Abstract
Purpose/Objective(s)
Mature data on tumor control and survival are presented from a randomized trial of the addition of a brachytherapy boost to long-course neoadjuvant chemoradiation (CRT) for locally advanced rectal cancer.
Methods and Materials
Between March 2005 and November 2008, 248 patients withT3-4N0-2M0 rectal cancer were prospectively randomized to either long-course preoperative CRT (50.4Gy in 28 fractions, peroral UFT and L-leucovorin) alone or the same CRT schedule plus a brachytherapy boost (10Gy in 2 fractions). Primary trial endpoint was pathological complete response (pCR) at time of surgery; secondary endpoints included overall survival (OS), progression-free survival (PFS) and freedom from locoregional failure.
Results
Results for the primary endpoint have previously been reported. This analysis presents survival data for the 224 patients in the Danish part of the trial. 221 patients (111 control arm, 110 brachytherapy boost arm) had data available for analysis, with a median follow-up of 5.4 years. Despite a significant increase in tumor response at the time of surgery, no differences in 5-year OS (70.6% vs 63.6%, HR=1.24, p=0.34) and PFS (63.9% vs 52.0%, HR=1.22, p=0.32) were observed. Freedom from locoregional failure at 5 years were 93.9% and 85.7% (HR=2.60, 1.00–6.73, p=0.06) in the standard and in the brachytherapy arm, respectively. There was no difference in the prevalence of stoma. Explorative analysis based on stratification for tumor regression grade and resection margin status indicated the presence of response migration.
Conclusions
Despite increased pathological tumor regression at the time of surgery, we observed no benefit on late outcome. Improved tumor regression does not necessarily lead to a relevant clinical benefit when the neoadjuvant treatment is followed by high-quality surgery.
Introduction
Preoperative chemoradiotherapy (CRT) reduces the incidence of local recurrences after surgery for locally advanced rectal cancer; although an effect on overall survival has not been demonstrated (1, 2). Various approaches have been investigated in order to optimize the preoperative treatment: these include chemotherapy intensification, such as addition of second drug (3–6); biologically targeted treatment (7); chemotherapy timing and prolongation (8, 9); and timing of surgery relative to neoadjuvant treatment (10, 11).
While a small number of randomized studies have compared short-course radiotherapy (RT) with long-course CRT (12, 13) and one has examined the combined effect of chemotherapy and RT intensification (3), the investigation of RT dose-escalation alone in the long-course setting has been very limited – the one exception being the Lyon R96-02 trial (14), which used contact therapy as a boost modality in order to increase the rate of sphincter-saving operations. This is thus an evident area for research. However, increasing the radiation dose carries an enhanced risk of early and late radiation-induced morbidity, and the choice of radiation technique for dose escalation should be carefully considered. We conducted a prospective, randomized trial to examine the effect of tumor dose-escalation by addition of a high-dose rate brachytherapy boost to a standard long-course preoperative CRT regimen for locally advanced rectal cancer. The primary study endpoint was tumor response at the time of surgery and the results for this endpoint have previously been reported (15): No significant improvement in pathological complete response (pCR) was demonstrated (18% in both arms), but an increase in near-complete tumor response was seen, most markedly in T3 tumors. A subsequent analysis, pooling patients from this and a previous phase II trial (16) established a clear relationship between tumor dose and regression at the time of surgery (17). We here present the secondary 5-year efficacy endpoints for the patients treated in the Danish part of the phase III trial (90% of all patients).
Methods and materials
Details of patients and methods have been reported in the primary trial publication (15). In short, patients were included if they had histopathologically confirmed adenocarcinoma of the rectum, which were less than 10 cm from the anal verge, and had a circumferential resection margin as estimated on magnetic resonance imaging (MRI) of less than 5 mm, according to national guidelines. They were classified as T3-4N0-2M0 tumors based on MRI of the pelvis, rectal ultrasound, chest and abdominal computed tomography (CT) scans, and rectoscopy. The trial protocol was approved by the regional research ethics committee (protocol ID VF20050006), and all patients provided written informed consent. The trial included patients from one Danish and one Canadian center, but patients in the Canadian part of the trial were only available for assessment of the primary trial endpoint
Randomization
Patients were randomized to either external CRT alone (Arm A) or external CRT plus an additional 10Gy high-dose-rate brachytherapy boost (Arm B) in a 1:1 ratio. Randomization was based on a pre-defined, computer-generated list concealed to treating physicians. Allocation to treatment arm was not blinded to patients and treating physicians, but allocation was blinded to the pathologist scoring for tumor response.
Treatment
Preoperative CRT was delivered in a single institution. CT-based conformal treatment plans using 6 and/or 18 MV photon beams were used for external RT. The planning target volume (PTV) consisted of the tumor and mesorectum (lower border 3 cm below the tumor), presacral lymph nodes, superior rectal, median, and internal iliac lymph nodes, and obturator lymph nodes, with a 1cm isotropic margin to account for internal motion and setup uncertainties. The PTV was prescribed 50.4Gy to the ICRU reference point in 28 fractions (5 fractions weekly) external beam RT in both trial arms.
