Abstract
Endocrine erythropoietin (Epo), which is synthesized in the kidney or liver of adult mammals, controls erythrocyte production and is regulated by the stress-responsive transcription factor Hypoxia Inducible Factor 2 (HIF-2). We previously reported that the lysine acetyltransferase Cbp is required for HIF-2α acetylation and efficient HIF-2 dependent Epo induction during hypoxia. We now show these processes require acetate-dependent acetyl CoA synthetase 2 (Acss2). In Hep3B hepatoma cells and in Epo-generating organs of hypoxic or acutely anemic mice, acetate levels increase and Acss2 is required for HIF-2α acetylation, Cbp/HIF-2α complex formation and recruitment to the Epo enhancer, and efficient Epo induction. In acutely anemic mice, acetate supplementation augments stress erythropoiesis in an Acss2-dependent manner. In acquired and genetic chronic anemia mouse models, acetate supplementation also increases Epo expression and resting hematocrits. Thus, a mammalian stress-responsive acetate switch controls HIF-2 signaling and Epo induction during pathophysiological states marked by tissue hypoxia.
Keywords: Epo, HIF-2, Cbp, p300, Acss2
Introduction
Oxygen deprivation induced by hypoxia activates stress responses, one of which for vertebrates is increased red blood cell production or erythropoiesis 1,2. The discovery of erythropoietin (Epo), an endocrine growth factor synthesized in the adult kidney and liver, began with elegant physiological experiments demonstrating an erythropoiesis-promoting blood-borne factor, continued with the characterization and purification of the Epo protein, and culminated with cloning of the Epo gene 3. Mapping of the hypoxia-responsive enhancer region in the Epo gene identified a molecular handle subsequently used to purify Hypoxia Inducible Factor 1 (HIF-1) 4, a sentinel regulator of the mammalian hypoxia response.
HIF-1 is a heterodimeric stress-responsive transcription factor comprised of an oxygen-sensitive alpha subunit and an oxygen-insensitive beta subunit. During hypoxia, HIF-1α is regulated in a novel manner 5 through inhibition of two oxygen-dependent modifiers, prolyl hydroxylases (PHDs) that decrease HIF-1α stability and an asparagine hydroxylase (Factor Inhibiting HIF-1, FIH1) that prevents coactivator/HIF-1α interactions 6. Mining of the human genome led to identification of HIF-2α, the second vertebrate HIF family member 7-9. Although structurally similar to HIF-1α, genetic ablation studies in cells and mice indicated that HIF-2α, rather than HIF-1α, is the key regulator of endocrine Epo 10-15.
HIF-2α undergoes the same oxygen-dependent mechanisms of activation as HIF-1α, yet HIF-2α protein increases under hypoxia are relatively modest in some cell lines. This suggests that post-translational mechanisms other than protein stability play an important role in HIF-2 signaling. Indeed, acetylation and deacetylation of HIF-2α are required for efficient HIF-2 signaling during hypoxia in Hep3B cells and in mice. HIF-2α acetylation is mediated by the lysine acetyltransferase and coactivator Creb binding protein (Cbp), which also provides a molecular platform for stable Cbp/HIF-2α complex formation, whereas Sirtuin 1 (Sirt1) is a specific deacetylase for acetylated HIF-2α 10,16. Notably, the lysine acetyltransferase and coactivator p300, which is closely related to Cbp, does not acetylate HIF-2α and is a minor component of HIF-2 signaling in Hep3B cells and liver. However, p300 also complexes with HIF-2α during hypoxia 16. We were interested in further exploring these differences in signaling function for Cbp and p300 in HIF-2 signal transduction.
Results
Acss2 controls HIF-2 signaling in hypoxic cells
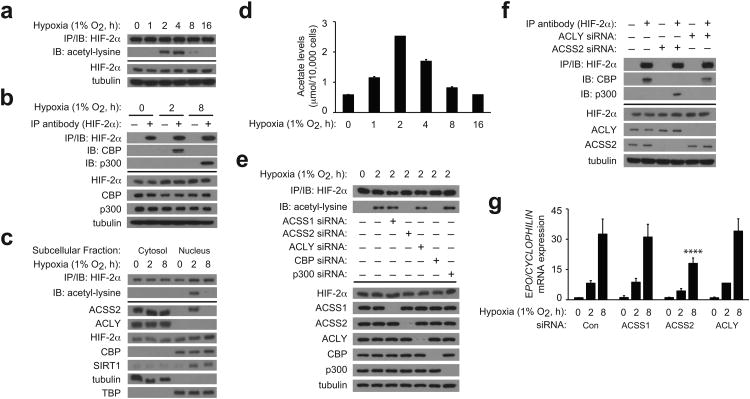
We first defined the temporal aspect of HIF-2α acetylation and Cbp/HIF-2α complex formation in Hep3B cells. HIF-2α acetylation and Cbp/HIF-2α complexes peak early (2 h) whereas p300/HIF-2α complexes form late (8 h) following hypoxia onset (Fig. 1a,b). Because Cbp/HIF-2α complex formation requires both Cbp enzymatic (i.e. acetyltransferase activity) and HIF-2α substrate determinants (i.e. lysine residues) of the acetylation reaction 16, we reasoned that availability of acetyl CoA, the other required substrate in the Cbp acetylation reaction, might control dynamic Cbp/HIF-2 interactions during hypoxia.
Figure 1. Acss2 controls HIF-2 signaling in hypoxic cells.
(a) Time course of endogenous HIF-2α acetylation during hypoxia following immunoprecipitation (IP) of HIF-2α from whole cell extracts and detection of acetylated lysines by immunoblotting (IB). (b) Endogenous CBP/HIF-2α or p300/HIF-2α complex formation after hypoxia exposure. (c) Subcellular localization of ACSS2, ACLY, HIF-2α, CBP, and SIRT1 following hypoxia exposure. Also shown is endogenous acetylated HIF-2α present in each fraction. (d) Acetate levels in cells during hypoxia (n=3 biological replicates/time-point; single measurement/replicate; mean/SD). (e) Contribution of ACSS1, ACSS2, ACLY, CBP and p300 to endogenous HIF-2α acetylation during early (2 h) hypoxia. (f) Contribution of ACLY and ACSS2 to endogenous CBP/HIF-2α or p300/HIF-2α complex formation during early (2 h) hypoxia. (g) Contribution of ACSS1, ACSS2, and ACLY to EPO gene expression following hypoxia exposure (single pool of triplicate biological replicates/manipulation; triplicate measurements/pool; mean/SD). Separate analyses of 0, 2, and 8 h hypoxia samples was performed by two-way ANOVA with Dunnett's multiple comparison post-hoc test using control siRNA as reference within each group; only results for 8 h hypoxia are indicated (****P≤0.0001). All experiments were performed with Hep3B cells and key aspects of the above findings have been confirmed by at least one additional independent experiment.
Acetyl CoA is synthesized de novo by pyruvate dehydrogenase, ATP citrate lyase (Acly), or acetate-dependent acetyl CoA synthetases (Acss). During hypoxia, pyruvate dehydrogenase is inactivated by the HIF-1 target gene pyruvate dehydrogenase kinase 17,18 whereas Acly, a major source of cytosolic acetyl CoA, remains active 19. Acss1 generates mitochondrial acetyl CoA 20 while Acss2 generates cytosolic acetyl CoA 21-23. Because Acss2 and Acly may reside in the nucleus 24, we asked whether their subcellular locations as well as that of Cbp, Sirt1, and HIF-2α change during hypoxia (Fig. 1c). Acss2 is nuclear-localized during early hypoxia (2 h), coincident with peak acetylation and Cbp/HIF-2α complex formation, but is cytosolic during late hypoxia (8 h). Acly is mostly cytosolic, Cbp is restricted to the nucleus, and HIF-2α is present in both the cytosol and nucleus, but their distribution does not change appreciably during hypoxia. Nuclear Sirt1 levels increase during hypoxia, which may be a combination of increases in Sirt1 mass during hypoxia 25 as well as an indirect result of passenger effects.
The dynamic behavior of Acss2 subcellular location during hypoxia led us to consider it a candidate acetyl CoA generator for Cbp-mediated HIF-2α acetylation and therefore a regulator of HIF-2 signaling. We hypothesized that acetate, which is generated by hypoxic cells 26, might function as a biochemical cue to activate Acss2. Indeed, acetate levels increase by an early (2 h) hypoxia time-point, and then decline to basal levels (Fig. 1d). As we have shown previously, acetylation of HIF-2α is dependent upon Cbp and not p300 (Fig. 1e). Knockdown of Acss2, but not of Acss1 or Acly, also eliminates HIF-2α acetylation as well as Cbp/HIF-2α complex formation in early hypoxia, which shifts instead to p300/HIF-2α complex formation (Fig. 1e,f). Finally, Acss2 is required for induction of the HIF-2 target gene Epo during hypoxia (Fig. 1g).
We asked whether Acss2 is required for HIF-α or coactivator (p300 or Cbp) recruitment using chromatin immunoprecipitation (ChIP) assays (Supplementary Fig. 1a). HIF-2α and HIF-1α recruitment to the Epo enhancer is evident during early (2 h) and late (8 h) hypoxia. Acss2 knockdown eliminates HIF-2α, but not HIF-1α, recruitment to the Epo enhancer as well as Cbp recruitment, but does not affect p300 recruitment. Furthermore, Acss2 knockdown eliminates HIF-2α recruitment of Cbp during early (2 h) hypoxia as assessed by sequential ChIP, but does not affect HIF-2α recruitment of p300 during late (8 h) hypoxia (Supplementary Fig. 1b). Conversely, Acss2 knockdown eliminates Cbp recruitment of HIF-2α during early (2 h) hypoxia, but likewise does not reduce p300 recruitment of HIF-2α (Supplementary Fig. 1c).
HIF-1α also regulates Epo gene expression in Hep3B cells. However, Acss2 knockdown does not affect HIF-1α recruitment of Cbp or p300 to the Epo enhancer or to the promoter of the HIF-1 selective target gene Pgk1during hypoxia (Supplementary Fig. 1d,e). Acss2 knockdown has no effect upon HIF-1α or HIF-2α recruitment to a non-hypoxia responsive promoter, Rpl13a (Supplementary Fig. 1f). In all chromatin immunoprecipitation experiments, a control immunoprecipitation with a non-specific antibody fails to generate a signal.
Several hypoxia-inducible genes are co-regulated by HIF-1α and HIF-2α (Epo, VegfA, Pai1, Glut1), by HIF-2α (Mmp9), or by HIF-1α (Pgk1) (Supplementary Fig. 2a–c). For target genes regulated by HIF-2α, knockdown of Acss2, but not of Acss1 or Acly, blunts induction following hypoxia exposure (Supplementary Fig. 2d). In contrast, induction of a HIF-1α target gene is unaffected by knockdown of these acetyl CoA generators (Supplementary Fig. 2e).
