045), increased severity of current celiac symptoms (p<0.001), and lower perceived importance of treatment (p<0.001) remained associated with poor adherence after controlling for age and gender (Table 3).
Table 3.
Celiac patient factors associated with poor adherence to GFD
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β coefficient | 95% CI | β coefficient | 95% CI | |
| Income* <$200,000 | 1.90 | 0.04, 3.76 | 0.92 | (0.02, 1.81) |
| Unemployed | 1.43 | (0.002, 2.87) | NS | NS |
| Worse symptoms | 0.17 | (0.13, 0.20) | 0.19 | (0.15, 0.22) |
| Lower perceived treatment importance | 0.07 | (0.05, 0.09) | 0.09 | (0.07, 0.11) |
| Greater treatment burden | 0.03 | (0.02, 0.04) | NS | NS |
NS, not significant
Income in US dollars
Perceived Importance of following the GFD
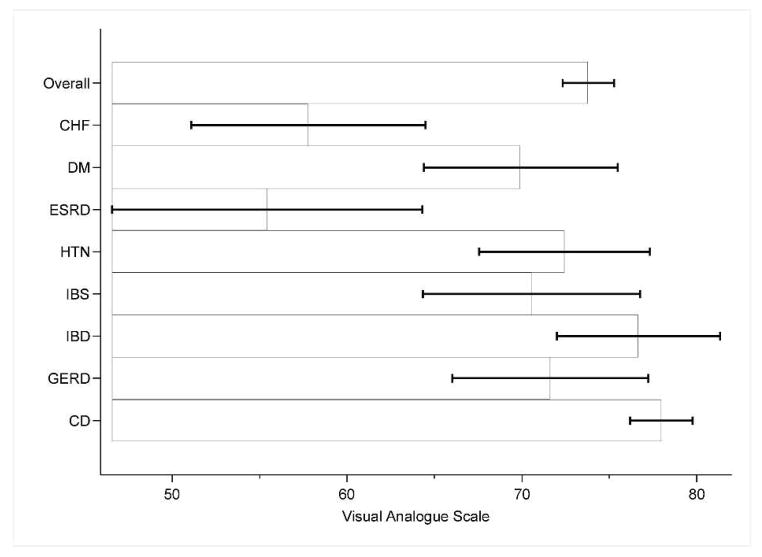
Most participants reported high importance of following a GFD, with a mean score for treatment importance of 93.80 (95% CI 91.83–95.77). In univariate linear regression, difficulty with eating outside the home (p=0.026), female gender (94.89 vs. 90.29, p=0.050), greater adherence (p<0.001), and increased severity of current celiac symptoms (p=0.024) were associated with higher perceived importance of following a GFD. Only greater adherence (p<0.001) and increased severity of current celiac symptoms (p<0.001) continued to have significance in multivariate linear regression model (Table 4). In comparison to other diseases, only ESRD rated treatment as more important (mean score 94.67), however the difference was only statistically significant between CD and IBS (mean score 79.42, p=0.016). Mean perceived importance of treatment was 90.14 for DM, 88.89 for HTN, 88.83 for CHF, 87.95 for IBD and 86.49 for GERD. The reported perceived importance of treatment for CD of 93.80 was higher than all other groups in aggregate (mean 87.90, p<0.001).
Table 4.
