Summary
Objectives
Monoamine oxidase A (MAOA) modulates metabolism of serotonin and dopamine metabolism, neurotransmitters involved in regulation of appetite and food intake. The gene coding for MAOA contains a 30-bp tandem repeat (uVNTR) polymorphism in its promoter region that has been previously identified to be associated with obesity with mixed findings in the literature. Our goals were to replicate the population effects of this functional polymorphism on obesity risk, and to further explore gender differences and interaction effects with negative stressors.
Methods
Analyses were conducted with data on genotypes, measured weight and height, and self-reported behavioral characteristics among 1,101 Chinese adolescents 11-15 years old living in Wuhan, China.
Results
Girls with the high activity allele had significantly lower BMI (β=-0.25±0.98, p=0.011) compared to those with the low activity allele. Experience of negative familial stressors(e.g., death or illness of family members, hit or scolded by parents and increased quarreling with parents, parents argued frequently) significantly weakened this protective genetic effect on BMI (p for interaction=0.043). Stratified analyses showed a significant protective genetic effect on BMI only within the stratum of low stress level (β=-0.44±0.14, p=0.002). No similar effect was observed among boys.
Conclusions
Our findings confirm the genetic effects of MAOA uVNTR polymorphism on BMI in a Chinese adolescent population and suggest potential genetic interactions with negative familial stressors.
Keywords: MAOA Polymorphism, Familial Stressors, BMI, Chinese Adolescents, Gene-Environment Interaction
Introduction
Obesity has emerged as a worldwide epidemic and is a public health problem in many industrialized countries and recently in developing countries undergoing rapid economic transition, such as China(1). An alarming increase in pediatric obesity occurred between 1985 and 2006 with the prevalence of overweight and/or obesity of 15.4% in 7- to 17-year old boys and 11% in girls(2, 3). The prevalence is even higher in the developed metropolitan areas of China(4, 5). Obese children and adolescents are more likely to maintain their obesity into adulthood, which may contribute to enhanced morbidity and susceptibility to chronic diseases such as cardiovascular disease, diabetes, and certain cancers in later life (6-8).
Susceptibility to obesity is determined by both joint effects of genetic and environmental factors with existence of potential complex gene by environment interactions, in which environmental factors may prompt gene-regulated phenotype expression. In previous studies, chronic stress due to either stressful life events or daily hassles has been linked to the etiology of obesity as a promoting factor by interacting with both mechanisms of energy intake (increase of appetite and energy intake) and expenditure (decrease of physical activity) (9-11). The underlying biological mechanisms may largely be attributed to the involvement of dopamine and serotonin in regulation of mood, diet, physical activity and inactivity. Monoamine oxidase A (MAOA) is one of the major enzymes responsible for the degradation of neurotransmitters including serotonin (5-HT) and dopamine (DA), rendering them inactive in the synapses of the brain (12, 13). Dysregulation in brain monoamine levels may potentially influence energy balance as these amines are associated with feeding behaviors and enjoyment of food (14, 15). The MAOA linked polymorphic region (MAOA-LPR) has been identified to affect the MAOA transcriptional activity and contains a 30-bp variable number tandem repeat (uVNTR) polymorphism in its promoter region (16-19). The 30-bp repeated sequence presents in 2, 3, 3.5, 4, or 5 copies; alleles with 3.5 or 4 repeats have been reported to be 2–10 times more efficient than those with 3 copies in transcription (16, 20, 21). Several recent studies indicate initial evidence of a genetic effect such that the high-activity MAOA uVNTR polymorphism may be associated with lower risks of obesity and decreased BMI even though findings were varied across populations (22-25). Although recent genome-wide association studies (GWAS) for obesity did not find evidence for MAOA among populations with European origins, there is a need for studies on various ethnic groups as well as for tests of gene-environment interactions. Replication studies with population-based samples are clearly warranted and necessary to quantify the population effects of relevant genetic variants to the risk of obesity. Furthermore, additional investigation to identify complex gene by environment interactions is crucial to gain insight on the causes of obesity, and thus, potential avenues of prevention.
Stress, especially academic stress, family conflict and disruption of family life, among Chinese adolescents has become an increasing problem that is associated with depression, anxiety, conduct disorders, suicide attempts and drug abuse (26-29). Limited research has focused on the investigation of population-level gene-environmental effects of stress and genetic variants on obesity among adolescents (11, 30). In this paper, we aimed to replicate the population effects of the functional polymorphism of the monoamine-regulating gene on risks of obesity among Chinese adolescents 11-15 years old. In addition, we further explored gender differences and interactive effects with multiple-domains of negative stressors and hypothesized that exposure to negative stressors would prompt gene-regulated obesity.