Acss2 regulates hypoxia-induced renal Epo expression in mice
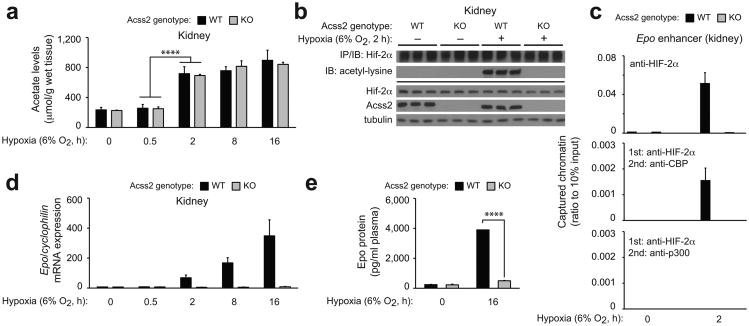
We deleted the majority of the Acss2 gene in mice (Supplementary Fig. 3a). Mixed strain Acss2 wild-type (WT), but not knockout (KO), mice demonstrate Acss2 immunoreactivity in renal cortex and medulla as well as in the liver (Supplementary Fig. 3b,c). Following hypoxia exposure, acetate levels in Acss2 WT and KO kidneys increase by 2 h and remain elevated (Fig. 2a). HIF-2α isolated from these kidneys is acetylated on lysine residues in an Acss2-dependent manner (Fig. 2b). Hypoxia exposure results in HIF-2α recruitment to the Epo enhancer in Acss2 WT, but not KO, kidneys, and sequential ChIP reveal that Cbp, but not p300, is present when HIF-2α occupies the Epo enhancer in mouse kidney after 2 h hypoxia (Fig. 2c).
Figure 2. Acss2 regulates hypoxia-induced renal Epo expression in mice.
(a) Acetate levels in kidneys of Acss2 wild-type (WT) or knockout (KO) mice exposed to normoxia or hypoxia (n=3 biological replicates/group, 3 male mice for 8 h time-point, 2 male and 1 female mice for other time-points; single measurements/replicate; mean/SD). Comparison of means was performed by two-way ANOVA with Dunnett's multiple comparison post-hoc test (****P≤0.0001). (b) Acetylation of endogenous HIF-2α isolated by immunoprecipitation (IP) from kidney extracts of Acss2 WT or KO mice and detected by immunoblotting (IB) with anti-acetylated lysine or anti-HIF-2α antibodies. (c) Recruitment of HIF-2α as well as coupled recruitment of HIF-2α with CBP or p300 to the Epo enhancer in kidneys of mixed strain Acss2 WT or KO mice maintained under normoxia or short-term (2 h) continuous hypoxia (n=4 biological replicates/group, 2 male and 2 female mice/group; duplicate measurements/replicate; mean/SEM). (d) Epo gene expression in kidneys of Acss2 KO and WT mice following normoxia or hypoxia exposure (n=3 biological replicates/group, 3 male mice for 8 h time-point, 2 male and 1 female mice for other time-points; triplicate measurements/replicate; mean/SEM). (e) Epo protein measurements of plasma from Acss2 KO and WT mice housed under normoxia or 16 h hypoxia (n=3 biological replicates/group; 2 male, 1 female mice/group; duplicate measurements/replicate; mean/SEM). All comparisons by unpaired Student's t-test with Holm-Sidak correction (****P≤0.0001). Key aspects of the above findings have been confirmed by at least one additional independent experiment, except for (c).
We asked if Cbp/HIF-2α complex formation at the Epo enhancer in mice during hypoxia correlates with Epo gene expression. Similar to global HIF-2α KO mice 11, Epo mRNA synthesis in kidneys of Acss2 KO mice is markedly blunted after hypoxia exposure (Fig. 2d) as is the increase in plasma Epo protein levels that is otherwise observed in Acss2 WT mice after 16 h of hypoxia exposure (Fig. 2e). However, the short-term duration of hypoxia (16 h) is not sufficient to result in an increase in red blood cell mass other than the initial rise in hematocrit levels during the early (2 h) hypoxia phase, an immediate physiological response 27 that is observed in both Acss2 WT as well as KO mice (Supplementary Fig. 3d).
Acute anemia induces Acss2-dependent HIF-2 signaling in mice
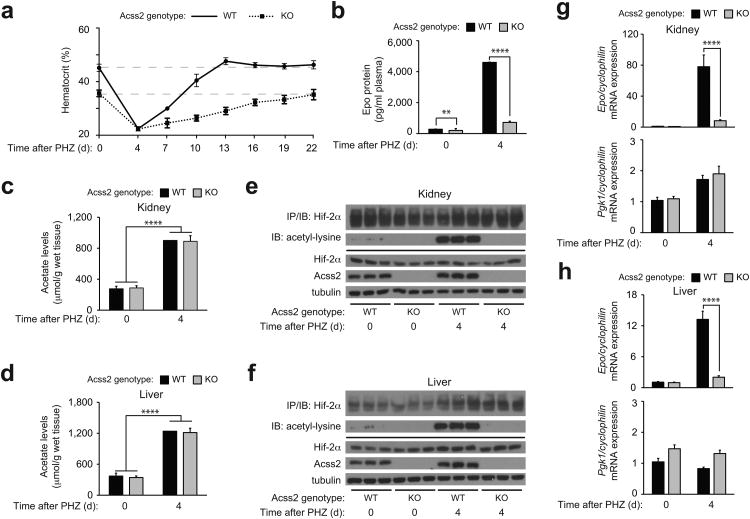
To determine if Acss2/HIF-2 signaling is relevant under pathophysiological states marked by more prolonged periods of tissue hypoxia, we used phenylhydrazine (PHZ) to induce hemolytic anemia in Acss2 WT or KO mice 28, which results in a comparable degree of severe anemia by 4 days after PHZ injection (Fig. 3a). We noted that the recovery of Acss2 KO mice takes substantially longer (18 days) than Acss2 WT mice (9 days), consistent with reduced plasma Epo levels observed in Acss2 KO relative to WT mice at the hematocrit nadir (Fig. 3b).
Figure 3. Acute anemia induces Acss2-dependent HIF-2 signaling in mice.
(a) Serial hematocrits (n=6 biological replicates/group, 4 male and 2 female mice/group; single measurements/replicate; mean/SD) and (b) plasma Epo protein measurements (n=8 biological replicates/group, 8 male mice/group; duplicate measurements/replicate; mean/SEM) of mixed strain Acss2 wild-type (WT) or knockout (KO) mice after phenylhydrazine (PHZ) treatment. Acetate levels in (c) kidneys and (d) livers of Acss2 WT or KO mice following PHZ treatment (n=10 biological replicates/group, 5 male and 5 female mice/group; single measurements/replicate; mean/SD). All comparisons by unpaired Student's t-test with Holm-Sidak correction (**P≤0.01, ****P≤0.0001). Immunoblot (IB) of acetylated lysine residues following immunoprecipitation (IP) with anti-HIF-2α antibody using (e) kidney or (f) liver extracts derived from three Acss2 WT or KO mice chosen at random from each group in (b). Epo and Pgk1 gene expression in (g) kidneys and (h) livers of same mice in (c) and (d) (n=10 biological replicates/group, 5 male and 5 female mice/group; duplicate measurements/replicate; mean/SEM). All comparisons by two-way ANOVA followed by Tukey's multiple comparison post-hoc test (****P≤0.0001). Key aspects of the above findings have been confirmed by at least one additional independent experiment, except for (c) and (d).
Acetate levels at the anemia nadir increase to comparable levels in Acss2 WT and KO kidney and liver (Fig. 3c,d). HIF-2α acetylation in kidneys and livers of severely anemic Acss2 WT mice also increases, but is undetectable in Acss2 KO mice (Fig. 3e,f). Finally, Epo mRNA levels are markedly induced in kidneys and livers of anemic Acss2 WT mice, but are only slightly elevated in these same organs of Acss2 KO mice (Fig. 3g,h). The absence of Acss2 has no effect on expression of the HIF-1 target gene Pgk1 in kidneys or livers of anemic Acss2 KO versus WT mice (Fig. 3g,h).
An acetate switch regulates Cbp/HIF-2 interactions in cells
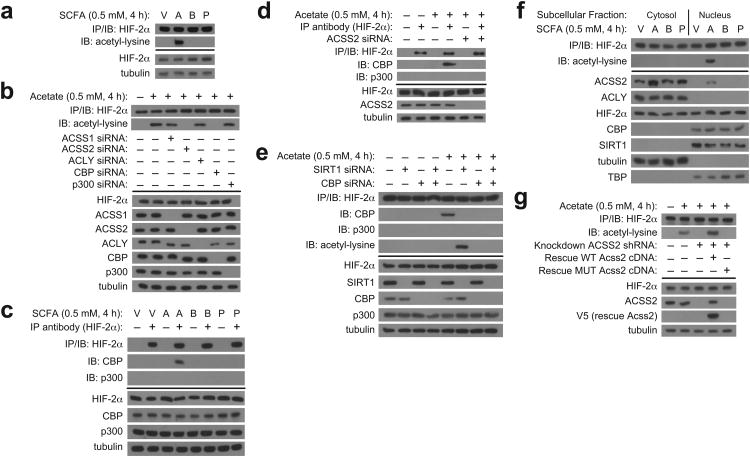
We reasoned that acetate generated by hypoxic cells triggers Cbp-mediated HIF-2α acetylation 26. Indeed, HIF-2α acetylation is increased in cells incubated with early (2 h) hypoxia conditioned medium or with acetate-supplemented medium, but not with other short chain fatty acid (SCFA) such as butyrate or propionate (Supplementary Fig. 4a, Fig. 4a). HIF-2α acetylation induced by acetate treatment requires Acss2 and Cbp (Fig. 4b). Similarly, Cbp/HIF-2α complex formation is observed in cells incubated with early (2 h) hypoxia conditioned medium or with acetate-supplemented medium, but not other SCFA (Supplementary Fig. 4b, Fig. 4c). Stable Acss2-dependent Cbp/HIF-2α complex formation induced by acetate requires Acss2 as well as Sirt1 and Cbp (Fig. 4d,e), similar to requirements under hypoxia 16. Acetate supplementation results in Acss2 nuclear localization (which is also seen after hypoxia exposure) (Fig. 4f), but the other SCFA do not have this effect. Finally, the loss of acetate-induced HIF-2α acetylation following Acss2 knockdown is rescued by expression of an shRNA-resistant wild-type, but not mutant, Acss2 cDNA (Fig. 4g).
Figure 4. An acetate switch regulates Cbp/HIF-2 interactions in cells.
