Abstract
Single photon emission computed tomography (SPECT) or positron emission computed tomography (PET) imaging agents for neurodegenerative disease have a significant impact on clinical diagnosis and patient care. The examples of Parkinson’s Disease (PD) and Alzheimer’s Disease (AD) imaging agents described in this paper provide a general view on how imaging agents, ie radioactive drugs, are selected, chemically prepared and applied in humans. Imaging the living human brain can provide unique information on the pathology and progression of neurodegenerative diseases, such as AD and PD. The imaging method will also facilitate preclinical and clinical trials of new drugs offering specific information related to drug binding sites in the brain. In the future, chemists will continue to play important roles in identifying specific targets, synthesizing target-specific probes for screening and ultimately testing them by in vitro and in vivo assays.
1) What are radiopharmaceuticals and why are they needed?
Radiopharmaceuticals, often referred to as radiolabeled diagnostic drugs, radioactive tracers or probes, are injected intravenously into patients for diagnostic purposes in conjunction with single photon emission computed tomography (SPECT) or positron emission computed tomography (PET).1 Molecular imaging, which enables the visualization of the cellular function and molecular processes in living humans, tissues or organisms, is a discipline at the intersection of molecular biology and in vivo imaging. Although PET and SPECT imaging are most prevalent, other imaging modalities, such as magnetic resonance imaging (MRI), x-ray computer tomography (CT), ultrasound or optical imaging could also be used for molecular imaging. The contrast agents for MRI, CT and ultrasound imaging are at a higher chemical concentration (μM to mM range) than those used for PET and SPECT (pM to nM). Molecular imaging merges many research disciplines with the potential for integrating diagnosis and drug development, as well as opening up the possibility of individualized molecular medicine.2
In the foreseeable future, it is likely that neurodegenerative diseases will be diagnosed and monitored with targeted imaging agents used in conjunction with either PET or SPECT scanners.3 Both SPECT and PET are tomographic imaging techniques. They use sensitive detectors to collect gamma rays emitted from the injected radioprobes into patients designed to create maps of cellular distribution and function. It is not possible to obtain information about the location and quantity of specific receptors or binding sites in the living human brain by other methods (i.e. magnetic resonance imaging MRI, or computer tomography, CT).4
It is well recognized that PET has higher resolution, higher sensitivity and is better for quantification than SPECT imaging is. On the other hand, there are more hospitals equipped with SPECT scanners. Therefore, it is more practical to design agents for use with SPECT. Readers interested in learning more about the physics and instrumentation of PET and SPECT are encouraged to consult the book by Cherry et al.5 With the recent development of combined SPECT/CT and PET/CT scanners, clinicians are able to compare three-dimensional anatomical CT images with PET or SPECT scans, which provide information about biochemical function. This is a powerful tool for the evaluation of CNS function in both normal and disease states.
An effective radiopharmaceutical for neurodegenerative diseases has several characteristics: First of all, it is labeled with a short-lived isotope, which provides gamma ray emission. Isotopes, such as 99mTc which emits medium energy photons (140 KeV), are useful for SPECT imaging; while positron emitting isotopes, such as 18F, emit a positron, which collides with near by electrons. The resulting annihilation reaction between a positron and an electron produces two 511 KeV photons for PET imaging. Most of the diagnostic procedures using radiopharmaceuticals are accomplished within a day and a short-lived radioisotope reduces the ionizing radiation dose to patients. Secondly, it can be injected at very low doses (picogram to nanogram). Therefore, the risk of chemical toxicity is minimal. Thirdly, its distribution in the body is based on specific processes or targeting mechanisms. For example, in brain imaging it means mapping brain function, such as the regional blood perfusion, glucose metabolism or targeting specific neuroreceptors, transporters or other binding sites.6 This is particularly useful for imaging changes in the receptors and binding sites associated with neurodegenerative diseases. Finally, since radiopharmaceuticals are classified as radioactive drugs, they are regulated by the FDA.1 This is often unfamiliar to chemists. For those who are interested in knowing more about the FDA regulation of radiopharmaceuticals, references are included.7
Radioactive drug manufacturing is very different from that of commonly used ethical drugs. There is essentially no time limitation on fulfilling the demands for cGMP preparation and quality control in the manufacture of most drugs. In contrast, the short-lived isotopes in radiopharmaceuticals put a severe time constraint on the process. All the manufacturing and quality control needs to be completed in a relatively short time (minutes), before the isotope decays away. The preparation of radiopharmaceuticals for commercial distribution usually starts with a large amount of radioactivity (for an 18F tracer it is often > 50 GBq, sufficient for 20 to 30 patient doses) and the reaction requires significant shielding. The synthesis proceeds inside a hot cell (lead shielded radiation containment chamber) by automated remote control processes in a clean and sterile environment. In addition, because most radiopharmaceuticals are delivered by an iv injection, the release criteria are usually more stringent than that applied to oral drugs.1,7
2) Examples of isotopes for PET and SPECT imaging
Many gamma-emitting isotopes are available for SPECT imaging. Two radionuclides, 99mTc and 123I, are commonly utilized in SPECT imaging because they emit medium gamma energy (100 to 300 KeV) and have suitable physical half-lives. (Table 1). Radioiodination is readily achieved by either oxidative iodination or by an iododestannylation reaction. One of the major drawbacks of using 123I labeled compounds for routine imaging is the difficulty in obtaining the isotope. It is produced by cyclotrons or by accelerators and is therefore not readily available, requiring overnight shipment from production sites. On the contrary, 99mTc, a radionuclide commonly used in nuclear medicine clinics, has several well-known advantages. These include favourable physical characteristics, T1/2 = 6 hr, with a gamma ray emission of 140 KeV and wide availability through the use of 99Mo/99mTc generators and simple kit formulations providing easy preparation procedures for various 99mTc based radiopharmaceuticals. The use of small molecules labeled with technetium has been reviewed previously.8, 9
Table 1.