Chemotherapy consisted of daily peroral Tegafur-Uracil (3×100 mg/m2) and peroral L-leucovorin (3×7.5 mg) given on days when external RT or brachytherapy were delivered.
The high-dose-rate brachytherapy boost was delivered in 2 fractions on week 4 and 6 of the treatment course, using a rigid, single-channel endorectal applicator (18). Dose was prescribed 1.0cm from the applicator surface, and was planned such as to provide a uniform dose distribution along the central axis. Patients in Arm B who could not comply with brachytherapy were prescribed an external boost of 6Gy or 12Gy, delivered with 2Gy per fraction, according to protocol.
Total mesorectal excision (TME) surgery was performed 8 weeks after end of CRT. Adjuvant chemotherapy was delivered at the discretion of the treating physician.
Follow-up
Patients were seen at surgical departments for on-protocol follow-up every 6 months for the first 3 years, and once a year in the 4th and 5th years. Any further follow-up was at the discretion of the treating surgeon. Additionally, all electronic patient records were reviewed at the time of final analysis (June 2013) in order to verify all reported events and to identify disease relapse and death not otherwise reported. For this final review, full electronic records were available for the majority of patients. For the remainders, national reimbursement codes were checked and supplementary information requested where necessary.
Endpoints
Tumor regression at the time of operation was evaluated by central histopathological review of the surgical specimens, and classified according to Mandard’s tumor regression grade (TRG1–5) (19, 20). Analysis of early endpoints, including tumor regression and resection margin status (R0-2), was reported in the primary publication (15). For this report, we classified patients according to major (TRG1–2; only rare, microscopic tumor cells left) and minor (TRG3–5) regression at the time of surgery, and the rate of R0 resections.
Late endpoints are defined in Table 1. Overall survival (OS), progression-free survival (PFS), freedom from distant metastasis and locoregional control were secondary trial endpoints. Additionally, freedom from secondary cancer was analyzed, and prevalence of stoma at last clinical follow-up was examined amongst patients surviving at least 2 years and patients surviving at least 5 years. Time was calculated from date of randomization to date of event or end of follow-up, except for the estimation of local recurrence rates (details below).
Table 1.
Definitions of late endpoints
| Endpoint | Event | Censoring |
|---|---|---|
| Overall survival | Death from any cause | At time of last follow-up |
| Progression-free survival | First clinical detection of disease progression (preferably by biopsy), i.e. distant metastasis, locoregional recurrence, or determination of inoperability; or death from any cause | At time of last follow-up |
| Locoregional control | Clinically proven (preferably by biopsy) local failure or disease recurrence in pelvic lymph nodes included in the original external beam treatment volume, irrespective of any distant failures (21) | At death or at the time of last follow-up |
| Freedom from distant metastases | Clinically proven (preferably by biopsy) distant progression, irrespective of any locoregional failures | At death or at the time of last follow-up |
| Secondary cancer | Any new primary malignant tumor (distinct from the rectal adenocarcinoma) | At death or at the time of last follow-up |