(a) Endogenous HIF-2α acetylation following immunoprecipitation (IP) of endogenous HIF-2α and detection by immunoblotting (IB) with anti-acetylated lysine or anti-HIF-2α antibodies after incubation using medium supplemented with vehicle (V; PBS) or the short chain fatty acid (SCFA) sodium acetate (A), sodium butyrate (B), or sodium propionate (P) prepared in PBS. (b) Contribution of ACSS1, ACSS2, ACLY, CBP and p300 to endogenous HIF-2α acetylation for cells incubated using complete medium supplemented with vehicle or acetate. (c) Endogenous CBP/HIF-2α complexes formed after treatment with SCFA-containing medium. (d) Contribution of ACSS2 to endogenous CBP/HIF-2α complex formation induced by acetate treatment. (e) Endogenous CBP/HIF-2α complexes and acetylated HIF-2α induced by acetate treatment following SIRT1, CBP, or combined SIRT1/CBP knockdown. (f) Subcellular localization of ACSS2, ACLY, HIF-2α, CBP, and SIRT1 following treatment with SCFA-containing medium. (g) Endogenous HIF-2α acetylation induced by acetate supplementation in stably transformed knockdown/rescue cells expressing either an shRNA-resistant wild-type (WT) or mutant (MUT) Acss2 rescue cDNA. All experiments were performed with Hep3B cells. Key aspects of the above findings have been confirmed by at least one additional independent experiment.
Exposure of cells to hypoxia, hypoxia-conditioned medium, or exogenous acetate all result in elevated intracellular acetate levels, but only medium of hypoxia and hypoxia-conditioned cells have altered acidity (Supplementary Fig. 4c). Cbp/HIF-2α complex formation is observed in vitro with early (2 h) hypoxia cell extracts and is absent following Acss2 depletion, but is restored with addition of acetyl CoA (Supplementary Fig. 4d). Although normally absent in Acss2-replete, late (8 h) hypoxia cell extracts, Cbp/HIF-2α complex formation is induced in vitro in these extracts by supplementing with either acetyl CoA or acetate and ATP, cofactors required by Acss2 21 that decrease during hypoxia 29 (Supplementary Fig. 4e).
Acss2 signaling in cells requires intact HIF-2 acetylation
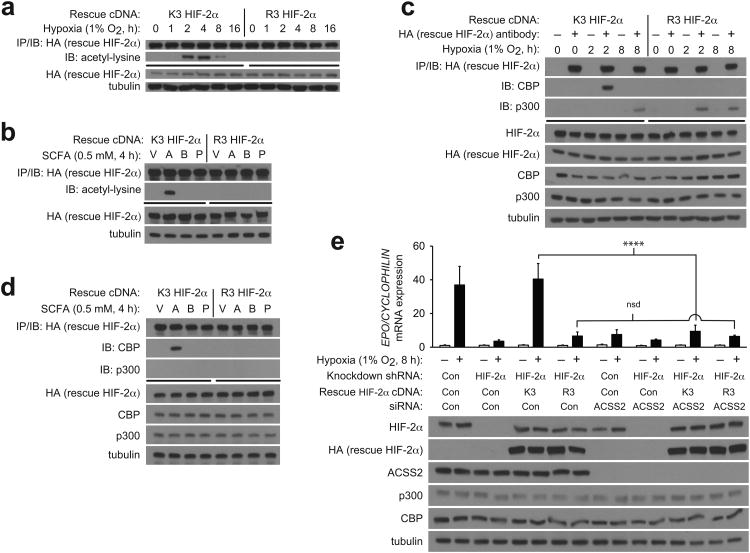
To ascertain whether acetate-induced Cbp/HIF-2α interactions requires HIF-2α lysine residues acetylated following hypoxia exposure, we exposed knockdown/rescue cells, expressing either rescue HIF-2α with intact lysine residues that are acetylated during hypoxia (K3) or a HIF-2α arginine substitution mutant incapable of acetylation (R3) 10, to hypoxia or medium supplemented with each SCFA. K3, but not R3, HIF-2α is acetylated (Fig. 5a,b) and complexes with Cbp (Fig. 5c,d) during hypoxia or after acetate supplementation. Finally, Epo mRNA induction is maximal and is markedly reduced by Acss2 knockdown following hypoxia exposure in K3 HIF-2α rescue cells (Fig. 5e). In comparison, Epo mRNA induction is blunted in R3 HIF-2α rescue cells following hypoxia exposure and Acss2 knockdown has no effect, consistent with Acss2 action on HIF-2 signaling being mediated through acetylation of the K3 lysine residues.
Figure 5. Acss2 signaling in cells requires intact HIF-2 acetylation.
Acetylation of ectopic rescue wild-type acetylase-sensitive (K3) or acetylase-insensitive (R3) human HIF-2α after immunoprecipitation (IP) of rescue HIF-2α, followed by immunoblotting (IB) of rescue HIF-2α or acetyl-lysine after (a) hypoxia exposure or (b) supplementation of medium with PBS vehicle (V), acetate (A), butyrate (B), or propionate (P). Immunoprecipitation of ectopic rescue K3 or R3 human HIF-2α after (c) hypoxia exposure or (d) supplementation with V, A, B, or P. (e) Measurements of EPO gene expression in stably transformed knockdown/rescue cells expressing ectopic rescue K3 or R3 human HIF-2α and transfected with control or Acss2 siRNA, followed by incubation under either normoxic or hypoxic conditions (single pool of triplicate biological replicates/manipulation, triplicate measurements/pool, mean/SD). All comparisons by two-way ANOVA with Tukey's multiple comparison post-hoc test (nsd, no significant difference; ****P≤0.0001). All experiments were performed with Hep3B cells. Key aspects of the above findings have been confirmed by at least one additional independent experiment.
Acetate facilitates recovery from anemia
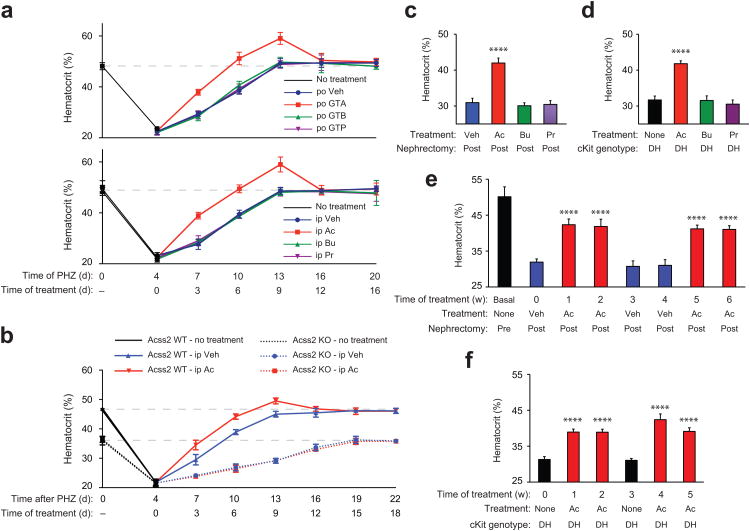
To determine if acetate augments recovery from acute anemia, we administered either vehicle (water) or a glyceryl triester of the short chain fatty acids (SCFA) acetate (GTA), butyrate (GTB), or propionate (GTP) to wild-type CD1 mice with phenylhydrazine (PHZ)-induced anemia. To determine if the route of administration influenced its action, we also delivered SCFA by intra-peritoneal injections. Erythropoiesis in acutely anemic wild-type CD1 mice is augmented after supplementation with oral or intra-peritoneal acetate, but not butyrate or propionate (Fig. 6a). Acetate supplementation also speeds recovery from PHZ-induced anemia in Acss2 WT, but not KO, mice on a mixed strain genetic background (Fig. 6b).
Figure 6. Acetate facilitates recovery from anemia.
(a) Hematocrits of CD1 wild-type female mice after phenylhydrazine (PHZ) treatment, followed by once daily per os (po) supplementation with water vehicle (Veh; n=7 biological replicates), glyceryl triacetate (GTA; n=6 biological replicates), glyceryl tributyrate (GTB; n=8 biological replicates), or glyceryl tripropionate (GTP; n=7 biological replicates) (single measurement/replicate; mean/SD), or followed by once daily intra-peritoneal (ip) supplementation with PBS vehicle (Veh), acetate (Ac), butyrate (Bu), or propionate (Pr) (n=5 biological replicates/group; single measurement/replicate; mean/SD). The dotted line indicates the mean hematocrit for control CD1 wild-type female mice (n=33 biological replicates). (b) Serial hematocrits of mixed strain Acss2 wild-type (WT) or knockout (KO) mice after PHZ treatment, followed by once daily ip Veh or Ac injections (n=4 biological replicates/group except for days 16, 19 and 22 where n=3 biological replicates for Acss2 KO mice treated with Ac following death of one mouse; single measurement/replicate; mean/SD). (c) Hematocrits of CD1 wild-type female mice with chronic renal failure (CRF) induced by 5/6 partial nephrectomy and supplemented with three (Monday, M/Wednesday, W/Friday, F) ip injections of Veh (n=11 biological replicates), Ac (n=11 biological replicates), Bu (n=8 biological replicates), or Pr (n=8 biological replicates) (single measurement/replicate; mean/SD). (d) Hematocrits of WBB6 F1 hybrid double heterozygous (DH) cKit mutant mice with no supplements (None) or supplemented with three (M/W/F) ip injections of Ac, Bu, or Pr (n=4 biological replicates/group; single measurements, mean/SD). (e) Serial hematocrits of CD1 wild-type female mice before (Pre) or after (Post) partial 5/6 nephrectomy, followed by thrice-weekly (M/W/F) ip supplements of Veh or Ac (n=8 biological replicates/group; single measurement/replicate; mean/SD). (f) Serial hematocrits of WBB6 F1 hybrid DH cKit mutant mice without or with thrice-weekly (M/W/F) ip Ac supplementation (n=4 biological replicates/group, 2 male and 2 female mice/group; single measurement/replicate; mean/SD). Hematocrits for (c) through (f) were obtained on the Monday following the indicated treatment period. Key aspects of the above findings have been confirmed by at least one additional independent experiment.
With respect to changes in hematological parameters, GTA treatment returns hematocrit levels to normal earlier (6 days) than control-treated anemic mice (9 days) (Supplementary Fig. 5a). Despite an initial overshoot, hematocrit levels return to normal within three days even with continued GTA administration. Reticulocyte levels, which increase in acutely anemic mice (day 4 post-PHZ), return to normal faster in GTA- versus vehicle-treated mice (Supplementary Fig. 5b). Increased (kidneys) or prolonged (livers) HIF-2α acetylation is observed after GTA treatment for acutely anemic CD1 mice (Supplementary Fig. 5c,d).
As for downstream effects on HIF-2 signaling, GTA supplementation results in increased Epo mRNA levels in kidneys and livers, which also remain elevated at least 3 days longer than vehicle-treated anemic mice (Supplementary Fig. 6a,b). Nevertheless, soon after hematocrit levels of GTA-treated anemic mice return to normal (Day 10 post-PHZ), Epo mRNA levels also return to basal levels in the kidney and liver (Day 13 post-PHZ) and remain so despite continued GTA administration (Days 16 and 20 post-PHZ). Acetate does not augment expression of the HIF-1 target gene Pgk1 gene in kidney or liver (Supplementary Fig. 6a,b).