Celiac patient factors associated with higher perceived importance of following GFD
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β coefficient | 95% CI | β coefficient | 95% CI | |
| Eating out | 6.06 | 0.74, 11.38 | NS | NS |
| Female gender | 4.60 | 0.01, 9.19 | NS | NS |
| Greater adherence | 1.92 | 1.40, 2.43 | 2.71 | 2.19, 3.23 |
| Worse symptoms | 0.24 | 0.03, 0.45 | 0.70 | 0.51, 0.89 |
NS, not significant
Overall and Disease Specific Health
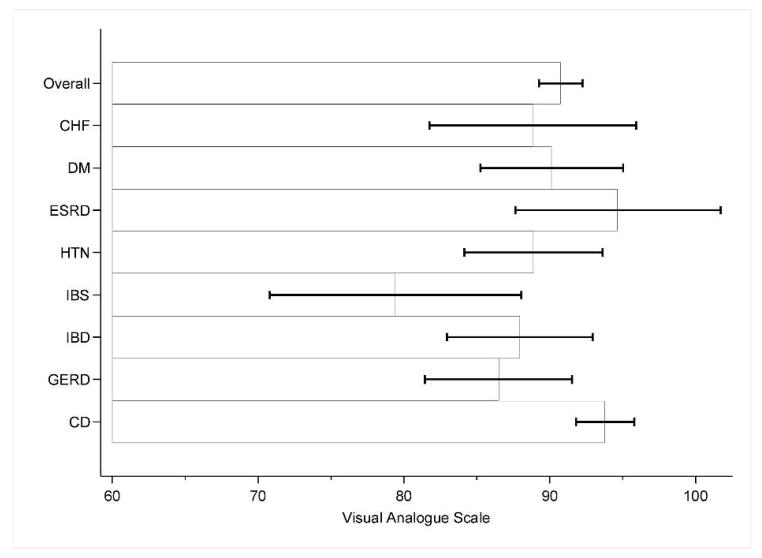
Participants with CD rated disease-specific health higher than any other group, with a mean score of 81.61 (0 “worst imaginable health state” and 100 “best imaginable health state”). This score was significantly higher than for ESRD (mean 59.44, p=0.003), IBS (mean 65.20, p=0.003), CHF (mean score 65.80, p=0.002), and, DM (mean 71.22, p=0.028). Disease-specific health was higher than that of the other chronic medical conditions as well, including HTN (mean 76.82), GERD (mean 74.56), and IBD (mean 74.34), but did not reach statistical significance. The reported disease-specific health for CD of 81.61 was higher than all other groups in aggregate (mean 71.72, p<0.001).
Similarly, participants with CD rated overall health higher than any other group, with a mean score of 77.97. Overall health was significantly greater for CD compared to ESRD (mean 55.44, p=0.001) and CHF (mean 57.80, p<0.001), but did not reach significance for HTN (mean 72.44), GERD (mean 71.62), IBD (mean 76.66), or IBS (mean 70.54). The reported overall health for CD of 77.97 was higher than all other groups in aggregate (mean 69.93, p<0.001).
In subgroup analysis, we further characterized IBD patients based on the use of biologic therapy and DM patients based on the use of insulin. 15% of IBD participants reported taking biologics and in this group treatment burden was 33.00 compared to 31.71 (p=0.89) in those not on biologic therapy and disease specific health and overall health were 76.40 and 79.80 respectively, compared to 73.96 (p=0.778) and 76.11 (p=0.576) for those not on biologic therapy. When compared to IBD participants not on biologics, celiac participants reported greater disease specific health (73.96 vs. 81.61, p=0.036), treatment burden (mean difference 31.71 vs. 44.90, p=0.002) and perceived importance of treatment (mean difference 87.26 vs. 93.80, p=0.027). No difference was observed between IBD participants on biologic therapy and celiac participants for disease specific health (76.40 vs. 81.61, p=0.479), treatment burden (33.00 vs. 44.90, p=0.243) and importance of treatment (92.11 vs. 93.80, p=0.808).
32% of diabetic participants reported taking insulin. Treatment burden (45.00 vs. 40.43, p=0.583) disease specific health (70.82 vs. 71.41, p=0.920) and overall health (67.50 vs. 71.06, p=0.554) did not differ for diabetics on insulin compared to those not on insulin. When compared to diabetics not on insulin, celiac participants reported greater disease specific health (71.41 vs. 81.61, p=0.004). Treatment burden (40.43 vs. 44.90, p=0.346) and perceived importance of treatment (87.72 vs. 93.80, p=0.101) did not differ between diabetics not on insulin and celiac participants. Disease specific health (70.82 vs. 81.61, p=0.060), treatment burden (45.00 vs. 44.90, p=0.989), and treatment importance (95.32 vs. 93.80, p =0.374) did not differ between diabetics on insulin and celiac participants.
Discussion
Adherence to a GFD is the only treatment for CD and failure to adequately treat is associated with morbidity. However, GFD adherence remain poor (40, 48) and studies examining factors that impact treatment burden and GFD adherence are limited (36, 38, 49). We sought to evaluate predictors of adherence and treatment burden, and to compare treatment burden in CD with other chronic diseases.