Radionuclides for SPECT imaging agents
| T1/2 | Gamma Ray | |
|---|---|---|
| 99mTc | 6 hr | 140 KeV |
| 123I | 13 hr | 159 KeV |
PET (positron emission tomography) imaging is a common diagnostic radiology tool. PET images are taken after an iv injection of a small amount of radioactive tracer. When a positron is emitted, it collides with a neighbouring electron and is annihilated, producing two 511 KeV gamma rays roughly 180 degrees apart. It is based on this physical principal and the positron coincident signals, that the point source of the gamma rays can be deduced by using a coincident circuit for detectors 180 degrees apart.5
There are two positron-emitting isotopes that are commonly used for brain imaging with PET, 11C (T1/2 = 20 min) and 18F (T1/2 = 110 min)(Table 2).5 The first known metabolic tracer for brain imaging was 11C glucose. Carbon can be easily substituted in natural metabolites or drugs in order to study metabolism and map in vivo drug action. However, due to its limited physical half-life, it is challenging to utilize 11C radiopharmaceuticals. Currently, only major medical centres and clinics with teams of skilled scientists can make a sufficient amount of 11C radioprobes for patient studies.
Table 2.
Radionuclides for PET imaging agents
| T1/2 | Gamma Ray | |
|---|---|---|
| 11C | 20 min | 511 KeV |
| 18F | 110 min | 511 KeV |
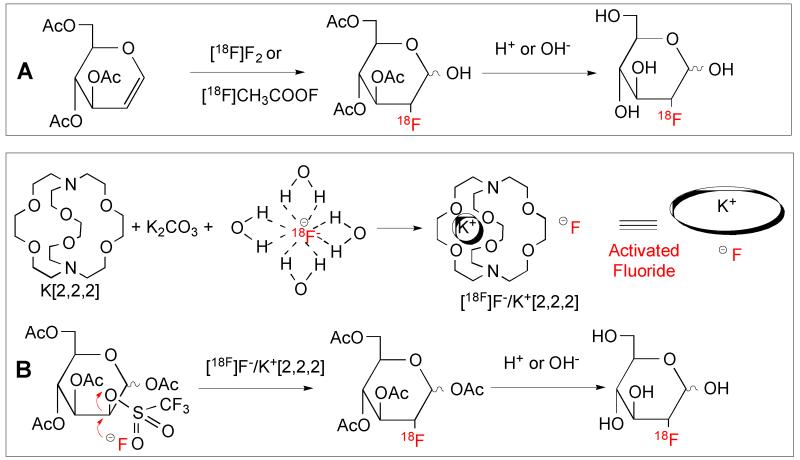
The half-life of 18F (110 min) is 5.5 times longer than that of 11C (T1/2 = 20 min, β+). Logistically, 18F tracers are more practical because they can be prepared by an off-site cyclotron and manufacturing and distribution can be centralized. Originally, [18F]FDG was prepared with fluorine gas ([18F]F2))Fig. 1A). The process is rather complicated and the labeling yield was not high. The reaction has been improved by a direct nucleophilic reaction using a fluoride ion, potassium carbonate and K[2.2.2]kryptofix, a cryptand, to activate the fluoride ion (See Fig. 1A and 1B).10, 11 This procedure can produce > 100 GBq of [18F]FDG per batch (sufficient for 20 to 30 patient doses) and is the method of choice for commercial production now.
Figure 1.
Radiolabeling reactions for production of [18F]FDG (2-fluoro-2-deoxy-D-glucose). A. The original method for preparation of [18F]FDG employed fluorine gas ([18F]F2. B. The improved synthesis of [18F]FDG uses [18F]fluoride ion, potassium carbonate and K[2.2.2], a cryptand, to activate the fluoride ion. This nucleophilic reaction leads to a cleaner product and is a more efficient and higher yield reaction.12
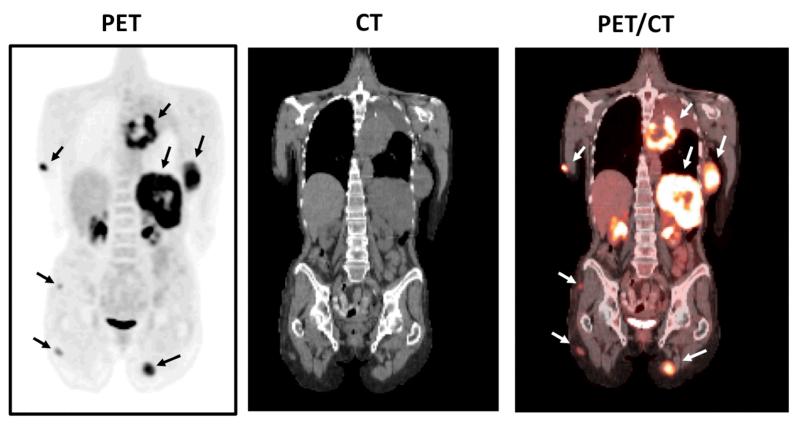
The only PET imaging agent that has widespread clinical application thus far is [18F]FDG (2-[18F]fluoro-2-deoxy-D-glucose), which highlights metabolism by indicating glucose usage. It has been extensively used as an imaging agent for studying glucose metabolism in the brain as well as in tumors.13 FDG-PET provides clinicians with a diagram of cancer cell activity within the body. PET-CT also gives a fusion image of glucose metabolism and anatomical location in one imaging session. This method is now the standard of care in oncology for diagnosis, staging, and monitoring therapy.14
3) In vivo imaging of Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disease which begins as a movement disorder and typically progresses to dementia. Early symptoms include tremor and dyskinesia. The pathology of PD is characterized by a selective loss of dopamine neurons in the basal ganglia and substantia nigra, which damage the nigrostriatal neural transmission system. Treatment of PD with L-DOPA and dopamine agonists has significantly improved the mobility of PD patients. Subthalamic nuclei stimulation (STN) has also been successful in slowing or reversing the deterioration of PD. Normally, diagnosis of PD is based on medical history as well as physical and neurological examination. However, the movement disorder may also be caused by factors other than nigrostriatal neuronal degeneration (idiopathic vs non-idiopathic). 15, 16
Since it is not always possible to make a final diagnosis based on neurological symptoms, it would be useful for clinicians to be able to monitor the status of monoamine neuron integrity with either PET or SPECT imaging.16 Imaging agents for Parkinson’s Disease could assist physicians in three critical ways: 1. differential diagnosis of idiopathic vs non-idiopathic PD; 2. early diagnosis of high risk patients (genetic predisposition or environmental exposure to toxin); 3. monitoring drug treatment.