Statistical analysis
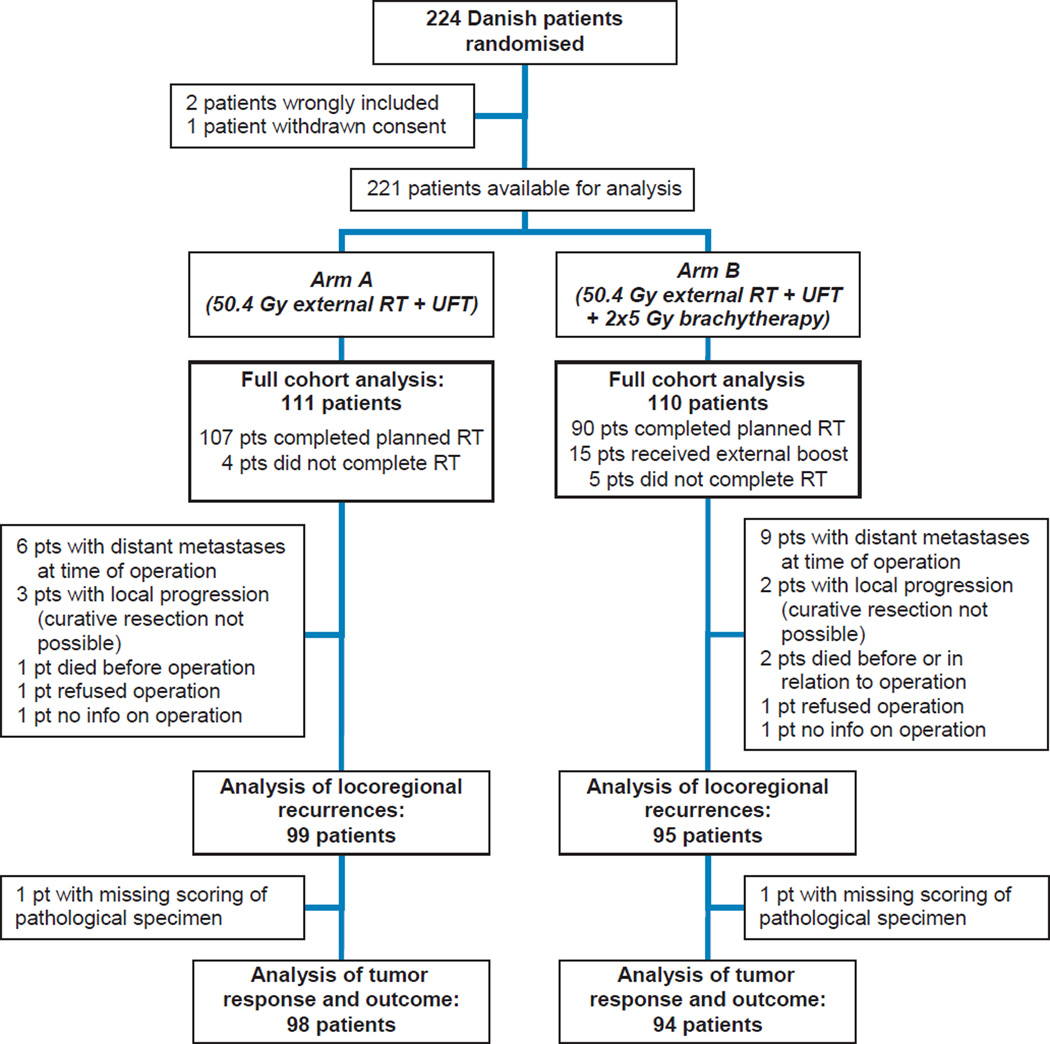
The analyses of OS, PFS and time to distant progression were conducted in the full cohort, which included all randomized patients in the Danish part of the study, excluding patients wrongly included (one metastatic cancer, one patient with prior cancer) or with withdrawn consent (one patient). Primary analysis of locoregional control was limited to patients who underwent curative resection; thus patients who had metastases detected or who were deemed not resectable at the time of surgery were excluded. Time to locoregional failure was calculated from date of surgery. In a secondary sensitivity analysis, locoregional control was estimated in the full cohort (21), with locally unresectable patients counted as failed on the date of surgery, and with time counted from date of inclusion.
Time-to-event endpoints were compared between trial arms using the Kaplan-Meier estimator and the Mantel-Cox log-rank, with stratification if indicated, as the test of statistical significant differences. Hazard ratios (HR) were calculated using Mantel-Haenzel type estimates. The robustness of the results for locoregional failure was checked by estimation of the cumulative incidence of locoregional failure in a competing risk framework (22), with death (from any cause) as a competing risk. For this analysis, trial arms were compared using Gray’s test (23).
Multivariate analysis was conducted using Cox proportional hazards regression. Factors to be included in the multivariate analysis were selected a priori as potential pre-treatment prognostic factors: Clinical tumor (cT) and lymph node (cN, N0 vs N+) categories, gender and age. The proportional hazards assumption of the Cox regression model was examined by testing for correlation between Schoenfeld residuals and time.
Finally, an unplanned, exploratory analysis was conducted: The effect of treatment arm on outcome when stratifying for pathological response grade and resection margin status (R status), was analyzed for patients who underwent curative resection (i.e. were deemed resectable and had no known metastases at the time of surgery). Time was calculated from the date of surgery.
Median follow-up was calculated using the Kaplan-Meier estimator of potential follow-up (24) – i.e. the Kaplan-Meier estimator with the status indicator reversed. Comparisons of distributions of factors between treatment arms were performed using Fisher’s exact test for dichotomized categorical variables and the Mann-Whitney U test for continuous variables. All significance tests were two-sided, and p-values of ≤0.05 were considered significant. Statistical analysis was conducted in R (25).
Results
The analyzed cohort comprised 221 patients; the difference between this cohort and the subset undergoing radical operation (194 patients) is outlined in Figure 1. Table 2 contains the patient characteristics as well as a summary of pathological tumor response and resection margin status. Pre-treatment characteristics and the number of patients receiving adjuvant chemotherapy were well-balanced between the arms. The brachytherapy arm had significantly more patients with major tumor response (TRG1–2), while there was no significant difference in the number of R0 resections.
Figure 1. Flow chart of patient inclusion, treatment and analysis.
RT: Radiotherapy. UFT: Tegafur-uracil
Table 2.