Our results demonstrate that celiac participants report a remarkably high treatment burden. In our study, celiac participants reported greater treatment burden than those with HTN and GERD and comparable to participants with CHF and ESRD. This underscores the difficulty of following the GFD and puts the high treatment burden of CD into context for practitioners who often have more experience with other diseases. Greater difficulty with treatment implies a need for non-dietary interventions as well as the need for patients to regularly follow up with clinicians, dieticians and other allied health professionals, while our data and others’ (50) suggest that patients with CD actually see physicians less often than those with other chronic medical conditions.
Our results also show that treatment burden is a predictor of poor adherence to a GFD. Those who report higher burden of following a GFD were more likely to have poor adherence to a GFD. An exception to this was in participants with high reported household income. These individuals were likely to report high treatment burden but were able overcome this and demonstrate greater adherence to the GFD. While our study was not designed to evaluate this relationship in detail, we hypothesize that low education increases burden though difficulty in managing the complexity of the GFD, while high income increase burden due to difficulty in following the diet during frequent travel and social outings. Similar to prior studies (41), celiac participants with poor GFD adherence had greater severity of symptoms likely due to gluten exposure. While the majority of participants reported overall high perceived importance of the GFD, the finding that participants who felt the GFD was not very important to their health had poor adherence is logical and reflects the internal validity of the survey measures.
It is encouraging to note that despite having reported quite a high treatment burden in CD when compared with other chronic medical conditions, participants with CD also reported a high disease-specific and overall health. It is notable that CD patients reported greatest disease-specific health, yet reported higher treatment burden compared to all other diseases except ESRD. The high burden of treatment may also be addressed by focusing on other alternative treatments such as novel medical therapy or even complementary alternative medicine (51).
Despite the use of validated measures and the relatively large size, we recognize a number of limitations. The data were collected from a dedicated celiac disease center at a major teaching hospital, which potentially limits the generalizability of the findings. Additionally, there was a substantial non-response rate that may bias results if respondents differ significantly from non-respondents, however, in the CD cohort respondent demographics were not significantly different from non-respondents. (Supplemental Table 1) Also, the response rate of 45% for the celiac disease patients compared with 29% for non-celiac disease patients raises the possibility of differential selection between these groups, which could bias results. Finally, while the VAS has been widely used and validated in other disease states (42–46), it is possible that the VAS may not measure the full spectrum of treatment burden, importance of treatment, and health state. In addition, participants were asked to rate treatment burden and health state without knowledge of the other disease states with which they were compared. While there is value in asking patients to rate their health compared to another distinct health state, it is difficult for individuals to gauge the impact of conditions they do not have. For this reason we chose to use the common and well validated anchors such as of ‘Best imaginable health’ and ‘Worst imaginable health’.
In conclusion, participants with CD report a remarkably high treatment burden similar to participants with ESRD and higher than many other chronic medical conditions. Conversely, CD participants in general reported high disease specific health state, which suggests that despite good long-term health outcomes, CD patients struggle with treatment. This study underscores both the limitations of the GFD as lone treatment as well as the need for attention to patient perceptions of recommended therapies (51). As the CD population expands and new therapies are proposed, attention to burden of treatment and disease will be vital for providing optimal care for this population.
Supplementary Material
Figure 2.
Perceived importance of treatment of celiac disease in comparison with non-celiac chronic illnesses
*CD= Celiac disease, HTN= Hypertension, DM=Diabetes Mellitus, CHF=Congestive heart failure, ESRD=End-stage renal disease, GERD=gastroesophageal reflux disease, IBD=inflammatory bowel disease, IBS=irritable bowel syndrome
**VAS: score of zero=not important at all, score of one hundred=very important
***Mean Scores: CD 93.8 (SD 18.6), HTN 88.9 (SD 20.4), GERD 86.5 (SD 19.7), IBD 88.0 (SD 19.9), CHF 88.8 (SD 22.4), IBS 79.4 (SD 25.5), DM 90.1(SD 20.4), ESRD 94.7 (SD 14.2)
****Based on results of post-hoc multiple comparisons, importance of treatment was statistically significant for the following groups: CD vs IBS (p value 0.016). Error bars represent 95% confidence intervals.