A useful PET or SPECT imaging agent for targeting a specific process or binding site of dopamine neurons should: 1. display high affinity (Ki < 10 nM); 2. exhibit high selectivity to the specific binding site (have at least one-hundred-fold lower affinity to other receptors); 3. be labeled with 18F or 123I quickly (radiosynthesis < 90 minutes) and efficiently (final radiochemical yield > 30%); 4. penetrate the intact blood-brain barrier and show a high uptake in the brain (> 1.0 %dose/g of striatum at 2 min after an iv injection in rats); 5. display a high selective uptake and retention in the striatum (striatum/cerebellum (= target/non-target) ratio > 3); 6. show high in vivo stability; 7. have kinetic properties which can be modelled to obtain quantitative information by in vivo imaging. All of these requirements need to be met in order to effectively and quantitatively map the neuronal function in the living human brain.16, 17
1. Imaging aromatic amino acid decarboxylase, AADC with 6-Fluoro-[18F]DOPA (FDOPA)
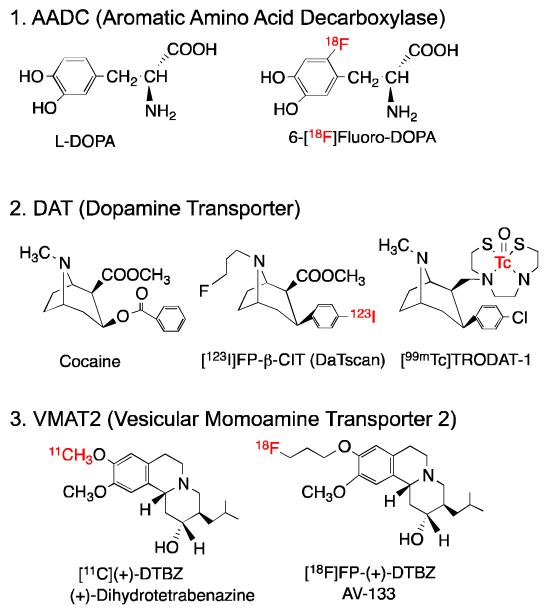
There are three types of PET and SPECT imaging agents currently available for testing nigrostriatal neuronal integrity and function (Fig. 3, 4 and 5).16, 18 The specific mechanisms of tracer uptake and retention are associated with different aspects of dopamine neuronal function. The enzymatic activity of aromatic amino acid decarboxylase, AADC, is responsible for in situ synthesis of dopamine as a neurotransmitter in the presynaptic neuron. 6-fluoro-[18F]FDOPA (FDOPA) is an analogue of L-DOPA and a false substrate for this enzyme, which converts FDOPA to the corresponding F-dopamine. The trapping of [18F]FDOPA in dopamine neurons reflects the in situ synthesis of endogenous dopamine within the synapse. [18F]FDOPA was the first PET imaging agent for PD. It was developed in the 1980s and remains commonly used. [18F]6-FDOPA provides a glimpse of neuronal activity, through the in situ enzymatic synthesis of dopamine catalysed by AADC (or the lack thereof) (Fig 5A).
Figure 3.
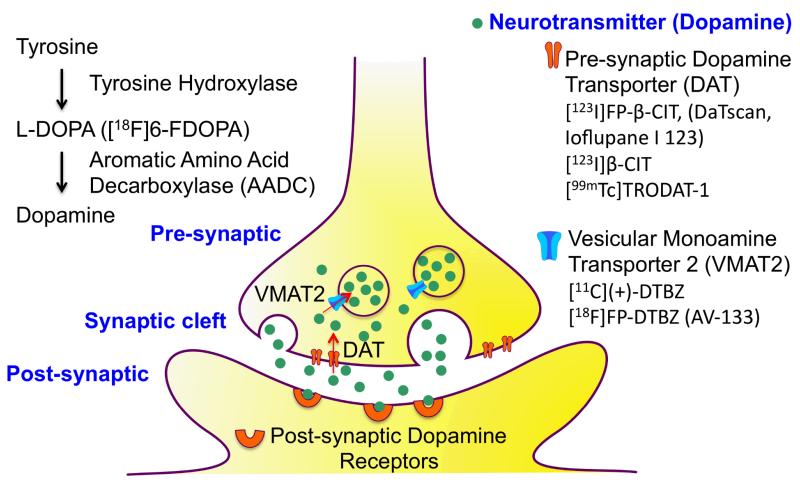
A schematic drawing of a dopamine neuron shows that the dopamine is synthesized in the pre-synaptic neuron starting from tyrosine to L-DOPA and then aromatic amino acid decarboxylase (AADC) catalyses the formation of dopamine. The SPECT/PET imaging agents for mapping dopamine neurons include: 1. 6-fluoro-[18F]DOPA (FDOPA), an analogue of L-DOPA which is a false substrate for AADC. 2. [123I]FP-β-CIT and [99mTc]TRODAT-1, which target the pre-synaptic dopamine transporter (DAT). 3. 9-fluoropropyl-(+)-DTBZ (AV-133), which binds to the vesicular monoamine transporter 2 (VMAT2).
Figure 4.
Chemical structures of three types of imaging agents for studying nigrastriatal neuron degeneration in Parkinson’s disease (PD): 1. Enzymatic activity (aromatic amino acid decarboxylase, AADC); 2. Dopamine transporters (DAT); 3. Vesicular monoamine transporters (VMAT2).
Figure 5.