Methods
Sample and Data
Secondary data were retrieved from the Wuhan Smoking Prevention Trial (WSPT) for Chinese adolescents in Wuhan, China, through two consecutive cycles of the Pacific Rim Transdisciplinary Tobacco Use Research Center (TTURC). During the first grant cycle (1999-2004), a school-based randomized control trial was implemented in 1999 with 7th grade students living in Wuhan, China to evaluate the effectiveness of a culturally appropriate school and family smoking prevention curriculum with a social normative approach for Chinese adolescents (31). Design and procedures of the prevention trial, school selection, and recruitment have been described in detail in two previous papers with baseline data (32) and intervention outcomes (31). The original cohort at baseline in 1999 consisted of 7th grade students randomly selected from 22 middle schools in urban and rural Wuhan with four classes randomly selected from each school. Among the selected classes in each school, two classes were further randomly selected to compose a subcohort for measurement of body weight and height at baseline. A total of 2179 (1156 boys and 1023 girls) healthy Chinese adolescents aged from 11 to 15 years (12.9± 0.7) completed weight and height measures. Socio-demographic, psychological and behavioral characteristics at baseline were measured with self-reported questionnaires. Questionnaire items were translated from English to Mandarin, then back translated to English by translators fluent in both languages and trained in behavioral research. Buccal cell samples were collected from students in 1999 as a source of DNA (33). A total of 1,101 students (530 girls and 571 boys) with genetic data, and measured weight, height and behavioral characteristics were included for the analysis in this paper. The study was approved by the Institutional Review Boards at both the University of Southern California and Wuhan Anti-Epidemic Station (now the Wuhan Center of Disease Control and Prevention).
Measures
Obesity Phenotype
Measurements of height and weight were collected using a standard calibrated scale and stadiometer, with subjects wearing light clothes with either thin socks or no shoes. Body weight was measured in pounds and subsequently converted to kilograms. Height was recorded to the nearest 0.1 centimeter. Body mass index (weight in kilograms divided by height in meters squared) was used to quantify underweight, overweight and obesity status. The BMI age-gender-specific reference chart recommended by the International Obesity Task Force (IOTF) reference for international populations was used to quantify overweight and obesity status (34). Age- and gender-specific BMI Z scores, which have been widely used in the pediatric obesity literature as optimal measures for adiposity in childhood and adolescence, were used for the analysis (35-37).
Stressful Life Events
Stressful life events were assessed through a checklist of 99 items, that were grouped into categories based on their domain (school, family, peers, and self) and valence (positive or negative). Positive events were those generally considered pleasant, while negative events were those generally considered unpleasant. Students were asked to indicate which of the events they had experienced in the past 6 months. For each event they experienced, they were asked to rate the severity of the event on a 4-point Likert-type scale ranging from 0 (no effect) to 3 (severe). Descriptions of the development of the checklist and items in each scale can be found elsewhere (29). To generate items for the life events scale, a pilot study was conducted with 75 male and 74 female seventh- to ninth-grade adolescents from three classrooms in a school in Wuhan, China (mean age of 14.0 years){Unger, 2001 #522}. Students were asked to generate open-ended responses to the questions measuring daily hassles or pleasures, and major life events during the past 6 months of their life, translated from Compas, Davis, Forsythe, and Wagner {Compas, 1987 #695}. Daily hassles can be events that irritate, annoy, or upset them or can cause problems, pressures, or difficulties for them. Daily pleasures can be events which made them feel happy, joyful, or at peace, which can happen once, twice, or many times during a month. Major positive or negative life events included those events which have had a large effect on their life or led to changes in how they feel about themselves, their health or well-being, their relationships with other people, or how well they did at school. Each of these major events had probably happened only once during the last 6 months but had a large effect on them when it occurred. All events listed by the students then were examined for content and were coded by two bilingual undergraduate students. Events that were similar in meaning but had slightly different wording were reworded to avoid generating numerous redundant life events. The initial inter-rater reliability was 91%. The two students discussed all the items they had coded differently and arrived at consensus for each item. Then these items were further examined by school staff and public health officials in Wuhan, China, who made minor wording modifications (to ensure that the content would be understandable by Chinese youth), combined several categories that seemed redundant, and added several new categories based on their previous research and discussions with students in Wuhan. The final list of life events was used as a life event checklist for the large-scale surveys.The domains of school, family, peers and individual (self) were chosen because they represent the most frequently reported sources of stress among Chinese adolescents (29, 38). This instrument measuring stressful life events was used to predict significant depressive symptoms as they related to smoking and alcohol use in this Wuhan adolescent cohort (26, 29). In this paper, we narrowed our analyses to focus on negative stressors (or stressful events), which included negative school stressors (e.g., too much homework, disliked school), negative family stressors (e.g., death or illness of family members, hit or scolded by parents and increased quarreling with parents, parents argued frequently), negative peer stressors (e.g., misunderstood by peers, lost face in public), negative health stressors (e.g., have taken sedative medicine, not had enough sleep), and negative violence stressors (e.g., were shocked by an event, witnessed a car accident). For each category of stressor, each student's score was the number of events in the category that the student reported. The unit weighting method (giving each event an equal weight rather than weighting items by their severity) was used because unit weighting methods produce smoother scale distributions and tend to correlate highly with weighted scales (39), and the scales weighted by event severity tended to have skewed distributions. Both average scores and a median-split (low vs. high level) of average scores were created for each category of negative stressors.