Figure 3.
Perceived disease-specific health of celiac disease in comparison with non-celiac chronic illnesses
*CD= Celiac disease, HTN= Hypertension, DM=Diabetes Mellitus, CHF=Congestive heart failure, ESRD=End-stage renal disease, GERD=gastroesophageal reflux disease, IBD=inflammatory bowel disease, IBS=irritable bowel syndrome
**VAS: score of zero=best imaginable health, score of one hundred=worst imaginable health
***Mean Scores: CD 81.6 (SD 18), GERD 74.6 (SD 18.4), HTN 76.8(18.4), IBD 74.3 (SD 24.9), CHF 65.8 (SD 19.4), IBS 65.2 (SD 20.7), DM 71.2 (SD 22.7), ESRD 59.4 (SD 22.2)
****Based on results of post-hoc multiple comparisons, disease specific health was statistically significant for the following groups: CD vs IBS (p value 0.003), CD vs ESRD (p value 0.003), CD vs DM (p value 0.028), CD vs CHF (p value 0.002). Error bars represent 95% confidence intervals.
Abbreviations
- CD
celiac disease
- CDAT
celiac dietary assessment tool
- CSI
celiac symptom index
- VAS
visual analog scale
- GFD
gluten free diet
- HTN
hypertension
- GERD
gastroesophageal reflux disease
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- ESRD
end stage renal disease
- DM
diabetes mellitus
- CHF
congestive heart failure
Footnotes
Conflicts of interest: Sveta Shah, Mona Akbari, Rohini Vanga, Joshua Hansen, Arjun Bhansali, Sohaib Tariq, Melinda Dennis: None
Ciarán P. Kelly: None
Daniel Leffler: consulting and/or research support from: Shire Therapeutics, Prometheus Laboratories, Alba Pharmaceuticals, Alvine Therapeutics
Author Contributions:
Sveta Shah: Study design and execution, analysis and interpretation of data, drafting of manuscript, and critical revision of the manuscript for important intellectual content of the manuscript
Mona Akbari: Study design and execution, analysis and interpretation of data, drafting of manuscript, and critical revision of the manuscript for important intellectual content of the manuscript
Rohini Vanga: Study execution
Ciarán P. Kelly: Study design and execution, interpretation of data, and critical revision of the manuscript for important intellectual content of the manuscript
Joshua Hansen: Study execution
Thimmaiah Theethira: Study execution
Sohaib Tariq: Study execution
Melinda Dennis: Study design, execution, and enrollment
Daniel Leffler: Study concept, design and execution, interpretation of data, and critical revision of the manuscript for important intellectual content of the manuscript
References
- 1.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Alimentary Pharmacology & Therapeutics. 2007;26(9):1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Archives of Internal Medicine. 2003;163(3):286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Farrell RJ, Kelly CP. Celiac sprue. The New England journal of medicine. 2002;346(3):180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107(10):1538–44. doi: 10.1038/ajg.2012.219. quiz 7, 45. [DOI] [PubMed] [Google Scholar]
- 5.Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18(42):6036–59. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahedi K, Mascart F, Mary JY, Laberenne JE, Bouhnik Y, Morin MC, et al. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. The American Journal of Gastroenterology. 2003;98(5):1079–87. doi: 10.1111/j.1572-0241.2003.07284.x. [DOI] [PubMed] [Google Scholar]
- 7.Hogberg L, Grodzinsky E, Stenhammar L. Better dietary compliance in patients with coeliac disease diagnosed in early childhood. Scandinavian Journal of Gastroenterology. 2003;38(7):751–4. doi: 10.1080/00365520310003318. [DOI] [PubMed] [Google Scholar]
- 8.Bardella MT, Molteni N, Prampolini L, Giunta AM, Baldassarri AR, Morganti D, et al. Need for follow up in coeliac disease. Archives of Disease in Childhood. 1994;70(3):211–3. doi: 10.1136/adc.70.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(4):445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA : the journal of the American Medical Association. 2009;302(11):1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 11.Leffler D, Kelly CP. Celiac disease: what the last few years have taught us. In: Howden CW, editor. Advances in Digestive Diseases. AGA Institute Press; 2007. p. 49. [Google Scholar]
- 12.Tontini GE, Rondonotti E, Saladino V, Saibeni S, de Franchis R, Vecchi M. Impact of gluten withdrawal on health-related quality of life in celiac subjects: an observational case-control study. Digestion. 2010;82(4):221–8. doi: 10.1159/000265549. [DOI] [PubMed] [Google Scholar]