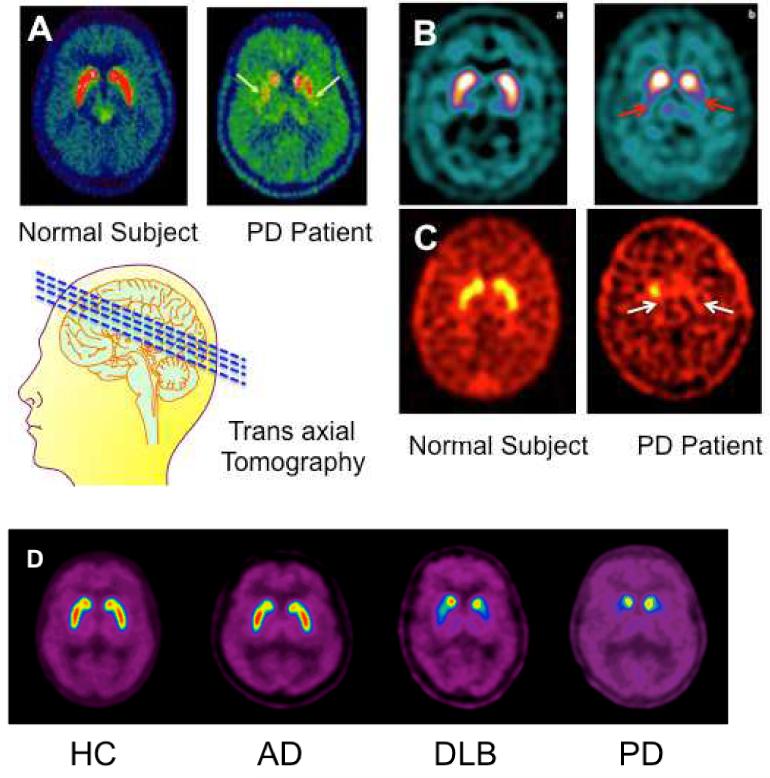
Transaxial images of normal control and PD patients using different imaging agents: 5A: [18F]6-FDOPA/PET imaging of differential activity of a presynaptic enzyme, aromatic amino acid decarboxylase (AADC) 18 (Reprinted with permission from Elsevier); 5B: [123I]F-β-CIT (DatScan)/SPECT (GE Health; http://www3.gehealthcare.com/en/Products/Categories/Nuclear_Imaging_Agents/DaTscan); 5C: [99mTc]TRODAT-1/SPECT (Authors thank Dr. David Mozley for providing the images); 5D: [18F]FP-(+)-DTBZ (AV-133). 28, 29 (Reprinted with permission from JAMA)
Yet, the images obtained with [18F]6-FDOPA do not provide a clear picture of the disease state. First of all, the enzyme, AADC, is localized not only in nigrastriatal dopamine neurons, but also in other types of brain cells. In the brains of PD patients, the AADC enzyme is often up-regulated to compensate for the low output of dopamine. Therefore, the [18F]6-FDOPA/PET images may underestimate the degree of neuronal loss due to compensatory changes.19 In addition, the tracer’s peripheral metabolites, O-methylated derivatives, are also taken up in the brain, contributing to higher background noise. Despite these drawbacks, [18F]6-FDOPA/PET imaging is still being used routinely in many clinics for diagnosis and monitoring of PD patients.
2. Imaging dopamine transporters (DAT)
Dopamine transporter (DAT) ligands are useful for evaluating changes in presynaptic DAT sites in vivo and in vitro. Patients with movement disorders, such as Parkinson’s disease (PD), have a selective loss of dopamine neurons in the basal ganglia and substantia nigra. The dopamine transporter (DAT) is located at the presynaptic neuron that removes dopamine from the synaptic cleft back into the neuron. The dopamine inside the neuron is then re-packaged into vesicles by the vesicular monoamine transporter 2 (VMAT2) (Fig. 3). When the dopamine neurons are functioning normally, there will be plenty of DAT available for binding to the high affinity DAT tracers. A high accumulation of radiolabeled tracers in the basal ganglia and substantia nigra of the brain, as measured by PET or SPECT, suggests that the neurons are intact. However, a reduction in the uptake of a DAT tracer usually suggests a loss of dopamine neurons.
Many of the agents for imaging DAT belong to a group of tropane derivatives, which have a backbone structure similar to that of cocaine and compete to the same dopamine transporter binding sites (Fig. 4). The design criteria for this group of DAT imaging agents are based on their structural similarity to cocaine and high affinity to the DAT binding sites in the brain. In the past two decades, an abundance of DAT imaging agents have been developed, most of which are tropane (or cocaine) derivatives. Although tropanes show excellent binding affinity to DAT (Ki < 10 nM), many of them also have varying degrees of binding affinity to serotonin and norepinephrine transporters. However, because the total number of DAT binding sites in the basal ganglia and substantia nigra area is much higher than the number of serotonin or norepinephrine transporters, interference from other binding sites is not significant.
[123I]FP-β-CIT, a tropane-based SPECT imaging agent, has been shown to be useful for imaging DAT, showing a strong correlation between a decrease in localization of the probe in the putamen area and PD symptoms.16,20 It is highly sensitive for detecting changes in dopamine neuronal degeneration. Currently, [123I]FP-β-CIT is the agent of choice for mapping the nigrastriatal dopamine neurotransmission system. In Europe and in the US, [123I]FP-β-CIT (DaTscan) is now commercially available for routine clinical use for diagnosing and monitoring the treatment of PD patients 20 (Fig. 5B).
However, as we noted previously, 123I labeled compounds are difficult to use routinely in the clinics due to difficulty in the logistics of supply and distribution. Since 123I is a cyclotron produced isotope, which costs more than a similar 99mTc labeled agent, it would be desirable to use a 99mTc probe for routine clinical studies. After a decade of continuing research in Tc-99m chemistry, [99mTc]TRODAT-1 (Fig. 5C) is the only agent which has shown reasonable success in imaging DAT for the diagnosis of PD.21 Currently, [99mTc]TRODAT is only available commercially in Taiwan. GE Healthcare Inc. has stopped developing it in favour of [123I]FP-β-CIT (DatScan).