We also examined whether acetate supplementation affects erythropoiesis in an acquired chronic anemia model, chronic renal failure (CRF) induced by partial nephrectomy 30,31, and a genetic chronic anemia model, double heterozygous (DH) cKit mutant mice 32,33. Acetate, but not butyrate or propionate, increases resting hematocrit levels in CRF as well as DH cKit mutant mice (Fig. 6c,d). Although hematocrit levels of CRF as well as DH cKit mutant, but not cKit wild-type, increase (Supplementary Fig. 7a,e), reticulocyte levels only increase for CRF mice, but decrease for DH cKit mutant mice, with acetate treatment (Supplementary Fig. 7b,f). Despite these differences, acetate treatment increases renal Epo mRNA levels in both models (Supplementary Fig. 7c,g). Acetate administration results in elevated acetate levels for CRF and DH cKit mutant mice as well as WT cKit mice (Supplementary Figs. 7d,h).
Cyclical acetate treatments of CRF as well as DH cKit mutant results in increased hematocrit levels (Fig. 6e,f) that parallel similar increases in plasma Epo protein (Supplementary Fig. 8a,b), which reverse with cessation of acetate supplementation. Finally, acetate treatment also results in increased acetylated HIF-2α protein levels in kidneys of CRF and DH cKit mutant mice, but not WT cKit mice (Supplementary Fig. 8c,d).
Discussion
Although exogenous Epo is an effective treatment for anemia, therapies that maintain physiological Epo regulation may reduce deleterious side-effects associated with bolus Epo treatment including hypertension, thrombosis, and possibly accelerated malignancies 34-36. HIF-2, a key regulator of endogenous Epo gene expression, may be a particularly efficacious target. A major finding from this study is that acetate, generated during hypoxia or anemia, is a signaling molecule used by a specific acetyl CoA generator, Acss2, to regulate interactions of HIF-2α with its selective acetyltransferase and coactivator, Cbp. Recruitment of Cbp/HIF-2α complexes to the Epo enhancer occurs in parallel with Cbp/HIF-2α complex formation in solution during early hypoxia and also depends upon Acss2. Interestingly, at the later phase of hypoxia, Cbp and HIF-2α remain associated with the Epo enhancer, but not with each other, which suggests that the Cbp/HIF-2α containing enhanceosome assembled at the Epo enhancer and likely other HIF-2 target genes undergoes further remodeling as hypoxia progresses.
The exact origin of acetate during hypoxia and anemia is unknown. The liver generates endogenous acetate 37 under ambient conditions; a similar process has not been reported for kidney. Kidney as well as liver exhibit zonal hypoxia, which place certain anatomical regions of these organs at risk to further changes in oxygen content 38,39. Acetate and acetyl CoA pools in cells rapidly cycle 40-42, so perturbations of this cycle during hypoxia and anemia may facilitate transient acetate increases in the kidney and liver 43. Histone deacetylation in acidic environments results in acetate release 44, which may also be relevant. Although increased SCFA levels may impair acetate utilization 45 or uptake 46, we find that addition of SCFA besides acetate does not trigger the Acss2 response. Acss2 is a proposed acetate generator 26, but is likely a minor source in normal cells as acutely hypoxia or anemic Acss2 WT and KO mice have similar acetate levels in kidney and liver.
Changes in intermediary metabolism during hypoxia initiate Acss2 activity, but also terminate its action as hypoxia continues. During early (2 h) hypoxia, HIF-2α acetylation as well as Cbp/HIF-2α complex formation, which occurs via HIF-2α binding independent of the carboxy terminal activation domain (CTAD), is stimulated by increased acetate levels and is regulated by Acss2 in Hep3B cells. During late (8 h) hypoxia, acetate levels return to baseline and ATP levels likely decrease, which limits Acss2 activity. In addition, Factor Inhibiting HIF-1 (FIH1) is inactive 47-49, which means that CTAD-dependent recruitment of p300 can commence.
The molecular and biochemical effects of acetate supplementation include increased HIF-2α acetylation, Epo mRNA levels, and plasma Epo protein levels, which result in an accelerated recovery from acute anemia. Continued acetate treatment has no further effect on hematocrit levels at or close to physiological levels, which indicates proper functioning of the homeostatic mechanisms for hematocrit control under these conditions. Thus, acetate augmentation of HIF-2 signaling in whole animals requires not only Acss2, but also the concomitant presence of hypoxemia. Whether this reflects a requirement for inactivation of PHDs or FIH1, or for production of other factors activated during anemia 50, remains to be determined.
Acetate supplementation may be useful in treating acute anemia in civilian and military scenarios and when patients have faith-based objections to blood transfusions 51. Chronic anemia associated with impaired Epo generation due to reduced HIF-2 signaling may also be impacted by the acetate switch, and may already be unwittingly exploited. End-stage renal disease (ESRD) patients often have anemia as well as blunted Epo production 11,13, and are dialyzed thrice-weekly with an acetate-containing dialysate that transiently increases plasma acetate levels 52,53. ESRD patients transitioned to an acetate-free dialysate 54 exhibit increased darbepoetin and iron requirements 55, consistent with expectations if HIF-2 signaling is impaired. The ability of acetate to increase Epo production in DH cKit mutant mice suggests that acetate may also be effective in treating some genetic disorders associated with impaired Epo, but intact HIF-2, signaling, which may be due to the ability of Epo to influence erythroid progenitor cell fates 56.
One proposed therapeutic role for acetate supplementation, which may also be mediated by Acss2, is in production of acetate-derived lipids21 that are used for myelin synthesis in neuropathophysiological states such as traumatic brain injury (TBI) 57. Notably, TBI, which results in tissue ischemia and hypoxia, results in Acss2 nuclear localization in astrocytes 57. HIF-2 is also expressed in astrocytes, where it regulates Epo gene expression and may alleviate neurological injury 58. The broad distribution of Acss2 in hepatocytes as well as in the renal cortex and medulla may signify an additional physiological role of Acss2/HIF-2 signaling, possibly relating to ischemic injury responses regulated by HIF-2 59,60. Whether Acss2 plays a protective role in the injury response of vital organs and, if so, whether the effect of Acss2 is mediated by anabolic contributions or by signaling events via HIF-2 or other acetylated signal transducers, is a topic for future studies.
Online Methods
Cell culture
We maintained Hep3B cells (Cat. No. HB-8064, ATCC, Manassas, VA) in complete DMEM media (Cat. No. SH30022, HyClone, Logan, UT), 10% fetal bovine serum (FBS; Cat. No. F4135, Sigma) with penicillin (100 U mL−1)/streptomycin (100 μg mL−1) (Cat. No. 15140-148, Gibco BRL, Carlsbad, CA) in a 5% CO2, 95% air incubator. We assayed cells periodically for mycoplasma. For passaging cells, we added 1 ml 0.25% Trypsin/2.21 mM EDTA/HBSS (Cat. No. 25-052-CI, Corning cellgro, Manassas, VA) to a freshly confluent 10 cm plate and waited 1 min at room temperature. Trypsinized cells from one 10 cm plate were added to complete media (1:3 ratio) and replated into three 10 cm plates. We used cells (unselected or selected) from between 15 and 25 passages in all experiments. For hypoxia treatments (1% O2, 5% CO2, 94% N2), we maintained cells, replaced media with deoxygenated media, and prepared extracts within a humidified environmental chamber (Coy Laboratory Products, Inc., Grass Lake, MI). For short chain fatty acid (SCFA) addition, we added the indicated sterile stocks of SCFA (acetate, proprionate, butyrate) to complete medium (final concentration 0.5 mM) and harvested cells after the indicated time under normal oxygen conditions.
Lentiviral generation
We previously described the wild-type (WT) acetylase-sensitive (K3) and acetylase-insensitive (R3; K385R, K685R, K741R) human HIF-2α plasmids 10,16. All human HIF-2α rescue cDNA constructs contain a carboxy terminal hemagglutinin A (HA) epitope tag. We generated wild-type (WT) and mutant (MUT) Acss2 from a mouse Acss2 cDNA. We constructed MUT Acss2 cDNA by zipper PCR so that it contains deletions of sequences corresponding to coding exons 3 through 7, and thereby encodes a truncated and non-functional Acss2 protein not detected by immunoblotting. The rescue HIF-2α and Acss2 cDNAs contain silent mutations that confer resistance to siRNA or shRNA directed against the endogenous human protein of interest. All Acss2 constructs contain an amino terminal V5 epitope tag.
The lentiviral knockdown constructs express multiple miR30 shRNAs 61 using Pol II promoters 62 for efficient knockdown. For knockdown only studies (control and Acss2), lentiviral (LTV) shuttle expression vectors (derivatives of pLenti6/V5-GW/lacZ, Invitrogen that are neomycin instead of blasticidin resistant) harbor a firefly luciferase cDNA followed by a concatamer of four different shRNAs directed against the gene of interest. For knockdown/rescue studies, LTV shuttle expression vectors encode an siRNA/shRNA-resistant mouse Acss2 or human HIF-2α cDNA followed by an IRES:firefly luciferase:shRNA concatamer cassette (WT and MUT Acss2) or an IRES:DsRed:shRNA concatamer cassette (K3 and R3 HIF-2α). The seed sequences for the four human ACSS2 shRNA are as follows: 5′-GTAATAGCCATCCTGGTCCCGC-3′, 5′-AACTTGGTCACCTTGTATTTGT-3′, 5′-TGGTATGTGATCTGAGTGGTCT-3′, 5′-GTATCCAGGAAACTTCTTAAAG-3′. The seed sequences for the four human HIF-2α shRNA are based on previous siRNAs 14,63-65 and are as follows: 5′-TTCATACTCCAGCTGTCGCTTA-3′, 5′-TAAGTCTATCCGGGCTTACTAC-3′, 5′-TCTGTGTCCATGGCGAAGAGCG-3′, 5′-ACTGCTATCAAAGATGCTGTTA-3′.
We used the LTV packaging plasmid psPAX2 (Addgene plasmid 12260) and the envelope plasmid pMD2.G (Addgene plasmid 12259) in conjunction with the shuttle expression plasmids to generate lentivirus. We generated concentrated lentiviral stocks from a 10 cm plate of HEK293T cells co-transfected with 3 μg LTV shuttle expression vector, 2.25 μg plasmid psPAX2 and 0.75 μg pMD2G using Lipofectamine 2000 (Cat. No. 11668019, Invitrogen) using Lenti-X Concentrator (Cat. No. 631232, Clontech). We aliquoted the virus immediately and stored at –80° C until use.