MAOA Promoter uVNTR Polymorphism
DNA was successfully extracted from all buccal cell samples (33). The GeneScan method was used to determine the allele size of MAOA Promoter uVNTR Polymorphism. The MAOA promoter 30-bp repeat was amplified by PCR with oligonucleotide primers: VICMAOA_HermanF (5′-CAGAAACATGAGCACAAA CGCCTCAGC-3′) which was labeled with VIC and MAOA_mHermanR (5′-GACCGCCACTCAGAACGGACG-3′) (40). PCR reaction contained 50 ng of genomic DNA, 200nM of each primer, 1.25 unit of Amplitaq Gold polymerase from ABI with Premix Buffer E from Epicentre Biotechnologies in a total volume of 20 μ1. Cycling conditions included initial denaturation at 94 °C for 10 min, followed by 32 cycles of 34s at 94 °C, 34 s at 62 °C, 1.5min at 72 °C, and a final elongation step for 7 min at 72 °C. PCR products were assayed on Applied Biosystems 3130xl Genetic Analyzer with GS500 LIZ size standard (35-500 bp, ABI). Results were analyzed using GeneMarker v1.5 (SoftGenetics) software.
In addition, covariates considered in the analysis consisted of a student's self-reported age, pubertal status, residence (urban or rural), and report of parent's education levels. Pubertal status was assessed with the following questions: “How old were you when you had your first period? (for girls)”, and “How old were you the first time that your Adam's apple got bigger, or that your voice changed, or that you had started to grow a beard (for boys)?“. Subjects who reported “Hasn’t happened yet” were defined as “pre-pubertal”, while subjects giving a specific age were defined as “pubertal” (41, 42). Father's and mother's education levels were obtained by collapsing the highest levels of education received by either father or mother into three categories: less than high school, high school and college or above.
Data Analysis
Descriptive statistics (mean, standard deviation and percentage) were calculated to reflect the background characteristics of the sample. Alleles of MAOA uVNTR were categorized by the relative transcriptional activity into two categories, high function (3.5 or 4 repeats) versus low function (3 repeats or other alleles) according to the extant literature on this functional polymorphism of MAOA gene (15, 24, 25). As the effect of the rare alleles (2 or 5) on transcriptional activity remains inconclusive in the literature, sensitivity analyses were conducted with data excluding the 21 participants with the rare genotypes (16, 20).
Age- and gender-specific BMI Z scores were used for the association analyses of genetic main effects and gene-environment interactions. Considering the potential gender difference in the genetic effect of MAOA, only gender-specific analyses were performed for the MAOA uVNTR. In boys, the BMI Z score of the carriers of the high activity alleles (3.5 or 4 repeats) were compared to that of the low activity allele carriers (3 repeats or other alleles). In girls, the heredity model of MAOA uVNTR remains unclear, and we grouped the carriers of the low activity allele, whether heterozygote or homozygote, and compared it to carriers of two high activity alleles (4R/4R or 4R/3.5R). This grouping strategy was based on the results of two previous investigations(43, 44). This grouping strategy was applied to both the marginal main effects and interactions of various domains of negative stressors with MAOA genotype on BMI.Mixed-effect models were used in the analysis with adjustment for the intra-class correlations due to the nested study sampling design (i.e. students nested within schools).
When a P value was less than 0.05 for the genotype-stressor interaction term was observed, an additional 2-degrees of freedom likelihood ratio test on both main genotype effect and its interaction with stressors was performed to investigate the overall role of the MAOA uVNTR in the phenotype. To clarify the interpretation of significant interaction effects, stratified analyses were conducted to get simple main effects within stratum of low and high stress levels. Age, pubertal status, and parental education were controlled as covariates in all models. All statistical analyses were carried out using SAS (version 9.1; SAS Institute, Cary, NC) and R (version 2.12.2; R Foundation for Statistical Computing).
Results
The general characteristics of this sample are summarized in Table 1 below. The majority of students were aged 12 or 13 years old, had entered puberty, had parental education attainment at high school, college level or above, and were classified as non-smokers, non-drinkers or being not overweight/obese. On average, boys were slightly older than girls (12.8±0.6 vs. 12.7±0.6, p=0.0003). In comparison to boys, relatively more girls reported having entered puberty, and relatively fewer girls were classified as being overweight or obese (p=0.03) or as occasional/regular smokers (p<0.0001). Significant gender differences in distributions of all categories of negative stressors were observed, in which girls reported slightly more experience with negative stressors than boys did.
Table 1. General Characteristics of the Sample.