- 13.Nachman F, Maurino E, Vazquez H, Sfoggia C, Gonzalez A, Gonzalez V, et al. Quality of life in celiac disease patients: prospective analysis on the importance of clinical severity at diagnosis and the impact of treatment. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009;41(1):15–25. doi: 10.1016/j.dld.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Nachman F, del Campo MP, Gonzalez A, Corzo L, Vazquez H, Sfoggia C, et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010;42(10):685–91. doi: 10.1016/j.dld.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Mustalahti K, Lohinierni S, Laippala P, Collin P, Maki M. Improvement of the quality of life of silent celiac disease patients during the gluten-free diet warrents screening. Gastroenterology. 2000;118(4):A369. [Google Scholar]
- 16.Vasquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, et al. Risk of fractures in celiac disease patients: a cross-sectional, case-control study. The American Journal of Gastroenterology. 2000;95(1):183–9. doi: 10.1111/j.1572-0241.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 17.Valdimarsson T, Lofman O, Toss G, Strom M. Reversal of osteopenia with diet in adult coeliac disease. Gut. 1996;38(3):322–7. doi: 10.1136/gut.38.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sategna-Guidetti C, Grosso SB, Grosso S, Mengozzi G, Aimo G, Zaccaria T, et al. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Alimentary Pharmacology & Therapeutics. 2000;14(1):35–43. doi: 10.1046/j.1365-2036.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 19.Mustalahti K, Collin P, Sievanen H, Salmi J, Maki M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet. 1999;354(9180):744–5. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- 20.Meyer D, Stavropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a north american adult population with celiac disease. The American Journal of Gastroenterology. 2001;96(1):112–9. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 21.Ciacci C, Maurelli L, Klain M, Savino G, Salvatore M, Mazzacca G, et al. Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. The American Journal of Gastroenterology. 1997;92(6):992–6. [PubMed] [Google Scholar]
- 22.Siniscalchi M, Iovino P, Tortora R, Forestiero S, Somma A, Capuano L, et al. Fatigue in adult coeliac disease. Alimentary Pharmacology & Therapeutics. 2005;22(5):489–94. doi: 10.1111/j.1365-2036.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- 23.Sher KS, Mayberry JF. Female fertility, obstetric and gynaecological history in coeliac disease: a case control study. Acta paediatrica (Oslo, Norway : 1992) Supplement. 1996;412:76–7. doi: 10.1111/j.1651-2227.1996.tb14258.x. [DOI] [PubMed] [Google Scholar]
- 24.Rujner J. Age at menarche in girls with celiac disease. Ginekologia polska. 1999;70(5):359–62. [PubMed] [Google Scholar]
- 25.Ferguson R, Holmes GK, Cooke WT. Coeliac disease, fertility, and pregnancy. Scandinavian Journal of Gastroenterology. 1982;17(1):65–8. doi: 10.3109/00365528209181045. [DOI] [PubMed] [Google Scholar]
- 26.Bona G, Marinello D, Oderda G. Mechanisms of abnormal puberty in coeliac disease. Hormone research. 2002;57 (Suppl 2):63–5. doi: 10.1159/000058103. [DOI] [PubMed] [Google Scholar]
- 27.Norgard B, Fonager K, Sorensen HT, Olsen J. Birth outcomes of women with celiac disease: a nationwide historical cohort study. The American Journal of Gastroenterology. 1999;94(9):2435–40. doi: 10.1111/j.1572-0241.1999.01370.x. [DOI] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129(2):454–63. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 29.Ciacci C, Cirillo M, Auriemma G, Di Dato G, Sabbatini F, Mazzacca G. Celiac disease and pregnancy outcome. The American Journal of Gastroenterology. 1996;91(4):718–22. [PubMed] [Google Scholar]
- 30.Lebwohl B, Granath F, Ekbom A, Smedby KE, Murray JA, Neugut AI, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Annals of Internal Medicine. 2013;159(3):169–75. doi: 10.7326/0003-4819-159-3-201308060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludvigsson JF. Mortality and malignancy in celiac disease. Gastrointestinal endoscopy clinics of North America. 2012;22(4):705–22. doi: 10.1016/j.giec.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Stevens L, Rashid M. Gluten-free and regular foods: a cost comparison. Canadian journal of dietetic practice and research : a publication of Dietitians of Canada = Revue canadienne de la pratique et de la recherche en dietetique : une publication des Dietetistes du Canada. 2008;69(3):147–50. doi: 10.3148/69.3.2008.147. [DOI] [PubMed] [Google Scholar]