Stable cell line generation
The day before transduction, we plated 2 × 105 Hep3B cells per well in 1 mL complete culture medium overnight in a 6-well plate. On the day of transduction, we replaced media with 1 mL of complete medium with 10 μg mL−1 polybrene (Cat. No. 107689, Sigma, St. Louis, MO). We thawed lentiviral particles to room temperature, mixed gently, and added to Hep3B cells (MOI=30). After 12 h, we replaced culture medium with 2 mL of complete medium containing 400 μg mL−1 G418 (Cat. No. SV3006901, HyClone), and replaced it every 2 days until one week after all control cells died. We maintained the initially positive-selected cells in 100 μg mL−1 G418 for 2 weeks, harvested into a smaller dish until confluent, split cells at 1:3 ratio and grew for at least ten passages under drug selection, and then froze the cells down until use. For experiments, we thawed cells, maintained cells in 100 μg mL−1 G418, split cells 1:3 for at least three passages when growing exponentially, trypsinized cells lightly at room temperature, and used cells in actual experiments without drug selection when freshly confluent. We used cells fifteen to twenty-five passages from initial drug selection.
siRNA/shRNA knockdown
For transient siRNA knockdown experiments, we used the following siRNAs (Thermo Fisher Scientific, Lafayette, CO): non-targeting control (Cat. No. D-001810-10-20), ACSS1 (Cat. No. L-008549-01-0005), ACSS2 (Cat. No. L-010396-00-0005), ACLY (Cat. No. L-004915-00-0005), p300 (Cat. No. L-003486-00-0005), CBP (Cat. No. L-003477-00-0005), SIRT1 (Cat. No. L-003540-00-0005), HIF-1α (Cat. No. 004018-00-0005), HIF-2α (Cat. No. L-004814-00-0005). We transfected siRNA into Hep3B cells using DharmaFECT1 (Cat. No. T-2001-03, Thermo Fisher Scientific) as quadruplicate biological replicates in a 12-well plate with three wells subsequently pooled for use in mRNA analyses and one well used for protein analyses.
For transient lentiviral transduction knockdown (control, ACCS2)/rescue (WT and MUT Acss2) experiments, we added 100 μL of concentrated lentiviral preparations to a 60 mm Hep3B plate, waited 6 h, then changed the medium to complete medium. After an additional 36 h, we performed the indicated experiment. For stable lentiviral transduction knockdown (control, HIF-2α)/rescue (K3 and R3 HIF-2α) experiments, we thawed, grew, split, and used cells as described above. Experiments were performed without drug selection as described above.
Immunoblotting
We used 20 μg Hep3B whole cell extracts, 20 μg Hep3B nuclear or cytosolic extracts, and 10 μg mouse kidney or liver extracts for immunoblotting 10,16. To prepare whole cell extracts from Hep3B cells, we used CytoBuster protein extraction reagent (Cat. No. 71009, Novagen, Gibbstown, NJ) with 1× protease inhibitor cocktail (Cat. No. P8340, Sigma, St. Louis, MO) and 1 mM PMSF (phenylmethylsulfonyl fluoride) (Cat. No. P7626, Sigma), and stored extracts at –80 °C until use. To prepare nuclear extracts from Hep3B cells, we used NE-PER® nuclear and cytoplasmic extraction reagents (Cat. No. 78833, Pierce, Rockford, IL) according to the manufacturer's protocol, and stored extracts at –80 °C until use. To prepare tissue extracts from liver or kidney, we used CytoBuster protein extraction reagent with 1× protease inhibitor cocktail and 1 mM PMSF, and stored extracts at –80 °C until use.
We used antibodies raised against the following antigens: human p300 (1:500 dilution; Cat. No. sc-584, Santa Cruz Biotechnology, Santa Cruz, CA), human CBP (1:500 dilution; Cat. No. 7389, Cell Signaling Technology, Danvers, MA), human SIRT1 (1:1,000 dilution; Cat. No. 07131, EMD Millipore, Billerica, MA), human ACLY (1:1,000 dilution; Cat. No. 3378, Cell Signaling Technology), human ACSS1 (1:1,000 dilution; Cat. No. SAB1400745, Sigma), human ACSS2 (1:500 dilution; Cat. No. ab66038, Abcam, Cambridge, MA), human HIF-1α (1:1,000 dilution; Cat. No 610958, BD Biosciences), human HIF-2α (1:1,000 dilution; Cat. No. NB10-132, Novus Biologicals, Littleton, CO), α-tubulin (1:10,000 dilution; Cat. No. T9026, Sigma), TATA-binding protein (TBP) (1:1,000 dilution; Cat. No. sc-204, Santa Cruz Biotechnology), acetyl-lysine (1:1,000 dilution; Cat. No. 9814, Cell Signaling Technology), HA epitope (1:5,000 dilution; Cat. No. H9658, Sigma), V5 epitope (1:10,000 dilution; Cat. No. R960-25, Invitrogen).
Acetate measurements
For cellular acetate measurements, we plated Hep3B cells at 80% confluency as triplicate biological replicates in 60 mm plates and grew them overnight in an incubator within the hypoxia workstation until the indicated time-point. At the time of harvest, we aspirated off media, added 1 mL ice-cold 0.1 N HCl and scraped cells. We transferred the lysate from each plate to an ice-cold microfuge tube and pelleted the cellular debris at 14,000 rpm, 4 °C, 10 min. We transferred 0.9 mL supernatant to a new, ice-cold microfuge tube, and then adjusted the pH to 6.5-7.0 with 5 N NaOH. We determined acetate concentrations from freshly prepared extracts in duplicates using the Megazyme Acetic Acid Rapid Kit (Cat. No. K-ACETRM, Megazyme, Ireland).
For hypoxic mouse kidneys, we exposed Acss2 wild-type and knockout mice to room air (21% O2) or hypoxia (6% O2) for 2 h and then euthanized under normoxic or hypoxic air mixtures to harvest kidney samples. For anemic mouse kidneys and livers, we euthanized under normoxic air mixtures to harvest kidney samples. To measure tissue acetate levels, we placed (∼0.1 g) weighed, freeze-clamped, powdered tissue into a cold microfuge tube on dry ice, and slowly added 500 μL ice-cold 1 N perchloric acid. We slowly thawed the samples on ice and then followed with homogenization (PowerGen 700D, Fisher Scientific). We pelleted the homogenate at 14,000 rpm, 4 °C, 10 min. We transferred 0.5 mL supernatant to a new, ice-cold microfuge tube, and then adjusted the pH to 6.5–7.0 with 10 N NaOH. We incubated the extract on ice for 30 min to precipitate proteins, and then centrifuged at 14,000 rpm, 4 °C, 10 min. We transferred the supernatant to a new tube and assayed fresh as single measurements described above and normalized to the weight of tissue used.
Endogenous HIF-2α acetylation/cells
We cultured Hep3B cells in a single 100 mm plate in complete medium supplemented with 5 μM sirtinol plus 10 mM NAM for 6 h under either normoxia or hypoxia. For acetylation assay using whole cell extracts, we lysed cells with CytoBuster protein extraction reagent supplemented with 1× protease inhibitor cocktail, 1 mM PMSF, 10 mM NAM, and 5 μM sirtinol. For acetylation assay with nuclear or cytosolic protein extracts, we fractionated cells and extracted the nuclear protein using a kit (Cat. No. 40010, Active Motif, Carlsbad, CA) supplemented with 1× protease inhibitor cocktail, 1 mM PMSF, 10 mM NAM, and 5 μM sirtinol. To immunoprecipitate endogenous HIF-2α, we incubated extracts with a monoclonal human HIF-2α antibody (Cat. No. NB100-132, Novus Biologicals) for 1 h and then immunoprecipitated using magnetic protein G beads (Cat. No. 54002, Active Motif). We immunoblotted for endogenous HIF-2α or acetyl lysine as described 10,16.
For short chain fatty acid (SCFA) experiments, we cultured a single 60 mm plate of Hep3B cells for 2 h in complete medium with 5 μM sirtinol plus 10 mM NAM under normoxia, and then incubated for 4 h with medium containing vehicle, sodium acetate, sodium proprionate, or sodium butyrate with 5 μM sirtinol plus 10 mM NAM. For conditioned media experiments, we cultured a single 60 mm plate of Hep3B cells for 4 h under normoxia in complete medium supplemented with 5 μM sirtinol plus 10 mM NAM, and then incubated for an additional 2 h under either normoxia (control media) or hypoxia (conditioned media). We replaced 2 mL of cell media of normoxia-maintained Hep3B cells (cultured for 4 h in complete medium with 5 μM sirtinol plus 10 mM NAM) with either 2 mL of control or conditioned media, and incubated the cells for 2 h. We then prepared whole cell extracts for immunoblotting of endogenous HIF-2α or acetyl lysine.
For transient transfection siRNA knockdown experiments, we first transfected Hep3B cells in a single 60 mm plate with siRNA, maintained the transfected cells in complete medium for 42 h under normoxia, changed medium to complete medium with 5 μM sirtinol plus 10 mM NAM, maintained cells for another 6 h under normoxia, exposed cells to the indicated condition, and then prepared whole cell extracts for immunoblotting of endogenous HIF-2α or acetyl lysine.
For lentiviral knockdown (control, Acss2) and knockdown/rescue (WT and MUT Acss2) experiments, 36 h after changing from polybrene-containing medium to complete medium (42 h after initial viral transduction), we cultured a single 60 mm plate of Hep3B cells for 2 h in complete medium with 5 μM sirtinol plus 10 mM NAM under normoxia, and incubated for 4 h with medium containing vehicle or sodium acetate with 5 μM sirtinol plus 10 mM NAM. We then prepared whole cell extracts for immunoblotting of endogenous HIF-2α or acetyl lysine.
Ectopic HIF-2α acetylation/cells
We housed a single 60 mm plate of stably transformed Hep3B cells expressing WT K3 or R3 HIF-2α under normoxia for 6 h in complete medium containing 5 μM sirtinol plus 10 mM NAM, and then we transferred cells to either normoxia or hypoxia for the indicated period. At the end of the incubation period, we lysed cells with CytoBuster protein extraction reagent supplemented with 1× protease inhibitor cocktail, 1 mM PMSF, 10 mM NAM, and 5 μM sirtinol. We purified ectopic HIF-2α:HA by HA-agarose pulldown, and then first immunoblotted using antibody recognizing acetylated lysine and second immunoblotted using antibody recognizing the HA epitope as previously described 10,16.
Endogenous HIF-2α acetylation/mice
We lysed mouse liver (∼50 mg) or kidney samples (∼100 mg) at the time of harvest in 0.5 mL CytoBuster protein extraction reagent supplemented with 1× protease inhibitor cocktail, 1 mM PMSF, 10 mM NAM, and 5 μM sirtinol. We incubated protein extract (500 μg) with a monoclonal human HIF-2α antibody for 1 h to bind endogenous HIF-2α protein and immunoprecipitated the complex using magnetic protein G beads (Cat. No. 54002, Active Motif). We then immunoblotted for endogenous HIF-2α or acetyl lysine as described 10,16.