| Overall (n=1,101) | Female (n=530) | Male (n=571) | p Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n(%) | n(%) | n(%) | |||||
| Age (years) | |||||||
| 11 | 21(1.9%) | 13(2.5%) | 8(1.4%) | <0.001 | |||
| 12 | 302(27.4%) | 162(30.6%) | 140(24.5%) | ||||
| 13 | 733(66.6%) | 346(65.3%) | 387(67.8%) | ||||
| 14 | 40(3.6%) | 6(1.1%) | 34(6%) | ||||
| 15 | 5(0.5%) | 3(0.6%) | 2(0.4%) | ||||
| Parental Education | |||||||
| Below High school | 153(13.9%) | 71(13.4%) | 82(14.4%) | 0.803 | |||
| High School | 637(57.9%) | 305(57.5%) | 332(58.1%) | ||||
| College or above | 311(28.3%) | 154(29.1%) | 157(27.5%) | ||||
| Pubertal Status | |||||||
| Pre-peri puberty | 481(43.7%) | 191(36%) | 290(50.8%) | <.0001 | |||
| Post-puberty | 620(56.3%) | 339(64%) | 281(49.2%) | ||||
| Cigarette Smoking | |||||||
| Non-smoker | 928(84.3%) | 486(91.7%) | 442(77.4%) | <.0001 | |||
| Occasional/regular smoker | 173(15.7%) | 44(8.3%) | 129(22.6%) | ||||
| Alcohol Drinking | |||||||
| Non-drinker | 954(86.7%) | 463(87.4%) | 491(86%) | 0.505 | |||
| Occasional/regular drinker | 147(13.4%) | 67(12.6%) | 80(14%) | ||||
| Overweight Status | |||||||
| Not overweight or obese | 933(84.7%) | 462(87.2%) | 471(82.5%) | 0.031 | |||
| Overweight or obese | 168(15.3%) | 68(12.8%) | 100(17.5%) | ||||
|
| |||||||
| mean(SD) | mean(SD) | mean(SD) | p Value | ||||
| BMI | 19.2(2.9) | 19.2(2.9) | 19.2(3) | 0.979 | |||
| BMI Z score | 0.1(0.9) | 0.1(0.9) | 0.1(1) | 0.410 | |||
| Negative Stressors (mean) | |||||||
| School | 0.8(0.4) | 0.8(0.4) | 0.7(0.4) | 0.003 | |||
| Family | 0.4(0.3) | 0.4(0.3) | 0.4(0.3) | 0.020 | |||
| Peer | 0.7(0.6) | 0.8(0.6) | 0.6(0.6) | <.0001 | |||
| Violence | 0.7(0.6) | 0.8(0.7) | 0.7(0.6) | 0.015 | |||
| Health | 0.6(0.5) | 0.7(0.5) | 0.5(0.4) | <.0001 | |||
| Total | 0.6(0.4) | 0.7(0.4) | 0.6(0.3) | <.0001 | |||
Allele frequencies of the MAOA polymorphisms for both boys and girls were 0.39 for 4 repeats, 0.60 for 3 repeats, 0.0006 for 3.5 repeats and 0.01 for other alleles. The allele frequencies fell within expected ranges, based on prior published reports in Asian and Chinese populations (45, 46). There was no deviation from Hardy-Weinberg Equilibrium (p>0.05). Genotype frequencies by gender and the bivariate associations with overweight status and BMI Z scores are summarized in Table 2. Among girls carrying genotypes of 4R/4R or 4R/3.5R, 2 out of 69 (2.94%) were overweight, comparing to the observation of 23 out of 196 (33.82%) being overweight among those 3R/3R or 3R/other carriers (OR=0.23 with 95% CI of 0.05-0.98, p=0.047). Across the entire BMI distribution, girls with genotypes of 4R/4R or 4R/3.5R had significantly lower BMI Z scores than those 3R/3R or 3R/others carriers (p=0.006). The distribution of weight status and BMI Z scores were not significantly different between boys with active alleles compared to their counterparts with low-active alleles.
Table 2. Genotype Frequencies of MAOA VNTR Polymorphisms and Bivariate Associations.
| Overall | Not Overweight | Overweight | OR (95% CI)* | p Value** | BMI Z score | P-value*** | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n(%) | n(%) | n(%) | Mean (SD) | ||||
| MAOA (Female) | |||||||
| 4R/4R or 4R/3.5R | 69 (13.02) | 67(14.50) | 2(2.94) | 0.23(0.05-0.98) | 0.047 | -0.22(0.90) | 0.006 |
| 4R/3R or 4R/Others | 265 (50.0) | 224(48.05) | 41(63.24) | 1.38(0.80-2.38) | 0.228 | 0.12(0.89) | 0.897 |
| 3R/3R or 3R/Others | 196 (36.98) | 173 (37.44) | 23(33.82) | 1 | - | 0.11(81) | - |
| MAOA (Male) | |||||||
| 4R | 339(59.37) | 278(58.28) | 61(59.81) | 0.98(0.63-1.52) | 0.921 | 0.09(0.96) | 0.65 |
| 3R or others | 232 (40.63) | 191(40.72) | 41(40.19) | 1 | 0.13(0.96) | ||
OR vs 3R/3R or 3R/Other category
P-value of Chi-square test or fisher's exact test
p-value compared to 3R/3R or 3R/other category
Table 3 presents results of marginal main effects of negative stressors and MAOA uVNTR polymorphisms on BMI Z scores. Girls who were exposed to high-levels of negative health stressors had significantly higher BMI Z scores (β=0.15±0.07, p=0.03) than their counterparts with low level exposure to negative health stressors. Moreover, girls with the high-activity allele had significantly lower BMI Z scores (β=-0.25±0.98, p=0.011) than those with the lowactivity allele. On the other hand, a significant effect of negative stressors on BMI Z scores was not detected in boys.