3. Imaging vesicular monoamine transporter 2 (VMAT2)
Radiolabeled [11C](+)-dihydrotetrabenazine ([11C](+)-DTBZ) was the first probe to be successfully tested in humans for PET imaging of VMAT2 sites in the basal ganglia area of the brain. Reserpine is a natural product, which inhibits the vesicular monoamine uptake within the synapse resulting in a depletion of monoamines in the CNS. The depletion of monoamines in the brain by reserpine is long-lasting. An analog of reserpine, tetrabenazine (TBZ, Nitoman®), displays a similar biological profile. TBZ was developed by Roche in the 1950s as an antipsychotic agent for schizophrenia to depress the monoamine levels in the brain.22 In contrast to reserpine, TBZ produces a short-lived depletion of all three monoamines, and it does not appear to irreversibly damage the transporter. Structure-activity relationship of TBZ was reported, it is likely that the R group at the C3 position of DTBZ may have sufficient bulk tolerance providing this ligand with high binding affinities. It was not until the 1980s that the mechanisms of action of TBZ were found to be associated with the binding of VMAT2 in the brain.23 It showed a high and specific binding to VMAT (Kd = 3 nM). The binding capacity in different regions of the brain was consistent with regional distribution of monoamine neurons. In unilaterally lesioned mice, it was demonstrated that [3H]DTBZ showed a diminished labeling on the side of striatum where dopamine neurons had been reduced by injection of varying amounts of 6-OH-dopamine in the nigra region. 24 It was thought that [3H]DTBZ would be useful as a biomarker for evaluation of monoaminergic innervation and would be of importance in studying patients with Parkinson’s disease as well as other patients with psychiatric diseases.
Indeed, a PET imaging agent, 11C-DTBZ, has been used in the diagnosis of Parkinson’s disease for the past decade.25,26 However, because 11C has a very short half-life (t1/2 = 20 min), 18F labeled analogues of DTBZ with a longer half-life (t1/2 =110 min) would be more practical for widespread use. 9-fluoropropyl-(+)-DTBZ (FP-(+)-DTBZ, AV-133), has shown good binding affinity towards VMAT2 in the rat brain, with the most intense labeling observed in monoaminergic neuron regions (caudate putamen, nucleus accumbens, substania nigra, dorsal raphe and locus coerules).27 Preliminary AV-133/PET imaging studies in humans detected reduced binding in the posterior putamen, anterior putamen and caudate nucleus of PD patients. A reduction of the VMAT2 binding in the caudate nuclei, as shown by AV-133, significantly correlated with the clinical severity of PD28. Since this tracer is capable of producing very high contrast images of the basal ganglia area, AV-133/PET imaging may be able to identify individuals with disorders characterized by degeneration of dopaminergic nigrostriatal afferents before they experience clinical symptoms. In addition, the agent may be helpful in differential diagnosis for dementia with Lewis body (DLB). The DLB patients are often presented as a dementia patient with co-morbidity of PD. The differentiation between PD and DLB patient is clinically useful, because the information not only confirms the diagnosis but also changes the course of treatment. This agent is currently under phase III clinical trial by the Eli Lilly and Company.
5A. [18F]6-FDOPA/PET imaging showed a dramatic reduction of enzyme activity in the PD patient as compared to the normal control18.
5B and 5C showed the images of normal control vs PD patient. In the normal subject, a high accumulation of the imaging agent was observed in caudate and putamen, where dopamine transporters are concentrated. The Parkinsonian patient displayed dramatically decreased uptake in this region of the brain (see arrows).
5D. AV-133/PET imaging of normal control (HC), Alzheimer’s disease (AD), dementia with lewis body (DLB) and Parkinson’s disease (PD). The images in this series of patients suggest that DLB and PD patients showed significant reduction of VMAT2 in the basal ganglia region of the brain, while the healthy subject and the AD patient showed no change. These images suggest that this method may be very useful not only for the diagnosis of PD but also for differentiating AD from DLB patients, as both types of patients may exhibit dementia.28 The DLB patients are often presented as a dementia patient with co-morbidity of PD.
4) In vivo imaging of human amyloid plaques in Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease affecting millions of people. As our population ages, there will be an increasing number of AD patients. Clinical symptoms of AD include cognitive decline, irreversible memory loss, disorientation, language impairment, etc. Major neuropathological observations from postmortem AD brains include the presence of plaques (β-amyloid, Aβ) aggregates, and neurofibrillary tangles (hyper phosphorylated Tau) (Fig. 6). Familial AD has been reported to have mutations in the genes encoding the β-amyloid precursor proteins (APP), presenilin 1 (PS1) and presenilin 2 (PS2). The exact mechanisms by which these mutations lead to the development of AD are not fully understood. However, there is a clear link between the development of AD and the observation of Aβ and Tau aggregates.30
Figure 6.
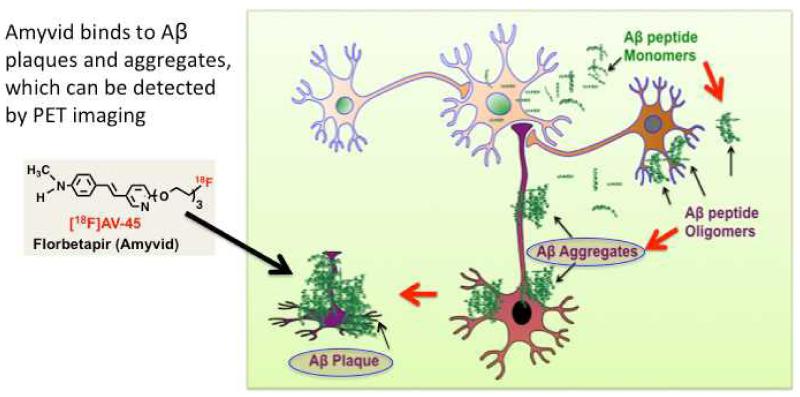
A schematic drawing for the β-amyloid hypothesis is shown. It is suggested that Aβ aggregates participate in the pathogenesis of AD. The Aβ peptides, produced by neurons and other brain cells, transform β-sheet structures into a variety of toxic assemblies, neurofibrillary tangles and neuritic plaques. The Aβ oligomers as well as aggregates damage the neurons. The presence of Aβ plaques in the brain may suggest a risk factor of developing Alzheimer’s disease (modified from31). (Reprinted with permission from ACS Med Chem Lett 2012. 3: p. 265-267; Copyright 2012 American Chemical Society.)