Immunoprecipitation experiments/cells
We immunoprecipitated endogenous HIF-2α proteins from a single 100 mm plate of Hep3B cells using a Universal Magnetic Co-IP kit (Cat. No.54002, Active Motif) according to the manufacturer's protocol. We first incubated whole cell extracts (500 μg) with magnetic protein G beads. We then incubated the cleared supernatants with antibodies to HIF-2α (endogenous) or HA (rescue HIF-2α protein), or with normal mouse (Cat. No. sc-2025, Santa Cruz Biotechnology) IgG, for 2 h before addition of magnetic protein G beads. After binding, we pelleted beads by centrifugation. After washing, we eluted the immunoprecipitated proteins and immunoblotted with antibodies to human p300 (1:500 dilution; Cat. No. sc-584, Santa Cruz Biotechnology), human CBP (1:500 dilution; Cat. No. 4772, Cell Signaling Technology), or HIF-2α (1:1,000 dilution; Cat. No. NB100-132, Novus Biologicals).
In vitro immunoprecipitation experiments/extracts
We first transfected Hep3B cells in a single 100 mm plate with siRNA, waited 48 h, exposed cells to the indicated condition, and prepared whole cell extracts using a Universal CoIP Kit (Cat. No. 54002, Active Motif) as described 10. For hypoxia samples, we performed all initial extract preparations in the hypoxia workstation. For all subsequent in vitro experiments, we performed manipulations under normal oxygen conditions. We next added acetyl CoA (final concentration 50 μM; Cat. No. A2056, Sigma), ATP (final concentration 100 μM; Cat. No. FLAAS-1VL, Sigma), or acetate (final concentration 50 μM; Cat. No. A2056, Sigma) to 50 μl whole cell extract and incubated the extract at 30 °C for 30 min. We then immunoprecipitated endogenous HIF-2α and performed immunoblotting for HIF-2α, CBP, or p300 as described 10,16.
Real-time PCR analyses
We determined the expression of endogenous Epo, VegfA, Pai1, Mmp9, Glut1, Pgk1, and cyclophilin B from a single pooled sample made from three individually transfected wells (biological replicates) of Hep3B cells in a 12-well plate for each condition or of endogenous Epo and cyclophilin B from the indicated number of individual mouse organs (biological replicates). We generated cDNA by reverse transcription of total RNA followed by real-time PCR analysis (rtRTPCR) performed and measured in triplicate (three technical replicates) using human or mouse specific primer pairs as previously described 10,16. We used the following human rtRTPCR primer pairs: Epo (forward) 5′-GAGGCCGAGAATATCACGACGGG-3′, EPO (reverse) 5′-TGCCCGACCTCCATCCTCTTCCAG-3′; VEGFa (forward) 5′-AGTAGCTGCGCTGATAGACATCCATGA-3′, VEGFA (reverse) 5′-CACCCATGGCAGAAGGAGGAGGGCAGAA-3′; PAI1 (forward) 5′-ATTCAAGCAGCTATGGGATTCAA-3′, PAI1 (reverse) 5′-CTGGACGAAGATCGCGTCTG-3′ [PrimerBank ID 10835159a2]; MMP9 (forward) 5′-GGGACGCAGACATCGTCATC-3′, MMP9 (reverse) 5′-TCGTCATCGTCGAAATGGGC-3′ [PrimerBank ID 4826836a2]; GLUT1 (forward) 5′-CTTTTCTGTTGGGGGCATGAT-3′, GLUT1 (reverse) 5′-CCGCAGTACACACCGATGAT-3′ [PrimerBank ID 5730051a2]; Pgk1 (forward) 5′-TTAAAGGGAAGCGGGTCGTTA-3′, Pgk1 (reverse) 5′-TCCATTGTCCAAGCAGAATTTGA-3′; Cyclophilin B (forward) 5′-ATGTGGTTTTCGGCAAAGTTCTA-3′, Cyclophilin B (reverse) 5′-GGCTTGTCCCGGCTGTCT-3′. We used the following mouse rtRTPCR primer pairs: Epo (forward) 5′-GAGGCAGAAAATGTCACGATG-3′, Epo (reverse) 5′-CTTCCACCTCCATTCTTTTCC-3′; Pgk1 (forward) 5′-CTCCGCTTTCATGTAGAGGAAG-3′, Pgk1 (forward) 5′-GACATCTCCTAGTTTGGACAGTG-3′; Cyclophilin B (forward) 5′-ATGTGGTTTTCGGCAAAGTTCTA-3′, Cyclophilin B (reverse) 5′-GGCTTGTCCCGGCTGTCT-3′.
Chromatin Immunoprecipitation (ChIP) Assays/cells
For each Hep3B sample analyzed in ChIP experiments, we seeded three individual 150 mm plates with 2 × 106 Hep3B cells per plate 48 h prior to use, exposed the cells to normoxia or hypoxia, and then pooled the three biological replicates together for chromatin preparations. We prepared a separate 150 mm plate in parallel for use in assessment of EPO induction after hypoxia exposure, which was confirmed by real-time RT-PCR, and for protein analyses. We performed sequential chromatin immunoprecipitation assays (Re-ChIP) using the Re-ChIP-IT™ magnetic chromatin re-immunoprecipitation kit (Cat. No. 53016, Active Motif). We used the following antisera for the first chromatin immunoprecipitation reaction: normal mouse IgG (1–2 mg mL−1; Cat. No. 2027, Santa Cruz Biotechnology, Inc.), normal rabbit IgG (1–2 mg mL−1; Cat. No. NI01, EMD Chemicals, Inc., Gibbstown, NJ), anti-human HIF-1 (250 μg mL−1; No 610958, BD Biosciences), anti-human EPAS1 (1 mg mL−1; Cat. No. NB 100-132, Novus Biologicals), anti-human p300 (2 mg mL−1; Cat. No. sc-584, Santa Cruz Biotechnology), or anti-human CBP (2 mg mL−1; Cat. No. 7389, Cell Signaling Technology). We used the following antisera for the second chromatin immunoprecipitation reaction: anti-human p300 (2 mg mL−1; Cat. No. sc-584, Santa Cruz Biotechnology), anti-human CBP (2 mg mL−1; Cat. No. 7389, Cell Signaling Technology), anti-human HIF-1 (250 μg mL−1; Cat. No. 610958, BD Biosciences), or anti-human EPAS1 (1 mg mL−1; Cat. No. NB 100-132, Novus Biologicals).
After the sequential ChIP, we analyzed the precipitated genomic DNA by quantitative PCR using an Applied Biosystems ABI Prism 7000 thermocycler (Applied Biosystems, Foster City, CA) and Power SYBR Green Master Mix (Cat. No. 4367659, Applied Biosystems) with the following human EPO enhancer primers: 5′- CTCTGTCCCACTCCTGGCAGCAGTG -3′ (forward) and 5′- CCTTGATGACAATCTCAGCGCACTG-3′ (reverse), or human PGK1 promoter primers: 5′- GGATCTTCGCCGCTACCCTTGTG -3′ (forward) and 5′- CTATTGGCCACAGCCCATCGCGGTC -3′ (reverse), or human RPL13A promoter primers: 5′- GAGGCGAGGGTGATAGAG -3′ (forward) and 5′- ACACACAAGGGTCCAATTC -3′ (reverse). We normalized captured genomic DNA to input material, and compared the normoxic and hypoxic samples.
Chromatin Immunoprecipitation (ChIP) Assays/mice
For mouse ChIP experiments, we exposed 4 Acss2 wild-type and 4 knockout mice to room air (21% oxygen) or hypoxia (6% oxygen) for 2 h as we have done previously 10,11, and then euthanized the mice under normoxic or hypoxic air mixtures to harvest the kidney samples. We minced 30 mg of fresh tissue to 1–3 mm3, transferred tissue into 10 mL of PBS plus 100 μl 1× protease inhibitor cocktail (Cat. No. P8340, Sigma), added formaldehyde (final concentration 1%), and rotated tubes at room temperature for 15 min. We stopped cross-linking with fresh glycine (final concentration 0.125 M). After 5 min at room temperature, we pelleted the samples in a centrifuge at 420 g at 4 °C, washed once with cold PBS plus protease inhibitors, and then repelleted. We resuspended the washed pellet in 1 mL of PBS on ice and ground the tissue using a micro-tissue grinder on ice. We pelleted cells again as above at 4 °C. We carried out ChIP using the ChIP-IT™ Express Magnetic assay kit (Cat. No. 53009, Active Motif). For sequential ChIP experiments, we prepared and analyzed Acss2 wild-type and knockout kidney samples using the reagents as described above. We analyzed precipitated genomic DNA by quantitative PCR in triplicate measurements for each sample using the following mouse Epo enhancer primers: 5′- CTGTACCTCACCCCATCTGGTC -3′ (forward) and 5′- CCCAGCTCACTCAGCACTTGTCC -3′ (reverse), or mouse Pgk1 promoter primers: 5′- GGCATTCTGCACGCTTCAA -3′ (forward) and 5′- GAAGAGGAGAACAGCGCGG -3′ (reverse). We normalized captured genomic DNA to input material and compared the normoxic and hypoxic samples.