Patient characteristics, tumor response, adjuvant chemotherapy and follow-up
| Arm A 50.4Gy external CRT |
rm B 50.4Gy external CRT + 2×5Gy brachytherapy |
p-value | |
|---|---|---|---|
| Gender | |||
| Female / Male | 43 (39) / 68 (61) | 38 (35) / 72 (65) | 0.58 |
| Age | |||
| Median (range) [years] | 62 (35–77) | 64 (38–78) | 0.09 |
| Disease category | |||
| - T3 / T4 | 90 (81) / 21 (19) | 93 (85) / 17 (15) | 0.59 |
| - N0 / N1–2 / ND | 10 (9) / 101 (91) / 0 (0) | 13 (12) / 95 (86) / 2 (2) | 0.51 |
| Pathological tumor response grade | |||
| TRG1–2 / TRG3–4 / ND | 27 (27) / 71 (72) / 1 (1) | 39 (41) / 55 (58) / 1 (1) | 0.05 |
| Resection margin statusa | |||
| R0 / R1 / ND | 89 (90) / 10 (10) / 0 (0) | 90 (95) / 4 (4) / 1 (1) | 0.17 |
| Adjuvant chemotherapy | |||
| Yes / No / ND | 12 (12) / 83 (84) / 4 (4) | 15 (16) / 78 (82) / 2 (2) | 0.54 |
| Follow-up | |||
| Median [years] | 5.4 | 5.4 | 0.74 |
No patient had an R2 resection. TRG: Tumor response grade according to Mandard19 (no patients had a TRG5 response). CRT: Chemoradiotherapy. Numbers for tumor response grade, margin status, and adjuvant chemotherapy are for patients undergoing curative resection. Numbers in brackets indicate percentages.
First patient was included in March 2005, last patient in November 2008. Clinical outcome data were updated in June 2013 (3.5 years after last enrolment), whereafter the database was locked. The median follow-up was 5.4 years, with no difference between the trial arms. A total of 75 patients (36 Arm A / 39 Arm B) experienced disease relapse, while 79 patients (36 Arm A / 43 Arm B) died. First site of relapse was locoregional for 10 patients (0 Arm A / 10 Arm B), distant for 57 patients (31 Arm A / 26 Arm B), and simultaneously local and distant for 8 patients (5 Arm A / 3 Arm B). For the patients who underwent curative resection, 17 locoregional failures (5 Arm A / 12 Arm B) were observed in total, including local failures after diagnosis of distant metastases.
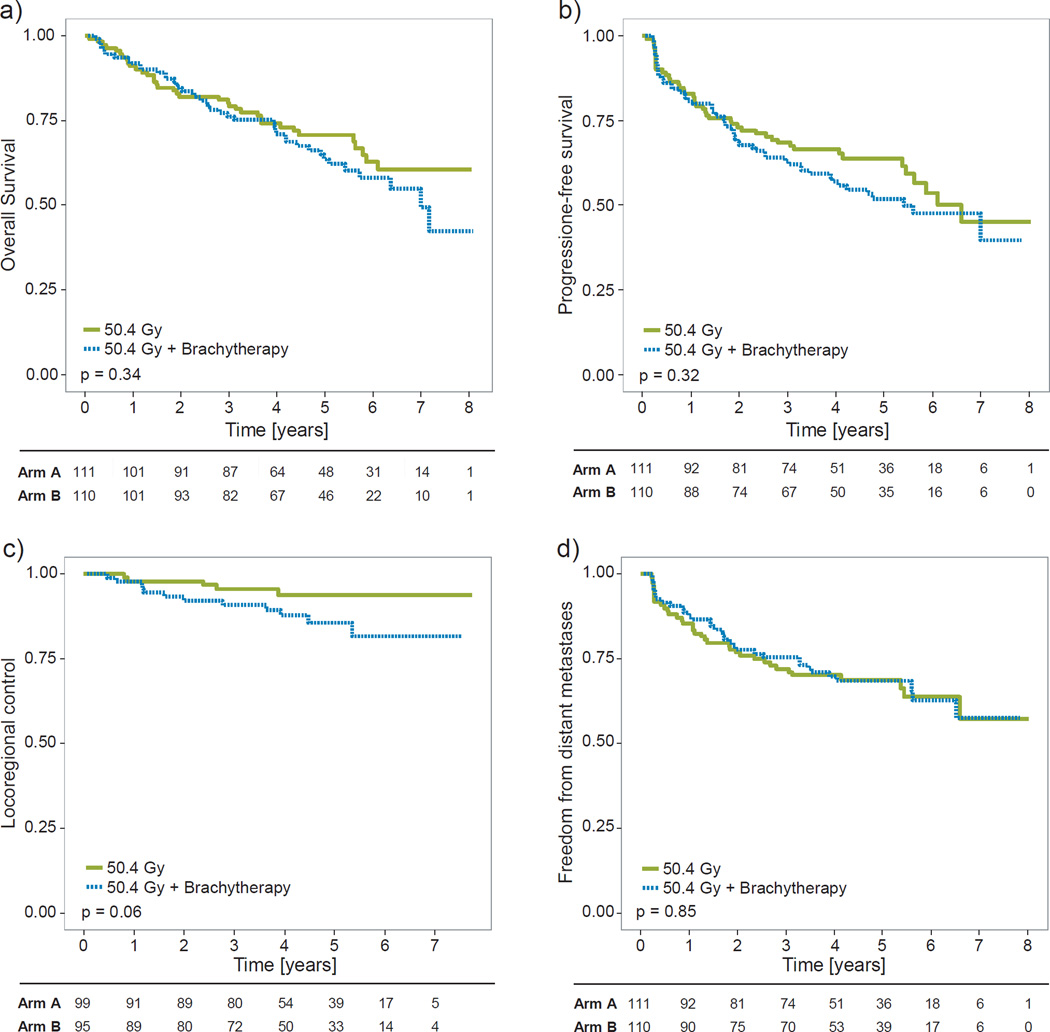
The analyses of OS, PFS, and freedom from distant metastasis demonstrated no significant differences between the trial arms, as illustrated in Figures 2a,b,d. 2- and 5-year OS were 82.0% and 70.6% for Arm A, and 84.5% and 63.6%, for Arm B, respectively (HR=1.24, 95% CI: 0.80–1.93, p=0.34). 2- and 5-year PFS were 73.0% and 63.9% (Arm A), and 68.7% and 52.0% (Arm B) (HR=1.22, 0.82–1.82, p=0.32). 2- and 5-year freedom from distant metastases were 76.8% and 68.7% (Arm A), and 77.6% and 68.4% (Arm B) (HR=1.05, 0.60–1.52, p=0.85).