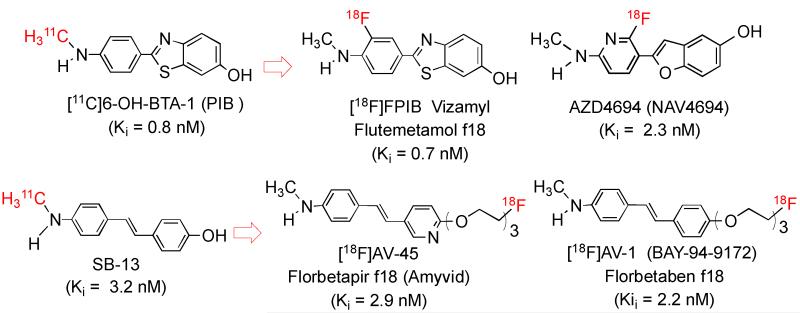
The development of Aβ plaque-specific imaging agents has been extensively reported and reviewed.32,33 The most well characterized PET imaging agent for Aβ plaques in the brain is [11C]6-OH-BTA-1, referred to as PIB or “Pittsburgh compound B,” which is a small, neutral and lipophilic 2-phenyl-benzothiazole derivative. It displays good binding affinity and excellent in vivo kinetics binding to Aβ plaques in the brain. In the past ten years, [11C]PIB has been used in PET imaging studies of thousands of AD patients.32,34 The success in using [11C]PIB as a tracer for imaging Aβ plaques in the brain of suspected AD patients has inspired researchers to further refine Aβ tracers. Many different core structures have been prepared and tested.32,33 Among the hundreds of potential ligands that show good binding to Aβ plaques, four 18F labeled tracers have been successfully tested in humans. The FDA has approved Amyvid and Vizamyl for human Aβ PET imaging. Two other tracers, Florbetaben (AV-1) and NAV4694 will probably be approved in the near future. (Figure 7). FPIB (flutemetamol, Vizamyl) is an analog of PIB (Fig. 7) which has excellent attributes for in vivo imaging of Aβ plaques35. The FDA approved flutemetamol f18 (Vizamyl) for routine clinical use on October 25th, 2013. Another Aβ plaque imaging agent, structurally similar to FPIB, AZD4694 (recently renamed NAV4694) has also shown excellent in vitro binding and promising in vivo kinetics in human studies.36
Figure 7.
Chemical structures of the benzothiazole series of AD imaging agents: [11C]PIB was the first agent to be tested in humans. [18F]FPIB was developed later, using the same core structure.32 The stilbene and styrylpyridine series of agents were discovered by modifying SB-13. Three units of polyethylene glycol chains were attached to the core, which reduce the lipophilicity without reducing brain penetration. This is a useful approach for adjusting the lipophilicity and providing a suitable position for fluorine substitution37.
Among the stilbene and styrylpyridine series of AD imaging agents, SB-13 was the first to be tested clinically in humans. Later, AV-1 (florbetaben) and AV-45 (florbetapir) were developed for commercialization. The chemical structures of benzothiazoles (PIB analogues) and stilbenes (SB-13 analogues) are similar in that both have a highly conjugated aromatic ring system37. They are also relatively planar molecules, an important attribute for insertion between the β-sheets of Aβ aggregates. Another common feature is an electron donating group (N-methylamine or hydroxyl group) at each end of the molecule38. They appear to compete for similar binding sites on the Aβ aggregates with very high binding affinity (Ki < 10 nM) (Fig. 7). The rigid stilbene and styrylpyridine structures have been utilized at the core of many specific imaging agents for Aβ plaques, including BAY 94-9172 (florbetaben f18, AV-1, currently being developed by Piramal Biotechnology) and Amyvid (florbetapir f18, AV-45, developed by Avid/Eli Lilly).37 Both agents display an excellent combination of in vitro and in vivo properties for Aβ plaque labeling.
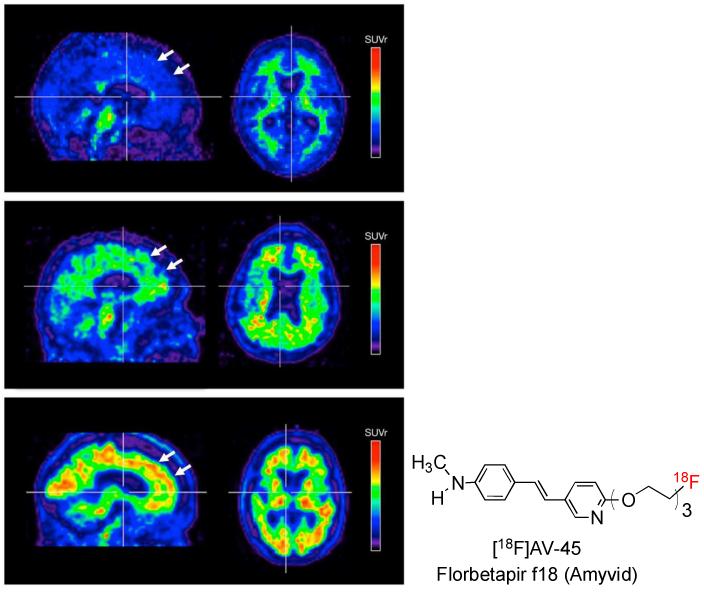
AV-45 binds with high affinity and selectivity to β-amyloid plaques composed of β-sheet aggregates of the Aβ peptide. AV-45 binding reflects the total β-amyloid deposition (plaque burden) in living human brains (Fig. 8). Results of a phase I clinical study of AV-45 in normal and AD subjects found clear differences in the kinetics of uptake in the brains of healthy control subjects and AD patients. The phase III clinical study (a PET-post-mortem study for AV-45 (florbetapir f18) concluded that florbetapir-PET was correlated with the presence and density of β-amyloid.39 Additional studies are required to understand the appropriate utilization of florbetapir-PET in the clinical diagnosis of Alzheimer’s disease or in predicting the progression to dementia. Ruling out the presence of Aβ plaques would naturally be the first useful clinical diagnosis. On April 6th, 2012, FDA approved Amivid (florbetapir f18) for commercialization. Amyvid is now being used for patient selection prior to enrollment in drug trials specifically designed to reduce the piling up of β-amyloid plaques in the brain. However, its clinical adoption is limited because Medicare has not yet approved payment for the scan. A serious discussion of how to best use this imaging method in connection with patient care is on-going.40
Figure 8.