Generation of Acss2 knockout mice
For Acss2 knockout (KO) mouse studies, we created Acss2 KO mice using SM-1 mouse embryonic stem cells (129S6/SvEvTac) by disruption of the Acss2 gene using a targeting construct containing ∼5 kb of intronic DNA just upstream of exon 3 and ∼1 kb of DNA just downstream of exon 16 separated by a neomycin resistance drug cassette, Pol II-neo-Bovine pA and two copies of HSV-TK outside the 3′ end of the targeting construct 66. The gene targeting and creation of chimeric mice were previously described 67. We mated chimeric founder mice with C57Bl/6J mice to generate mixed strain heterozygous Acss2 progeny, which maintained by random matings of heterozygous Acss2 mice for more than 10 generations. We then crossed the line with C57Bl6J/129 F1 hybrid mice and maintained on this mixed background for more than 10 generations. We generated mixed strain Acss2 wild-type (WT) and homozygous knockout (KO) mice from matings of heterozygous Acss2 mice, which were fertile and used to generate progeny for study use. We maintained mice under standard conditions (7 am/7 pm light/dark cycle) in the UTSWMC animal facilities and fed them ad lib with standard chow. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
Mouse experiments/hypoxia exposure
For hypoxia exposure experiments, we examined equal numbers of 10–11 week old wild-type versus homozygous Acss2 knockout mice under normoxia (room air, 21% oxygen) or the indicated period of short-term hypoxia exposure (6% oxygen; 0.5, 2, 8 16 h) we have done previously 10,11. After treatment, we euthanized mice and harvested kidneys for further characterization. We derived baseline hematocrits from normoxia mice. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
Mouse experiments/Phenylhydrazine (PHZ)-induced anemia
For Acss2 knockout mice anemia studies, after measuring baseline hematocrits via the tail vein, we injected 3–4 mo Acss2 wild-type (WT) or knockout (KO) mice (6 mice per group) with 40 mg kg−1 of phenylhydrazine hydrochloride (PHZ; Cat. No. 114745-5G, Sigma) in normal saline or an equal volume of normal saline two times at 24 h intervals, with equal numbers of male (4) and female (2) mice in each group. We bled mice from tails to measure hematocrits 48 h (day 2) and 96 h (day 4; nadir) after the initial PHZ injection. For acetate supplements, we administered 400 μL of a sterile 0.5 M stock solution of either PBS (vehicle) and sodium acetate/PBS (A, Ac, NaAc) as a single dose at approximately 9 am on the day of treatment by intra-peritoneal injection to PHZ-treated WT or KO Acss2 mice (three mice per group, all males) with hematocrits between 21% and 25% starting on day 4 following the initial PHZ dose. We measured hematocrits every 3 days beginning on day 4 after the initial PHZ injection, and on the final day of euthanasia when we euthanized mice for organ harvesting. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
For CD-1 mice/acetate treatment acute anemia studies, we injected 7–8 week old (20–24 g) CD1 female mice intra-peritoneal (Charles River Laboratories; Wilmington, MA) with 60 mg kg−1 of PHZ in normal saline (PHZ) or normal saline (control) two times at 24 h intervals. We bled mice from the tails to measure hematocrits 96 h (day 4) after the initial injection. For the PHZ treatment group, we first selected mice according to an acceptable range of anemia (∼21–24%). We then subdivided mice into groups of 5 mice (plus up to 3 extra mice to allow for losses or harvesting of mice during the protocol) for every collection day with similar starting mean hematocrit levels for each group (see Supplemental Table 1). We gavaged anemic mice per os (po) with vehicle (water), glyceryl triacetate (GTA; 90 μL/25 gm body weight; Cat. No. W200700, Sigma-Aldrich Chemicals, Saint Louis, MO) glyceryl tributyrate (GTB; 140 μL/25 gm body weight; Cat. No. W222305, Sigma), or glyceryl tripropionate (GTP; 115 μL/25 gm body weight; Cat. No. W328618, Sigma) using a 20 gauge stainless steel animal feeding tube (Cat. No. FTSS-20S-25, Instech Solomon). We performed gavage once a day in the morning from day 4 to day 20 until euthanasia. We euthanized mice and harvested organs for further characterization on day 4, 7, 10, 13, 16, and 20 after the initial PHZ injection, corresponding to day 0, 3, 6, 9, 12, and 16 of gavage. We gave a control treatment group (solvent injection) - which had approximately equal baseline hematocrits (∼49%) - acetate (GTA) or vehicle (water) by oral gavage once a day starting on day 4 following solvent injection and we harvested the mice on day 4, 7, 10, 13, 16, and 20 after the initial solvent injection. We noted no differences in hematocrit levels in this control group (data not shown).
For intra-peritoneal (ip) short chain fatty acid (SCFA) treatments, we prepared a sterile 0.5 M stock solution of each SCFA using sterile PBS and filter-sterilized using a 0.2 μM filter (Cat. No. 190-2520, Thermo Scientific). We injected mice intra-peritoneally with the indicated SCFA/PBS or vehicle (sterile PBS alone) solution. We did not blind investigators as to PHZ or gavage treatments because of institutional animal monitoring requirements, although we performed harvesting and blood draws of mice using only eartag identifiers. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
Mouse experiments/chronic renal failure (CRF)
Prior to nephrectomy, we collected blood by eye bleeds for baseline hematocrit, plasma creatinine, and blood urea nitrogen (BUN). We performed 5/6 nephrectomy on wild-type CD-1 female mice (n-49, 6 wo, 25-28 g; Charles River, Strain Code 022) 30. Four weeks after nephrectomy, we again collected blood and plasma from the surviving mice (n=33) for analysis. We excluded CRF mice with hematocrits less than 21% or greater than 25%. We supplemented three groups of CRF mice - which exhibited approximately equal composite hematocrit, plasma creatinine, and plasma BUN means (see Supplemental Table 1) - with vehicle alone (Group A), cyclical acetate or vehicle (Group B), or a single week of short chain fatty acids (Group C). For supplements, we administered 400 μL of a sterile 0.5 M stock solution of either PBS (vehicle), sodium acetate/PBS (A, Ac, NaAc), sodium butyrate/PBS (B, Bu, NaBu), or sodium propionate/PBS (P, Pr, NaPr) by thrice-weekly (Monday, M/Wednesday, W/Friday, F) intra-peritoneal single dose injections at approximately 9 am on the day of treatment for the indicated periods. We obtained hematocrits via eye bleeds on the Monday of each week, prior to supplement or as indicated. After completion of the treatment protocol, we harvested blood and kidneys for further characterization after euthanasia. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
Mouse experiments/double heterozygous cKit mutant
We obtained double heterozygous (DH) cKit mutant mice (KitW/KitW-v) on a WBB6 F1 hybrid background (Jackson Laboratories, Stock No. 100410) and wild-type controls from the same colony and housed under standard conditions. For treatments, we obtained baseline hematocrits via eye bleeding, and then we administered 400 μL of a sterile 0.5 M stock solution of either PBS (vehicle), sodium acetate/PBS (A, Ac, NaAc), sodium butyrate/PBS (B, Bu, NaBu), or sodium propionate/PBS (P, Pr, NaPr) for the indicated periods as thrice-weekly (Monday, M/Wednesday, W/Friday, F) intra-peritoneal injections as a single dose at approximately 9 am on the day of treatment. After completion of the treatment protocol, we harvested blood and kidneys for further characterization after euthanasia. The UTSWMC Institutional Animal Care and Use Committee approved all experiments.
Immunohistochemistry
We fixed tissues used for immunohistochemical analyses in 10% neutral-buffered formalin, paraffin-embedded, and sectioned at 5 μm. We mounted sections on poly-L–lysine coated glass slides, baked at 56 °C for 30 min, deparaffinized in xylene, and rehydrated through graded alcohols. We quenched endogenous peroxidase activity by incubating slides in 3% hydrogen peroxide (Cat. No. H1009-100ML, Sigma) in deionized water for 30 min and rinsing in deionized water twice for 5 min. For Acss2 antibody (Cat. No. 3658, Cell Signaling Technology) staining, we incubated slides with the BD Retrievagen A working solution (Cat. No. 550524, BD Biosciences, San Jose, CA) and heated to ∼90 °C (193 ° F) in a microwave oven for 10 min, followed by slow cooling to room temperature over 60 min. We then rinsed slides twice with deionized water, and next rinsed once with PBS for 10 min. We blocked non-specific binding by incubation with 10% goat serum (Cat. No. S-1000, Vector Laboratories, Burlingame, CA) in PBS for 60 min. Next, we incubated slides with Acss2 primary antibody (1:150 in 10% goat serum with PBS) overnight at 4 °C and then with biotin-conjugated goat anti-rabbit IgG (1:500 in 10% goat serum with PBS) for 2 h. After rinsing in PBS, we incubated slides with HRP conjugated streptavadin (1:750 in 10% goat serum with PBS) for 60 min at room temperature. After rinsing in PBS, we visualized immunoreactive deposits by incubation with 3′-amino-9-ethylcarbazole (Cat. No. 00-2007, Invitrogen) for 10 min (kidney sections) or 15 min (liver sections). Finally, we counterstained slides with hematoxylin QS (Cat. No. H-3404, Vector Laboratories) for 2 min, and cover-slipped using Aqua-Polymount (Cat. No. 18606, Polysciences, Inc.).
Plasma Epo protein measurements
To harvest plasma, we mixed 200 μL eye blood with 7.8 μL heparin (Cat. No. NC9593879, Fisher Scientific), incubated 30 min on ice, and spun down at 14,000 g for 10 min. We then removed the supernatant and stored in –80 °C until analysis. For plasma Epo protein measurements, we assayed 25 μL of plasma in duplicates using a Mouse Erythropoietin Quantikine ELISA Kit (Cat. No. MEP00B, R&D System).
Plasma chemistry, hematocrit, and reticulocyte measurements
To measure creatinine and blood urea nitrogen (BUN), we divided 50 μL plasma into two aliquots and measured in duplicates using a Vitros 250 Chemistry System machine (Ortho-Clinical Diagnostics, Inc.) with VITROS CREA or VITROS BUN/UREA slides running on VITROS Chemistry System performed in the UTSWMC Mouse Metabolic Phenotyping Core Facility.
We obtained spun hematocrits from tail-vein or eye bleeds 12. For reticulocyte measurements, we obtained dried blood smears and stained using new methylene blue solution (0.5 g new methylene blue, 1.4 g potassium oxalate, 0.8 g sodium chloride brought to 100 mL using deionized water and filtered using a 0.45 μm filter). We stained slides for 10 min, rinsed three times in PBS for 5 min each, and then cover-slipped. We counted cells and reticulocytes in three fields chosen at random (40× magnification, ∼500 to 1,000 cells/field) and summed to determine reticulocyte percentage.
Statistical analyses
The observations regarding acetate and Acss2 in HIF-2 signaling and Epo regulation are novel. As we collected molecular and biochemical data for the role of acetate/Acss2 switch in regulation of HIF-2 signaling and Epo regulation in Hep3B cells, we anticipated a similar role for the acetate/Acss2 switch in mice. Therefore, we considered the mouse experiments as exploratory studies aimed at identifying a meaningful biological role for acetate and Acss2 in HIF-2 signaling and Epo regulation. Accordingly, we were interested in sample sizes that allowed for detection of a very large effect size 68,69. We used one-way statistical testing in anticipation of similar results in mice as we noted in Hep3B cells. We chose α=0.10 and β=0.20 as an acceptable Type I and Type II statistical error, respectively, and therefore set power=0.80. Given the small sample size, we performed testing for both normal as well as non-normal distributions, but only report statistics for normal distributions.
We used GraphPad Prism and StatPlus to analyze data by t test or Mann-Whitney for two group comparisons and by one-way or two-way ANOVA for multiple comparisons followed by Tukey's or Dunnett's correction to provide coverage for Type I statistical error. When one group was used as a control to compare to all other groups, we used Dunnett's correction to minimize the effect of multiple comparisons on the p-value. Where all comparisons were performed, we used Tukey's correction.
Supplementary Material
Supplementary Figure 1: Acss2 regulates coupled Cbp/HIF-2 recruitment to chromatin.
Supplementary Figure 2: Acss2 acts selectively on HIF-2 signaling in cells.
Supplementary Figure 3: Acss2 is expressed in endocrine Epo synthesis sites in mice.
Supplementary Figure 4: Acetate is required for Cbp/HIF-2α interactions in cells.
Supplementary Figure 5: Acetate facilitates recovery from acute anemia.
Supplementary Figure 6: Acetate augments HIF-2 signaling during acute anemia.
Supplementary Figure 7: Acetate increases erythropoiesis in chronic anemia.
Supplementary Figure 8: Acetate alters HIF-2α acetylation patterns in anemic mice.
Supplementary Table 1: Renal and hematocrit assessment of chronic renal failure mice.
Acknowledgments
We thank R. Hogg, A. Das, J. Colunga, S. Nystrom, and E. Ballard for technical assistance. We thank D. Trono, École polytechnique fédérale de Lausanne for making the lentiviral packaging and envelope plasmids available to us through Addgene.