Figure 2. Comparison of clinical outcomes in the two trial arms.
a) Overall survival. b) Progression-free survival. c) Locoregional control. d) Freedom from distant metastases. Green: Arm A, patients treated with external chemoradiotherapy (CRT) alone. Blue: Arm B, patients treated with CRT plus 10Gy brachytherapy boost. Numbers under the plots are patients at risk. Significance levels (p-values) are from unstratified log-rank analyses. For locoregional control (c), stratifying for gender resulted in p=0.03 (see main text for details).
Multivariate analysis showed no significant effects of cT category, cN category, gender or age for either of the three endpoints (although cT4 tumors tended towards worse PFS, HR=1.50, p=0.10), and correcting for these factors did not change the effect of treatment allocation.
The unstratified log-rank test showed a trend towards worse locoregional control in the brachytherapy boost arm (Figure 2c), with 2- and 5 year locoregional control at 97.9% and 93.9% in Arm A, and 92.2% and 85.7% in Arm B (HR=2.60, 1.00–6.73, p=0.06). Cox multivariate regression demonstrated a significant effect of both treatment arm (p=0.04) and gender (p=0.003) on the risk of locoregional recurrence, but the test for proportional hazards showed a borderline (p=0.08) significant deviation from this assumption for both factors. Non-parametric log-rank test with stratification by gender confirmed the significantly higher rate of locoregional recurrences in the brachytherapy arm (HR=3.01, 1.16–7.84, p=0.03).
The sensitivity analysis of locoregional control in the full cohort showed very similar results in terms of HR and significance levels (HR=2.52, p=0.03, stratified analysis), but with a slightly lower estimated control (91% in Arm A, 83.2% in Arm B, at 5 years). Estimation of the cumulative incidence provided 5-year incidences of locoregional failure of 5.5% and 12.6%, respectively, with significance levels (p=0.03 for stratified test) consistent with the results of Kaplan-Meier analyses.
18 secondary cancers were detected during the follow-up period, with a 5-year risk of secondary cancer at 7.8% in Arm A and 8.9% in Arm B (p=0.61). The most frequent secondary cancer sites were colon (5) and lung (4). The prevalence of stoma was 66.1% amongst 2-year survivors, and 64.5% amongst 5-year survivors, with no difference between trial arms (p=1.00).