Results of a phase III clinical study suggest that the Amyvid/PET images are correlated with β-amyloid deposition seen in post-mortem studies. PET images are shown for three subjects (where red is highest uptake, blue the lowest): (top row) a normal subject with no β-amyloid plaques; (middle row) moderate loading of β-amyloid plaques associated with an early stage of Alzheimer’s Disease); and (bottom row) high β-amyloid plaques (white arrows) indicative of a late stage of Alzheimer’s Disease.14, 39 (Reprinted with permission from JAMA)
Researchers have placed a lot of effort into developing a cure for Alzheimer’s Disease. Most treatments have focused on stopping the formation of, or reversing, the Aβ aggregates. The two major approaches of the anti-plaque treatments have been the inhibition of gamma secretase (reducing Aβ production) and immunotherapy (removing Aβ from the brain).41 Despite the investment of years of effort and millions of dollars, no “magic bullet” has been found to alleviate the symptoms of Alzheimer’s Disease.42 As pharmaceutical companies experiment with various therapies, the use of amyloid plaque imaging agents, particularly Amyvid, could facilitate drug trials by selecting the patient population which may be benefited by the anti-Aβ plaque treatment. Advances in early diagnosis of β-amyloid formation and aggregation may also contribute to the development of inhibitors for Aβ aggregates as a treatment of AD.43
Although much has been already accomplished in the field of neuroimaging, there are many targets of neurodegenerative diseases, which remain to be explored. For PD imaging, it would be useful to develop an agent to image the presence of α-synuclein in the brain.44 In Alzheimer’s Disease imaging, researchers are already working to develop a probe for the second hallmark of AD, Tau aggregates.45 Imaging agents for these targets would be extremely useful to compliment the tracers currently being used for diagnosis of AD and PD.
5). Summary
Developing PET/SPECT imaging agents for neurodegenerative disease is a multidisciplinary research field. The examples of PD and AD imaging agents described in this paper suggest that chemists can continue to play important roles in identifying specific targets, synthesizing target-specific tracers for screening and ultimately testing them by in vitro and in vivo assays.
It is important to note that developing a tracer from lab bench to clinical trial and ultimate FDA approval takes a significant amount of resources far above and beyond the scope of a single research project. It is estimated that 50 to 100 million US dollars will be needed to develop one diagnostic compound. Basic research, supported by NIH grants and other government resources, plays a critical role in the initial proof of concept of new drug candidates, which might convince commercial companies to invest major resources, which are needed to obtain final FDA drug registration.
Key learning points.
What are radiopharmaceuticals and why are they needed?
Provide examples of isotopes for imaging and their application in PET and SPECT
SPECT/PET imaging agents are useful in diagnosis and monitoring disease progression
Provide specific examples on In vivo imaging of dopamine transporters for the diagnosis of Parkinson’s disease
Provide specific examples on in vivo imaging agents for mapping human β-amyloid plaques in Alzheimer’s disease
Figure 2.
FDG PET-CT fusion images of a cancer patient (melanoma) can provide both anatomical and functional information as well as pinpoint the cancerous tissue, which shows higher glucose metabolism (arrows). (Images provided by David Mankoff M.D. Ph.D. University of Pennsylvania).14 (Reprinted with permission from The American Association for the Advancement of Science)
Acknowledgments
Authors thank Dr. David Mankoff for supplying the FDG PET-CT images and Dr. Carita Huang for editorial assistance. This work was supported in part by grants from Stand-Up 2 Cancer (SU2C), PA Health Department, and National Institutes of Health (CA-164490; AG-022559; DK-081342).