- 33.Singh J, Whelan K. Limited availability and higher cost of gluten-free foods. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2011;24(5):479–86. doi: 10.1111/j.1365-277X.2011.01160.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2007;20(5):423–30. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 35.Whitaker JK, West J, Holmes GK, Logan RF. Patient perceptions of the burden of coeliac disease and its treatment in the UK. Alimentary Pharmacology & Therapeutics. 2009;29(10):1131–6. doi: 10.1111/j.1365-2036.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- 36.Edwards George JB, Leffler DA, Dennis MD, Franko DL, Blom-Hoffman J, Kelly CP. Psychological correlates of gluten-free diet adherence in adults with celiac disease. Journal of clinical gastroenterology. 2009;43(4):301–6. doi: 10.1097/MCG.0b013e31816a8c9b. [DOI] [PubMed] [Google Scholar]
- 37.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Medical care. 2004;42(3):200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 38.Leffler DA, Edwards-George J, Dennis M, Schuppan D, Cook F, Franko DL, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Digestive diseases and sciences. 2008;53(6):1573–81. doi: 10.1007/s10620-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leffler D. Celiac disease diagnosis and management: a 46-year-old woman with anemia. JAMA : the journal of the American Medical Association. 2011;306(14):1582–92. doi: 10.1001/jama.306.14.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Alimentary Pharmacology & Therapeutics. 2009;30(4):315–30. doi: 10.1111/j.1365-2036.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- 41.Leffler DA, Dennis M, Edwards George JB, Jamma S, Magge S, Cook EF, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(5):530–6. 6.e1–2. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox AR, Dragnev MC, Darcey CJ, Siegel CA. A new tool to measure the burden of Crohn’s disease and its treatment: do patient and physician perceptions match? Inflammatory bowel diseases. 2010;16(4):645–50. doi: 10.1002/ibd.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JY, Woloshin S, Laycock WS, Rothstein RI, Finlayson SR, Schwartz LM. Symptoms and treatment burden of gastroesophageal reflux disease: validating the GERD assessment scales. Archives of Internal Medicine. 2004;164(18):2058–64. doi: 10.1001/archinte.164.18.2058. [DOI] [PubMed] [Google Scholar]
- 44.Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS. The use of the EQ-5D preference-based health status measure in adults with Type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2011;28(4):395–413. doi: 10.1111/j.1464-5491.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 45.Ekman I, Granger B, Swedberg K, Stenlund H, Boman K. Measuring shortness of breath in heart failure (SOB-HF): development and validation of a new dyspnoea assessment tool. European journal of heart failure. 2011;13(8):838–45. doi: 10.1093/eurjhf/hfr062. [DOI] [PubMed] [Google Scholar]
- 46.Bushnell DM, Martin ML, Ricci JF, Bracco A. Performance of the EQ-5D in patients with irritable bowel syndrome. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2006;9(2):90–7. doi: 10.1111/j.1524-4733.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- 47.Leffler DA, Dennis M, Edwards George J, Jamma S, Cook EF, Schuppan D, et al. A validated disease-specific symptom index for adults with celiac disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(12):1328–34. 34.e1–3. doi: 10.1016/j.cgh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 48.Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128(4 Suppl 1):S135–41. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66(3):178–85. doi: 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- 50.Herman ML, Rubio-Tapia A, Lahr BD, Larson JJ, Van Dyke CT, Murray JA. Patients with celiac disease are not followed up adequately. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(8):893–9. e1. doi: 10.1016/j.cgh.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? Journal of gastrointestinal and liver diseases : JGLD. 2011;20(1):27–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.