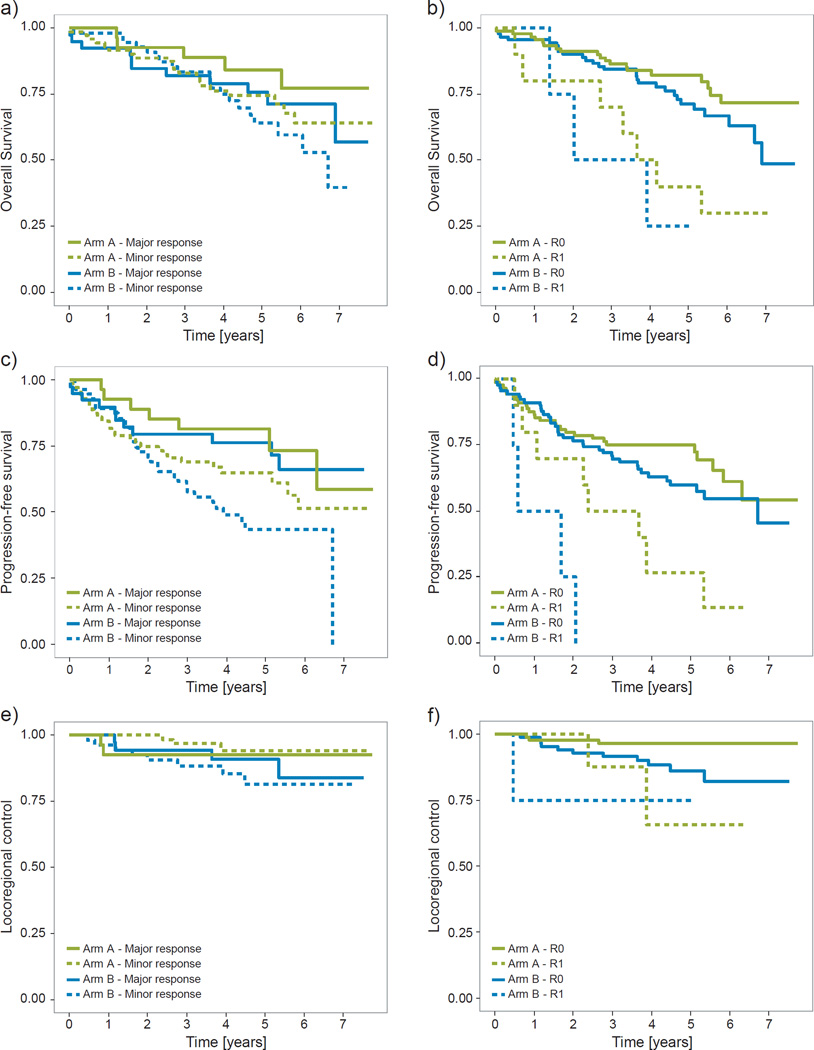
Figure 3 shows the results of the exploratory analysis of the effects on outcome of stratifying for tumor response and resection margins. Expectedly, patients with less tumor response at the time of surgery or with R1 resections did worse on almost all outcome measures (26–29). However, within response groups, the brachytherapy arm did notably worse, especially with respect to PFS. These differences between treatment arms, when stratifying for response grade or R-status, were only borderline significant, though, and only for some combinations of endpoints and classifications: for margin status (p=0.03) and tumor response (p=0.07) for locoregional control; and for margin status (p=0.08) when considering PFS.
Figure 3. Clinical outcome stratified for treatment arm and scoring of the surgical specimen.
a&b) Overall survival. c&d) Progression-free survival. e&f) Locoregional control. Green: Arm A, patients treated with external chemoradiotherapy (CRT) alone. Blue: Arm B, patients treated with CRT plus 10Gy brachytherapy boost. a,c,e) Solid lines indicate patients with major response at the time of surgery, tumor regression grade (TRG) 1–2; dashed lines indicate patients with minor response, TRG3–4. b,d,f) Solid lines indicate patients with negative surgery resection margins, R0; dashed lines indicate patients with positive resection margins, R1.
Discussion
This trial investigated dose-escalation of preoperative radiotherapy for locally advanced rectal cancer by addition of a brachytherapy boost. A dose response for tumor regression at the time of surgery has previously been demonstrated (17). The question addressed in this report was whether dose intensification also improves local control, OS and PFS. We were unable to show any improvement in long-term clinical outcome in the experimental arm, neither with respect to OS nor for disease control. Intriguingly, the boost cohort showed a trend towards an increase in locoregional recurrences, completely at odds with conventional wisdom surrounding radiation dose-response of malignant tumors.
The discussion of relevant endpoints for RT and CRT trials in rectal cancer has been ongoing for years (30). Early surrogate endpoints are desirable for obvious reasons, with results on OS and DFS from randomized trials often not available until almost a decade after opening a trial. A number of recent trials of long-course chemoradiotherapy optimization (including this one) have had tumor regression at the time of surgery as the primary endpoint (7, 9, 31, 32). However, while tumor regression is a prognostic marker (26–28) this does not imply that it is a valid surrogate of a relevant clinical endpoint in rectal cancer (28, 30, 33). Although there was no difference between the treatment arms for the primary trial endpoint (pCR), we found a significant increase in the rate of major response in the brachytherapy boost arm (41% vs 28%). This did not, however, translate into an effect on OS, PFS or locoregional control. These results indicate that major tumor response cannot be used as surrogate endpoint for loco-regional control in a radiation dose-intensification setting. Figure 3 demonstrates a possible mechanism behind some of these findings: Within each tumor response category, the brachytherapy arm performs notably worse. This may suggest the presence of response migration (34). The best (i.e. least biologically aggressive) tumors from the “minor response” category may be converted to “major response” by the brachytherapy boost. However, if the boost only alters the early response, then this response migration does not lead to a gain in long-term outcome. Consequently, both the “major response” and the “minor response” categories present with apparently worse prognosis for overall outcome – the first due to the addition of tumors with worse prognosis, and the second due the to selective removal of the least aggressive tumors (Figure 3), in parallel to the phenomenon of stage migration. The same holds true for positive / negative resection margins: Fewer patients presented with R1 resections in the brachytherapy arm (although not significantly so), but those who did had apparent worse outcome.