M.X., J.S.N., and R.C. conducted the majority of experiments; J.L. and R.D.G. contributed to the protein and viral studies; J.X. and C.-L.H. generated the partial nephrectomy chronic renal failure mice and assisted in their evaluation; H.W., Y.A.M., J.D.H. and R.E.H. generated the Acss2 KO mouse model; S.A.C. and R.E.H. developed as well as assisted with Acss2 immunohistochemistry studies; J.A.G. wrote the manuscript and prepared the figures; M.X., J.S.N., R.C., R.D.G., J.X., C.-L.H., J.D.H., S.A.C. and R.E.H. assisted with editing of the manuscript; R.C. and J.A.G. designed and supervised the project as well as analyzed the data.
These studies were supported by funds provided by the US Department of Veterans Affairs (I01BX000446 - J.G.), and the US National Institutes of Health (HL108104 - J.G.; HL20948 - J.H.; DK79328 – C-L.H.).
The abbreviations used are
- A, Ac
acetate
- Acly
ATP citrate lyase
- ACS
acetyl CoA synthetase
- Acss1
acetyl CoA synthetase 1
- Acss2
acetyl CoA synthetase 2
- B, Bu
butyrate
- CBP
Creb binding protein
- Con
control
- CTAD
carboxy (C)-terminal activation domain
- Epo
erythropoietin
- ESRD
end-stage renal disease
- FIH1
Factor Inhibiting HIF-1
- GTA
glyceryl triacetate
- GTB
glyceryl tributyrate
- GTP
glyceryl tripropionate
- HIF
Hypoxia Inducible Factor
- HRE
HIF-responsive element
- IB
immunoblot
- IP
immunoprecipitation
- KO
knockout
- MUT
mutant
- NAM
nicotinamide
- P, Pr
propionate
- PDH
pyruvate dehydrogenase
- PHZ
phenylhydrazine
- PMSF
phenylmethylsulfonyl fluoride
- SCFA
short chain fatty acids
- SD
standard deviation
- SEM
standard error of the mean
- Sirt1
Sirtuin 1
- V, Veh
vehicle
- WT
wild-type
Footnotes
The authors declare no competing financial interests.
References
- 1.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiological Reviews. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 2.Richalet JP. Oxygen sensors in the organism: examples of regulation under altitude hypoxia in mammals. Comparative Biochemistry & Physiology, Part A Physiology. 1997;118:9–14. doi: 10.1016/s0300-9629(96)00370-2. [DOI] [PubMed] [Google Scholar]
- 3.Koury MJ. Erythropoietin: the story of hypoxia and a finely regulated hematopoietic hormone. Exp Hematol. 2005;33:1263–1270. doi: 10.1016/j.exphem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–2019. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- 7.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamme I, et al. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor 1α and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 10.Dioum EM, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 11.Scortegagna M, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 12.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warnecke C, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. Faseb J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 15.Kapitsinou PP, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, et al. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem. 2012;287:30800–30811. doi: 10.1074/jbc.M111.244780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 20.Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- 21.Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2000;275:26458–26466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- 22.Loikkanen I, Haghighi S, Vainio S, Pajunen A. Expression of cytosolic acetyl-CoA synthetase gene is developmentally regulated. Mech Dev. 2002;115:139–141. doi: 10.1016/s0925-4773(02)00097-7. [DOI] [PubMed] [Google Scholar]
- 23.Barth C, Sladek M, Decker K. The subcellular distribution of short-chain fatty acyl-CoA synthetase activity in rat tissues. Biochim Biophys Acta. 1971;248:24–33. doi: 10.1016/0005-2760(71)90071-3. [DOI] [PubMed] [Google Scholar]
- 24.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869–13878. doi: 10.1074/jbc.M110.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshii Y, et al. Cytosolic acetyl-CoA synthetase affected tumor cell survival under hypoxia: the possible function in tumor acetyl-CoA/acetate metabolism. Cancer Sci. 2009;100:821–827. doi: 10.1111/j.1349-7006.2009.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord BI, Murphy MJ., Jr Hematopoietic stem cell regulation. II. Chronic effects of hypoxic-hypoxia on CFU kinetics. Blood. 1973;42:89–98. [PubMed] [Google Scholar]
- 28.Itano HA, Hirota K, Hosokawa K. Mechanism of induction of haemolytic anaemia by phenylhydrazine. Nature. 1975;256:665–667. doi: 10.1038/256665a0. [DOI] [PubMed] [Google Scholar]
- 29.Laderoute KR, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leelahavanichkul A, et al. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 2010;78:1136–1153. doi: 10.1038/ki.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brox AG, Zhang F, Guyda H, Gagnon RF. Subtherapeutic erythropoietin and insulin-like growth factor-1 correct the anemia of chronic renal failure in the mouse. Kidney Int. 1996;50:937–943. doi: 10.1038/ki.1996.394. [DOI] [PubMed] [Google Scholar]
- 32.Russell ES. Analysis of pleiotropism at the W-locus in the mouse; relationship between the effects of W and Wv substitution on hair pigmentation and on erythrocytes. Genetics. 1949;34:708–723. doi: 10.1093/genetics/34.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabaya K, et al. Improvement of anemia in W/WV mice by recombinant human erythropoietin (rHuEPO) mediated through EPO receptors with lowered affinity. Life Sci. 1995;57:1067–1076. doi: 10.1016/0024-3205(95)02052-k. [DOI] [PubMed] [Google Scholar]
- 34.Tonia T et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012;12:CD003407. doi: 10.1002/14651858.CD003407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y. Erythropoietin in cancer: a dilemma in risk therapy. Trends Endocrinol Metab. 2013;24:190–199. doi: 10.1016/j.tem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhou B, et al. Erythropoietin promotes breast tumorigenesis through tumor-initiating cell self-renewal. J Clin Invest. 2014;124:553–563. doi: 10.1172/JCI69804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita H, Kaneyuki T, Tagawa K. Production of acetate in the liver and its utilization in peripheral tissues. Biochim Biophys Acta. 2001;1532:79–87. doi: 10.1016/s1388-1981(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol. 2006;33:961–967. doi: 10.1111/j.1440-1681.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 39.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 40.Rabkin M, Blum JJ. Quantitative analysis of intermediary metabolism in hepatocytes incubated in the presence and absence of glucagon with a substrate mixture containing glucose, ribose, fructose, alanine and acetate. Biochem J. 1985;225:761–786. doi: 10.1042/bj2250761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crabtree B, Gordon MJ, Christie SL. Measurement of the rates of acetyl-CoA hydrolysis and synthesis from acetate in rat hepatocytes and the role of these fluxes in substrate cycling. Biochem J. 1990;270:219–225. doi: 10.1042/bj2700219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crabtree B, Marr SA, Anderson SE, MacRae JC. Measurement of the rate of substrate cycling between acetate and acetyl-CoA in sheep muscle in vivo. Effects of infusion of acetate. Biochem J. 1987;243:821–827. doi: 10.1042/bj2430821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crabtree B, Newsholme EA. Sensitivity of a near-equilibrium reaction in a metabolic pathway to changes in substrate concentration. Eur J Biochem. 1978;89:19–22. doi: 10.1111/j.1432-1033.1978.tb20891.x. [DOI] [PubMed] [Google Scholar]
- 44.McBrian MA, et al. Histone acetylation regulates intracellular pH. Mol Cell. 2013;49:310–321. doi: 10.1016/j.molcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon MJ, Crabtree B. The effects of propionate and butyrate on acetate metabolism in rat hepatocytes. Int J Biochem. 1992;24:1029–1031. doi: 10.1016/0020-711x(92)90369-c. [DOI] [PubMed] [Google Scholar]
- 46.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lando D, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bracken CP, et al. Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006;281:22575–22585. doi: 10.1074/jbc.M600288200. [DOI] [PubMed] [Google Scholar]
- 50.Wu DC, Paulson RF. Hypoxia regulates BMP4 expression in the murine spleen during the recovery from acute anemia. PLoS One. 2010;5:e11303. doi: 10.1371/journal.pone.0011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball AM, Winstead PS. Recombinant human erythropoietin therapy in critically ill Jehovah's Witnesses. Pharmacotherapy. 2008;28:1383–1390. doi: 10.1592/phco.28.11.1383. [DOI] [PubMed] [Google Scholar]
- 52.Mansell MA, Wing AJ. Acetate or bicarbonate for haemodialysis? Br Med J (Clin Res Ed) 1983;287:308–309. doi: 10.1136/bmj.287.6388.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki M, Hirasawa Y. Correction of metabolic acidosis and changes in plasma acetate levels in acetate and bicarbonate dialyses and acetate-free biofiltration. Contrib Nephrol. 1994;108:114–120. doi: 10.1159/000423364. [DOI] [PubMed] [Google Scholar]
- 54.Veech RL, Gitomer WL. The medical and metabolic consequences of administration of sodium acetate. Adv Enzyme Regul. 1988;27:313–343. doi: 10.1016/0065-2571(88)90024-6. [DOI] [PubMed] [Google Scholar]
- 55.Masuda A, et al. Effects of acetate-free citrate dialysate on glycoxidation and lipid peroxidation products in hemodialysis patients. Nephron Extra. 2012;2:256–268. doi: 10.1159/000342258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grover A, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J Exp Med. 2014;211:181–188. doi: 10.1084/jem.20131189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ariyannur PS, et al. Nuclear-cytoplasmic localization of acetyl coenzyme a synthetase-1 in the rat brain. J Comp Neurol. 2010;518:2952–2977. doi: 10.1002/cne.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 60.Kojima I, et al. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol. 2007;18:1218–1226. doi: 10.1681/ASN.2006060639. [DOI] [PubMed] [Google Scholar]
- 61.Sun D, Melegari M, Sridhar S, Rogler CE, Zhu L. Multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. Biotechniques. 2006;41:59–63. doi: 10.2144/000112203. [DOI] [PubMed] [Google Scholar]
- 62.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci U S A. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- 64.Aprelikova O, et al. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- 65.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 66.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 67.Shimano H, et al. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 69.Denenberg VH. A Primer for Behavioral Research. Mental Retardation and Developmental Disabilities Research Reviews. 1996;2:209–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Acss2 regulates coupled Cbp/HIF-2 recruitment to chromatin.
Supplementary Figure 2: Acss2 acts selectively on HIF-2 signaling in cells.
Supplementary Figure 3: Acss2 is expressed in endocrine Epo synthesis sites in mice.
Supplementary Figure 4: Acetate is required for Cbp/HIF-2α interactions in cells.
Supplementary Figure 5: Acetate facilitates recovery from acute anemia.
Supplementary Figure 6: Acetate augments HIF-2 signaling during acute anemia.
Supplementary Figure 7: Acetate increases erythropoiesis in chronic anemia.
Supplementary Figure 8: Acetate alters HIF-2α acetylation patterns in anemic mice.
Supplementary Table 1: Renal and hematocrit assessment of chronic renal failure mice.