References
- 1.Norenberg JP, Schwarz S, VanBrocklin H. J. Nucl. Med. 2011;52:16N. [PubMed] [Google Scholar]
- 2.Weissleder R. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 3.Hargreaves RJ, Rabiner EA. Neurobiol. Dis. 2013 doi: 10.1016/j.nbd.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Rahmim A, Zaidi H. Nucl. Med. Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 5.Cherry S, Sorenson J, Phelps M. Physics in Nuclear Medicine. W.B. Saunders; New York: 2003. [Google Scholar]
- 6.Kim E, Howes OD, Kapur S. Dialogues Clin. Neurosci. 2013;15:315–328. doi: 10.31887/DCNS.2013.15.3/ekim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz S, Norenberg J, Berridge M, Dragotakes S, Hung J, Link J, Mason NS, Mattmuller S, Nickel RA, Packard A, Paolino J, Petry N, Ponto J, Quinton TM, Seifert KL, Swanson D, Weiner RE, Zigler S. J. Nucl. Med. 2013;54:472–475. doi: 10.2967/jnumed.112.115089. [DOI] [PubMed] [Google Scholar]
- 8.Jurisson SS, Lydon JD. Chemistry Reviews. 1999;99:2205–2218. doi: 10.1021/cr980435t. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee SR, Maresca KP, Francesconi L, Valliant J, Babich JW, Zubieta J, Alberto R, Pak JK, van Staveren D, Mundwiler S, Benny P, Stichelberger A, Waibel R, Dumas C, Schubiger PA, Schibli R. Nucl. Med. Biol. 2005;32:1–20. doi: 10.1016/j.nucmedbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Fowler JS, MacGregor RR, Wolf AP, Farrell AA, Karlstrom KI, Ruth TJ. J. Nucl. Med. 1981;22:376–380. [PubMed] [Google Scholar]
- 11.Hamacher K, Coenen HH, Stocklin G. J. Nucl. Med. 1986;27:235–238. [PubMed] [Google Scholar]
- 12.M. H. S, Ametamey M, Schubiger PA. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 13.Gambhir SS. J. Nucl. Med. 2008;49(Suppl 2):1S–4S. doi: 10.2967/jnumed.108.053751. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Ploessl K, Kung HF. Science. 2013;342:429–430. doi: 10.1126/science.1245011. [DOI] [PubMed] [Google Scholar]
- 15.Seibyl JP. Semin. Nucl. Med. 2008;38:274–286. doi: 10.1053/j.semnuclmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Seibyl J, Russell D, Jennings D, Marek K. Semin. Nucl. Med. 2012;42:406–414. doi: 10.1053/j.semnuclmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Villalobos A, Beck EM, Bocan T, Chappie TA, Chen L, Grimwood S, Heck SD, Helal CJ, Hou X, Humphrey JM, Lu J, Skaddan MB, McCarthy TJ, Verhoest PR, Wager TT, Zasadny K. J. Med. Chem. 2013;56:4568–4579. doi: 10.1021/jm400312y. [DOI] [PubMed] [Google Scholar]
- 18.Brooks DJ, Pavese N. Prog. Neurobiol. 2011;95:614–628. doi: 10.1016/j.pneurobio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Frey KA. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:715–717. doi: 10.1007/s00259-002-0815-4. [DOI] [PubMed] [Google Scholar]
- 20.Seifert KD, Wiener JI. Am J Neurodegener Dis. 2013;2:29–34. [PMC free article] [PubMed] [Google Scholar]
- 21.Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, Stern MB, Doty RL, Mozley PD, Wintering N, Duda JE, Weintraub D, Moberg PJ. Neurology. 2005;64:1716–1720. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- 22.Saner A, Pletscher A. J. Pharmacol. Exp. Ther. 1977;203:556–563. [PubMed] [Google Scholar]
- 23.Scherman D, Raisman R, Ploska A, Agid Y. J. Neurochem. 1988;50:1131–1136. doi: 10.1111/j.1471-4159.1988.tb10583.x. [DOI] [PubMed] [Google Scholar]
- 24.Kilbourn M, Frey K. Eur. J. Pharmacol. 1996;307:227–232. doi: 10.1016/0014-2999(96)00252-x. [DOI] [PubMed] [Google Scholar]
- 25.Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. J. Cereb. Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 26.Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Ann. Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- 27.Kung M, Hou C, Goswami R, Ponde D, Kilbourn M, Kung H. Nucl. Med. Biol. 2007;34:239–246. doi: 10.1016/j.nucmedbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura N, Villemagne V, Drago J, Pejoska S, Dhamija R, Mulligan R, Ellis J, Ackermann U, O’Keefe G, Jones G, Kung H, Pontecorvo M, Skovronsky D, Rowe C. J. Nucl. Med. 2010;51:223–228. doi: 10.2967/jnumed.109.070094. [DOI] [PubMed] [Google Scholar]
- 29.Villemagne VL, Okamura N, Pejoska S, Drago J, Mulligan RS, Chetelat G, Ackermann U, O’Keefe G, Jones G, Gong S, Tochon-Danguy H, Kung HF, Masters CL, Skovronsky DM, Rowe CC. Arch. Neurol. 2011;68:905–912. doi: 10.1001/archneurol.2011.142. [DOI] [PubMed] [Google Scholar]
- 30.Querfurth H, LaFerla F. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 31.Kung H. ACS Med Chem Lett. 2012;3:265–267. doi: 10.1021/ml300058m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis CA, Mason NS, Lopresti BJ, Klunk WE. Semin. Nucl. Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng F, Goodman MM. Curr. Top. Med. Chem. 2013;13:909–919. doi: 10.2174/1568026611313080004. [DOI] [PubMed] [Google Scholar]
- 34.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang G.-f., Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 35.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks D. Ann. Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 36.Rowe CC, Pejoska S, Mulligan RS, Jones G, Chan JG, Svensson S, Cselenyi Z, Masters CL, Villemagne VL. J. Nucl. Med. 2013;54:880–886. doi: 10.2967/jnumed.112.114785. [DOI] [PubMed] [Google Scholar]
- 37.Kung H, Choi S, Qu W, Zhang W, Skovronsky D. J. Med. Chem. 2009;53:933–941. doi: 10.1021/jm901039z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kung H, Lee C, Zhuang Z, Kung M, Hou C, Plossl K. J. Am. Chem. Soc. 2001;123:12740–12741. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- 39.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, Reiman EM, Sabbagh MN, Sadowsky CH, Fleisher AS, Murali Doraiswamy P, Carpenter AP, Clark CM, Joshi AD, Lu M, Grundman M, Mintun MA, Pontecorvo MJ, Skovronsky DM. Alzheimers Dement. 2013;9:S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callaway E. Nature. 2012;489:13–14. doi: 10.1038/489013a. [DOI] [PubMed] [Google Scholar]
- 42.Tayeb HO, Murray ED, Price BH, Tarazi FI. Expert Opin. Biol. Ther. 2013;13:1075–1084. doi: 10.1517/14712598.2013.789856. [DOI] [PubMed] [Google Scholar]
- 43.Nordberg A, Rinne J, Kadir A, Langstrom B. Nature Reviews Neurolgy. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- 44.Neal KL, Shakerdge NB, Hou SS, Klunk WE, Mathis CA, Nesterov EE, Swager TM, McLean PJ, Bacskai BJ. Mol. Imaging Biol. 2013;15:585–595. doi: 10.1007/s11307-013-0634-y. [DOI] [PubMed] [Google Scholar]
- 45.Villemagne VL, Furumoto S, Fodero-Tavoletti M, Harada R, Mulligan RS, Kudo Y, Masters CL, Yanai K, Rowe CC, Okamura N. Future Neurology. 2012;7:409–421. [Google Scholar]