There is limited existing, clinical evidence for the benefit of dose-escalation in preoperative treatment of rectal cancer. A meta-analysis of randomized trials of preoperative RT (without chemotherapy) suggested a minimum dose of ~30Gy / 15 fractions to effectively prevent local recurrences (35), but the analysis was limited by the potential bias resulting from higher radiation doses being prescribed in later time periods, where RT and surgery techniques were more developed, and no trials considered doses above ~45Gy. The only prior randomized trial to isolate the effect of dose-escalation is the Lyon R 96-02 randomized trial (14, 36), which compared external beam RT (39Gy in 13 fractions) with external beam plus contact x-ray therapy (85Gy in 3 fractions) and found improved sphincter preservation with higher doses. However, the Lyon trial predominantly included less advanced tumors, with no cT4 and 25% T2 tumors, as well as the exclusion of tumors with >2/3 circumferential involvement. This, as well as the completely different boost technique, makes comparison with the current trial difficult.
The rationale for this phase III trial came from a phase II trial of high-dose CRT for locally advanced T3 rectal cancer (16), where updated follow-up data show a locoregional recurrence rate at 5 years of 4.5%, and a 5-year OS of 65% (unpublished data). As such, the current report not only demonstrates the problems with the use of non-validated, early (surrogate) endpoints, but also provides yet another example of a promising phase II trial which failed to deliver in phase III. A possible adverse effect of several fractions of high-dose-rate brachytherapy, perhaps combined with the surgical intervention, could maybe explain this failing, but any discussion of such mechanisms is purely speculative. A study of germline genetic markers in the trial patients is ongoing, and may in due course provide additional insight. Clearly, one should also bear in mind that local recurrence was not a primary trial endpoint, and there is a risk that the results represent chance findings due to multiple testing. Specifically, the relatively high 14% locoregional failure rate in the brachytherapy arm could be a ‘fluke’ due to the limited number of patients studied. The difference between the arms for locoregional control was only just significant, depending on the factors controlled for – on the other hand, the HR in favor of “no boost” stayed consistent independently of the cohort studied (patients undergoing surgery or full cohort) and whether competing risks were accounted for or not. Hence the likelihood that brachytherapy improves the locoregional control, in a setting with locally advanced disease and modern surgery practices (recurrence rate in control arm ~6%), is probably very low – based on the observed, unstratified rates, the probability of a “true” improvement in 5-year locoregional control of >3% is less than 1%. The use of brachytherapy as a boost treatment for locally advanced rectal cancer patients in the preoperative setting thus cannot be recommended based on the findings of this trial. The role of brachytherapy in other patient groups – such as patients with early rectal cancer treated with definite CRT or patients treated with palliative intent – remains open.
Unfortunately, the trial suffers from missing data on late toxicity. A planned collection of physician-scored toxicity in surgical departments during follow-up visits was not successful. Less than 40% of the patients had toxicity scored at all, and only ~25% had (one or more) evaluations conducted beyond the first year of followup. This made any attempt at reporting late radiation-induced side-effects meaningless. The crude prevalence of stoma use for 2- and 5-year survivors does indicate that sphincter preservation is not severely worsened by the brachytherapy boost – but also that the increased rate of tumor response did not, ultimately, improve the rate of sphincter saving. A quality-of-life study, including patient reported outcome, amongst disease-free survivors is currently being planned.
Conclusion
In conclusion, despite a statistically significant improvement in pathological tumor response from the addition of a brachytherapy boost to preoperative CRT for locally advanced rectal cancer, a corresponding improvement in OS, PFS or locoregional control was not seen. Thus, an increase in pathological tumor regression at the time of surgery did not indicate a benefit on late clinical outcome. TRG score and R0 resection are well-established prognostic factors, but their utility as surrogate endpoints for long-term patient benefit remains to be defined.
Summary.
This report presents mature data on tumor control and survival from a randomized trial of the addition of a brachytherapy boost to long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Despite improvement in tumor response at the time of surgery, no benefit on late outcome was observed. Tumor regression score and R0 resection are prognostic factors, but do not seem to represent useful surrogate endpoints.