Table 3. Marginal Main Effects of Stressors and MAOA VNTR Polymorphisms on BMI with adjustment for covariates.
| Female | Male | |||
|---|---|---|---|---|
|
| ||||
| β (SE) | p | β (SE) | p | |
| Negative Stressors’ Effects on BMI Z Scores | ||||
| School | 0.03(0.07) | 0.648 | 0.06(0.08) | 0.416 |
| Family | 0.05(0.07) | 0.428 | -0.005(0.08) | 0.953 |
| Peer | 0.05(0.07) | 0.467 | 0.05(0.08) | 0.550 |
| Violence | 0.03(0.07) | 0.658 | -0.03(0.08) | 0.696 |
| Health | 0.15(0.07) | 0.028 | 0.09(0.08) | 0.284 |
| Total | 0.10(0.07) | 0.133 | 0.01(0.08) | 0.857 |
| MAOA VNTR | -0.25(0.98) | 0.011 | -0.03(0.080) | 0.697 |
Age, pubertal status, and parental education were adjusted in the models.
Median-split of average scores of negative stressors were used (0 for low and 1 for high level of stressors).
MAOA VNTR polymorphisms were coded as 1 for high activity (3.5 or 4 repeats in males; 4/4 or 4/3.5 genotypes in females) and 0 for low activity (3 or other alleles in males; 3/3, 3/other or 4/other in females).
In addition, a significant genotype X stressors interaction was observed between MAOA uVNTR polymorphism and exposure to negative familial stressors among girls (Table 4 and Figure 1). Experience of negative familial stressors significantly weakened the protective genetic effect on BMI Z scores (β=0.40±0.20 for interaction, p-value for interaction=0.043). The significance of interaction remained in the two-degrees of freedom likelihood ratio tests for BMI Z scores (p=0.009 for likelihood ratio test; β for gene main effect=-0.45±0.14, p=0.001; β for gene X stressors interaction=0.40±0.20, p=0.043). Stratified analyses showed a significant protective genetic effect on BMI Z score only within the stratum of low stress level (β=-0.44±0.14, p=0.002).Among boys, the interaction term between gene and exposure to negative violence stressors was significant (p=0.04), but the significance disappeared in the two-degrees of freedom likelihood ratio test (p=0.07). Consistent results were observed in the sensitivity analysis after excluding the 21 individuals with rare alleles (data not shown). In our study sample, there were 32 (2.91%) underweight adolescents including 14 (2.64%) girls and 18 (3.15%) boys. We re-ran the analysis with data excluding these underweight cases, and similar results were observed (data not shown).
Table 4. Genotype X Stressors Interaction Effects on BMI.
| Female | Male | |||
|---|---|---|---|---|
|
| ||||
| β (SE) | p | β (SE) | p | |
| MAOA X Negative Stressors Effects on BMI Z Scores | ||||
| School | 0.15(0.20) | 0.440 | -0.12(0.16) | 0.465 |
| Family | 0.40(0.20) | 0.043 | -0.14(0.16) | 0.383 |
| Simple main effect in low stress level stratum | -0.44(0.14) | 0.002 | ||
| Simple main effect in high stress level stratum | -0.06(0.14) | 0.66 | ||
| Peer | 0.22(0.20) | 0.277 | -0.16(0.16) | 0.329 |
| Violence | 0.29(0.20) | 0.144 | 0.33(0.16) | 0.04 |
| Health | -0.22(0.20) | 0.258 | 0.19(0.16) | 0.245 |
| Total | 0.15(0.20) | 0.444 | -0.01(0.16) | 0.946 |
Age, pubertal status, and parental education were adjusted in the models.
Pubertal status was not included in the model.
Median-split of average scores of negative stressors were used (0 for low and 1 for high level of stressors).
MAOA VNTR polymorphisms were coded as 1 for high activity (3.5 or 4 repeats in males; 4/4 or 4/3.5 genotypes in females) and 0 for low activity (3 or other alleles in males; 3/3, 3/other or 4/other in females).
Figure 1. Interaction between the MAOA uVNTR and negative family stressor in females*.
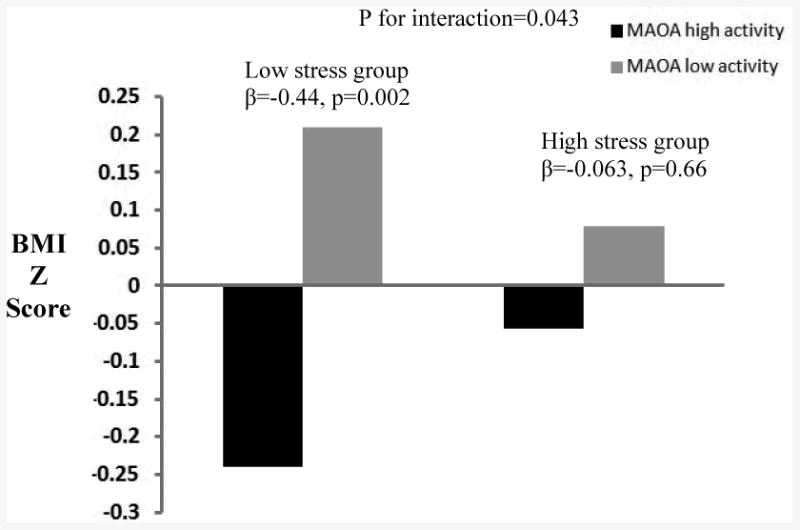
*Age, pubertal status, and parental education were adjusted in the models.
Median-split of average scores of negative stressors were used (0 for low and 1 for high level of stressors). MAOA VNTR polymorphisms were coded as 1 for high activity (3.5 or 4 repeats in males; 4/4 or 4/3.5 genotypes in females) and 0 for low activity (3 or other alleles in males; 3/3, 3/other or 4/other in females).
Discussion
The first major finding of our study was the significant effects of negative health stressors on BMI. Exposure to negative health stressors resulted in higher BMI for girls only. We also looked at the frequency distributions of each negative health stressor. The percentages of having the following negative health stressors were: 53.8% for changes of the body during puberty, 47.9% for having gained weight, 45.0% for not having enough time to play or relax, 44.7% for not having enough sleep, 20.88% for having lost weight, 14.3% for having had acute/serious disease(s), 12.13% for having had accident(s), and 1.46% for taking sedative medicine. Our results were consistent with De Vriendt et al's recent report from the Healthy Lifestyle in Europe by Nutrition in Adolescence cross-sectional study, in which perceived stress was significantly associated with increased measures of general and abdominal adiposity in girls only {De Vriendt, 2012 #706}. Although a well-defined association is not described in the literature, chronic stress may be a factor interfering with energy balance and the maintenance of weight status, that is, an increase of appetite and energy intake and decrease of physical activity (9-11). It is widely accepted that diverse threatening stimuli or stressors could have an effect on the activation of major stress pathways or brain systems, such as the hypothalamic-pituitary-adrenal (HPA) axis and the sympathoadrenal medullary (SAM) systems, that would result in increased neuroendocrine and autonomic arousal and negative moods, leading to the use of substances like food, nicotine, and alcohol to self-medicate the resultant distress (47, 48). These neurotransmitter systems could account for a neuroendocrine connection between stress and food intake regulation, and are hypothesized to regulate behavioral and metabolic responses associated with the development of obesity (49). Even though we did not find significant results in all categories of negative stressors, significant findings from our analysis may provide preliminary evidence linking negative health stressors to the risk of obesity. Future research on biomarkers of neurotransmitters as well as food consumption and other weight-regulated behaviors may help clarify the pathways from stressors to the development of obesity.
Our second major finding is significant association of MAOA genotype with BMI among girls only. Specifically, girls with the high-activity allele had significantly lower BMI. In a limited but growing body of literature, the association of variation within the MAOA gene with obesity phenotypes was supported by a whole genome linkage study implicating a locus for obesity on the p arm of the X chromosome (50), as well as a study in which low activity MAOA alleles were associated with obesity in one familial data set (22). More interestingly, significant association was also detected between the MAOA gene polymorphism and BMI in a large cohort of Caucasian females, with the high-activity uVNTR genotype being less frequent(23). Consistent with those investigations, our study provides additional evidence on the potential role of MAOA uVNTR in BMI. The fact that the effect of MAOA uVNTR was observed only in females is intriguing. This finding is consistent with Need et al (23) in which the association was also observed among females only. However several other studies also reported the associations of the high-activity MAOA uVNTR polymorphism with lower risks for obesity and decreased BMI although the association was observed in males only(22-25). Ducci et al reported a significant association between BMI and the MAOA uVNTR polymorphism, with the low-activity allele associated with a higher BMI among a sample of primarily non-obese male participants with and without a history of alcohol dependence (24). In subsamples of the National Longitudinal Study of Adolescent Health (Add Health), significant associations were found between the MAOA promoter uVNTR and categories of BMI (obese and overweight + obese) among White and Hispanic, but not African-American, males in a US cohort of young adolescents and adults (25). The potential mechanism of the observed gender difference is still unclear. Some studies suggest that an age-related effect of ovarian hormones (such as estrogen) on MAOA gene expression may explain the observed findings in the literature. Specifically, ovarian steroids can decrease MAOA expression, which, in turn, may lead to an elevated level of serotonin in circulation (25, 51, 52). Furthermore, there is also evidence on sex differences in the way that serotonin influence the development of obesity and body fat distribution (53). Complex biological crosstalk might lead to different effects of the MAOA uVNTR in different genders, particularly in different age groups. More studies, preferably with larger sample sizes and both genders, are warranted to further investigate the role of MAOA uVNTR in different genders.
Finally, our findings provide a new insight into potential genotype interactions with negative familial stressors that require replication in future research. The protective effects of the high-activity MAOA genotype on BMI in girls were contingent on exposure to negative familial stressors, in which negative familial stressors significantly weakened this protective genetic effect. Very limited efforts in the past have been made to explore potential gene by environment interaction in this literature. Fuemmeler et al reported a significant MAOA genotype by depressive symptoms interaction from their analyses of the Add Health data (15). In their study, males with a MAOA high-active allele and high depressive symptoms were at decreased risk for obesity (OR 0.22; 95% CI 0.06–0.78) and overweight plus obesity (OR 0.48; 95% CI 0.26–0.89) (15). Other reports on gene-environment interactions were mainly from the research on the role of MAOA in the development of antisocial behaviors (54-56). Carriers of low-activity MAOA alleles with adverse experiences early in life, such as childhood maltreatment, were more likely to develop antisocial problems in their later lives (54-56). To our knowledge, our study is the first to demonstrate a significant interaction between the MAOA uVNTR genotype by negative stressors as they related to risk for obesity. Further replication studies are needed to confirm our results.