Acknowledgements
ALA is supported by CIRRO - The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research, and by the Region of Southern Denmark. IRV is supported by the Global Excellence in Health program of the Capital Region of Denmark. SMB is supported in part by grant no. P30 CA 134274-04 from the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
The authors have no conflicts of interest to disclose.
References
- 1.Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 2.Gérard J-P, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 3.Gérard J-P, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 4.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J. Clin. Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 5.Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: First results of the PETACC-6 randomized phase III trial. J. Clin. Oncol. 2013 ASCO Annu. Meet. Proc. 2013;31:3531. (abstract). [Google Scholar]
- 6.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant therapy for rectal cancer: Mature results from NSABP protocol R-04. J. Clin. Oncol. 2014;32 abstr 390. [Google Scholar]
- 7.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J. Clin. Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 8.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J. Clin. Oncol. 2006;24:668–674. doi: 10.1200/JCO.2005.04.4875. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imagingdefined, l. J. Clin. Oncol. 2010;28:859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 10.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J. Clin. Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br. J. Surg. 2010;97:580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 12.Bujko K, Nowacki MP, Nasierowska-Guttmejer a, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 13.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 14.Gerard J-P, Chapet O, Nemoz C, et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the Lyon R96-02 randomized trial. J. Clin. Oncol. 2004;22:2404–2409. doi: 10.1200/JCO.2004.08.170. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen A, Ploen J, Vuong T, et al. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:949–954. doi: 10.1016/j.ijrobp.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Jakobsen A, Mortensen JP, Bisgaard C, et al. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int. J. Radiat. Oncol. Biol. Phys. 2006;64:461–465. doi: 10.1016/j.ijrobp.2005.07.969. [DOI] [PubMed] [Google Scholar]
- 17.Appelt AL, Pløen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:74–80. doi: 10.1016/j.ijrobp.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen JW, Jakobsen A. The importance of applicator design for intraluminal brachytherapy of rectal cancer. Med. Phys. 2006;33:3220–3224. doi: 10.1118/1.2207143. [DOI] [PubMed] [Google Scholar]
- 19.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Bouzourene H, Bosman FT, Seelentag W, et al. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–1130. [PubMed] [Google Scholar]
- 21.Marsh PJ, James RD, Schofield PF. Definition of local recurrence after surgery for rectal carcinoma. Br. J. Surg. 1995;82:465–468. doi: 10.1002/bjs.1800820412. [DOI] [PubMed] [Google Scholar]
- 22.Chappell R. Competing risk analyses: how are they different and why should you care? Clin. Cancer Res. 2012;18:2127–2129. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988;16:1141–1154. [Google Scholar]
- 24.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing. R Found. Stat. Comput. 2013 [Google Scholar]
- 26.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 27.Dhadda AS, Dickinson P, Zaitoun AM, et al. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur. J. Cancer. 2011;47:1138–1145. doi: 10.1016/j.ejca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y-C, Hsieh C-C, Chuang J-P. Prognostic Significance of Partial Tumor Regression After Preoperative Chemoradiotherapy for Rectal Cancer: A Meta-analysis. Dis. Colon Rectum. 2013;56:1093–1101. doi: 10.1097/DCR.0b013e318298e36b. [DOI] [PubMed] [Google Scholar]
- 29.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008;26:303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 30.Glynne-Jones R, Mawdsley S, Pearce T, et al. Alternative clinical end points in rectal cancer--are we getting closer? Ann. Oncol. 2006;17:1239–1248. doi: 10.1093/annonc/mdl173. [DOI] [PubMed] [Google Scholar]
- 31.Valentini V, Coco C, Minsky BD, et al. Randomized, multicenter, phase IIb study of preoperative chemoradiotherapy in T3 mid-distal rectal cancer: raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5-fluorouracil + radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:403–412. doi: 10.1016/j.ijrobp.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:1367–1375. doi: 10.1016/j.ijrobp.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnetain F, Bosset JF, Gerard JP, et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3–4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: surrogacy in question? Eur. J. Cancer. 2012;48:1781–1790. doi: 10.1016/j.ejca.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Brincker H. Problems associated with comparisons of response-defined subsets of patients in randomized trials. Treatment-related bias and response migration. Acta Oncol. 1987;26:425–428. doi: 10.3109/02841868709113711. [DOI] [PubMed] [Google Scholar]
- 35.Viani GA, Stefano EJ, Soares FV, et al. Evaluation of biologic effective dose and schedule of fractionation for preoperative radiotherapy for rectal cancer: meta-analyses and meta-regression. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:985–991. doi: 10.1016/j.ijrobp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Ortholan C, Romestaing P, Chapet O, et al. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e165–e171. doi: 10.1016/j.ijrobp.2011.12.002. [DOI] [PubMed] [Google Scholar]