Before definitive conclusions can be made about the role negative stressors and MAOA have on regulating weight and risk of obesity, we acknowledge certain limitations of our study. While we focused our investigation on a single functional polymorphism in a fairly large and homogeneous sample (i.e. Chinese Han ethnicity only), we must consider the genotype by stressors interaction as preliminary due to a lack of replication. In addition, we did not have measures to determie abdominal adiposity, such as waist circumference, or other markers of total adiposity such as body fat mass measured by skinfold thickness or bioelectrical impedance analysis, which would also be relevant to chronic stress. As pointed out by prior researchers, there is no standardized methodology for the assessment of adolescent psychosocial stress, although checklists of stressful life events have been widely used to characterize chronic stress among adolescents {De Vriendt, 2011 #704; Grant, 2004 #516; Unger, 2001 #522}. The stressor checklists can be implemented on a large scale and are cost-effective. However, it would have been ideal to have had biological markers of stress, such as saliva or hair cortisol assessment, to objectively quantify the presence of stress. Due to the self-report nature of the stressful life event checklist, the possibility of self-presentation bias may not be completely eliminated even though the students were assured that their responses would be kept confidential during the survey administration. The potential risks of Type I error inflation due to multiple testing in the genetic analyses may not be completely ignored, as the p value for the interaction effect was 0.043. However, the p values for the marginal genetic main effect (p=0.011) and the simple main effect within the low stress level stratum in girls (p=0.002) remained statistically significant after conservative Bonferroni correction. Moreover, although the power of our sample to detect the main effect of MAOA is acceptable (0.69 for the association of MAOA uVNTR with BMI in females, for example), the power to detect the interaction between MAOA uVNTR and stressors is poor given that the sample size was further stratified by levels of stress. For example, the estimated power for the simple main effect of MAOA uVNTR on BMI in females was 0.62 and 0.2 for the low and high family stress level strata respectively. Undoubtedly, future studies with larger sample sizes are warranted to validate our findings and identify new clues for the potential contribution of MAOA and stressors to BMI.Other than these concerns, our findings replicate the genetic main effects of MAOA uVNTR polymorphism on BMI in a Chinese adolescent population and convey the potential to derive new insights about interactions with negative familial stressors. The results highlight the need for future research to examine the roles of genes, stressful environments, and their interactions potentially leading to the development of overweight and obesity and other energy-balance behaviors (e.g. diet and physical activity) as measured by objective indices of neuroendocrine/hormonal changes in homeostatic systems.
What is already known about this subject?
Initial evidence from recent studies indicated a relationship between the high-activity MAOA uVNTR polymorphism and lower risk of obesity even though findings were varied across populations, and hence replication studies with population-based samples are clearly warranted.
What this study adds?
Our findings confirmed the genetic effects of MAOA uVNTR polymorphism on body mass index in a Chinese adolescent population.
This study demonstrated a significant gene-environment interaction with negative familial stressors in girls only, which provides a new insight on the causes of obesity, and thus, potential avenues of prevention.
Acknowledgments
This research was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases (Grant # R21DK088313, PI of Xie), and was also partially supported by the Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health (ZO1 ES49019), and the Claremont Graduate University/University of Southern California Transdisciplinary Tobacco Use Research Center (TTURC) funded by the National Institutes of Health (Grant #2 P50 CA084735-06, PI of Johnson). The authors thank the director and project staff at the Centers for Disease Control and Prevention in Wuhan city, People's Republic of China, for assistance with project coordination and data collection. We also thank the principals, physicians, and teachers in the participating schools for their cooperation.
Footnotes
Conflicts of Interest Statement: The authors declare that there are no conflicts of interest.
References
- 1.Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr Rev. 1998;56(4 Pt 1):106–14. doi: 10.1111/j.1753-4887.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 2.Ji CY, Sun JL, Chen TJ. [Dynamic analysis on the prevalence of obesity and overweight school-age children and adolescents in recent 15 years in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2004:103–8. [PubMed] [Google Scholar]
- 3.Cui Z, Huxley R, Wu Y, Dibley MJ. Temporal trends in overweight and obesity of children and adolescents from nine Provinces in China from 1991-2006. Int J Pediatr Obes. 2010;5(5):365–74. doi: 10.3109/17477166.2010.490262. [DOI] [PubMed] [Google Scholar]
- 4.Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008;9(Suppl 1):14–21. doi: 10.1111/j.1467-789X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 5.Xie B, C C, Spruijt-Metz D, Reynolds K, Palmer PH, Gallaher P, Sun P, Qian G, Johnson CA. Socio-Demographic and Economic Correlates of Overweight Status in Chinese Adolescents. American Journal of Health Behavior. 2007;31(4):339–352. doi: 10.5555/ajhb.2007.31.4.339. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103(6 Pt 1):1175–82. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 7.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature Preventive Medicine. 1993;22(2):167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337(13):869–73. doi: 10.1056/NEJM199709253371301. see comments. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Tsigos C, Chrousos GP. Stress, obesity, and the metabolic syndrome: soul and metabolism. Ann N Y Acad Sci. 2006;1083:xi–xiii. doi: 10.1196/annals.1367.025. [DOI] [PubMed] [Google Scholar]
- 11.De Vriendt T, Moreno LA, De Henauw S. Chronic stress and obesity in adolescents: Scientific evidence and methodological issues for epidemiological research. Nutr Metab Cardiovasc Dis. 2009 doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet. 1999;65(3):593–8. doi: 10.1086/302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih JC. Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. Neurotoxicology. 2004;25(1-2):21–30. doi: 10.1016/S0161-813X(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 14.Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31(3):120–9. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Fuemmeler BF, Agurs-Collins T, McClernon FJ, Kollins SH, Garrett ME, Ashley-Koch AE. Interactions between genotype and depressive symptoms on obesity. Behav Genet. 2009;39(3):296–305. doi: 10.1007/s10519-009-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103(3):273–9. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 17.Levy ER, Powell JF, Buckle VJ, Hsu YP, Breakefield XO, Craig IW. Localization of human monoamine oxidase-A gene to Xp11.23-11.4 by in situ hybridization: implications for Norrie disease. Genomics. 1989;5(2):368–70. doi: 10.1016/0888-7543(89)90072-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZY, Powell JF, Hsu YP, Breakefield XO, Craig IW. Organization of the human monoamine oxidase genes and long-range physical mapping around them. Genomics. 1992;14(1):75–82. doi: 10.1016/s0888-7543(05)80286-1. [DOI] [PubMed] [Google Scholar]
- 19.Serretti A, Cristina S, Lilli R, Cusin C, Lattuada E, Lorenzi C, et al. Family-based association study of 5-HTTLPR, TPH, MAO-A, and DRD4 polymorphisms in mood disorders. Am J Med Genet. 2002;114(4):361–9. doi: 10.1002/ajmg.10356. [DOI] [PubMed] [Google Scholar]
- 20.Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8(4):621–4. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 21.Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105(6):542–51. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- 22.Camarena B, Santiago H, Aguilar A, Ruvinskis E, Gonzalez-Barranco J, Nicolini H. Family-based association study between the monoamine oxidase A gene and obesity: implications for psychopharmacogenetic studies. Neuropsychobiology. 2004;49(3):126–9. doi: 10.1159/000076720. [DOI] [PubMed] [Google Scholar]
- 23.Need AC, Ahmadi KR, Spector TD, Goldstein DB. Obesity is associated with genetic variants that alter dopamine availability. Ann Hum Genet. 2006;70(Pt 3):293–303. doi: 10.1111/j.1529-8817.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 24.Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11(9):858–66. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- 25.Fuemmeler BF, Agurs-Collins TD, McClernon FJ, Kollins SH, Kail ME, Bergen AW, et al. Genes implicated in serotonergic and dopaminergic functioning predict BMI categories. Obesity (Silver Spring) 2008;16(2):348–55. doi: 10.1038/oby.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booker CL, Unger JB, Azen SP, Baezconde-Garbanati L, Lickel B, Johnson CA. Stressful life events and smoking behaviors in Chinese adolescents: a longitudinal analysis. Nicotine Tob Res. 2007;9(11):1085–94. doi: 10.1080/14622200701491180. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Kurita H, Uchiyama M, Okawa M, Liu L, Ma D. Life events, locus of control, and behavioral problems among Chinese adolescents. J Clin Psychol. 2000;56(12):1565–77. doi: 10.1002/1097-4679(200012)56:12<1565::AID-7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Tein JY. Life events, psychopathology, and suicidal behavior in Chinese adolescents. J Affect Disord. 2005;86(2-3):195–203. doi: 10.1016/j.jad.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Unger JB, Li Y, Johnson CA, Gong J, Chen X, Li C, Trinidad DR, Tran NT, Lo AT. Stressful life events among adolescents in Wuhan, China: Associations with somking, alcohol use, and depressive symptoms. Int J Behav Med. 2001;8(1):1–18. [Google Scholar]
- 30.Redden DT, Allison DB. Nonreplication in genetic association studies of obesity and diabetes research. J Nutr. 2003;133(11):3323–6. doi: 10.1093/jn/133.11.3323. [DOI] [PubMed] [Google Scholar]
- 31.Chou CP, Li Y, Unger JB, Xia J, Sun P, Guo Q, et al. A randomized intervention of smoking for adolescents in urban Wuhan, China. Prev Med. 2006;42(4):280–5. doi: 10.1016/j.ypmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Unger JB, Yan L, Chen X, Jiang X, Azen S, Qian G, et al. Adolescent smoking in Wuhan, China: baseline data from the Wuhan Smoking Prevention Trial. Am J Prev Med. 2001;21(3):162–9. doi: 10.1016/s0749-3797(01)00346-4. [DOI] [PubMed] [Google Scholar]
- 33.London SJ, Xia J, Lehman TA, Yang JH, Granada E, Chunhong L, et al. Collection of buccal cell DNA in seventh-grade children using water and a toothbrush. Cancer Epidemiol Biomarkers Prev. 2001;10(11):1227–30. [PubMed] [Google Scholar]
- 34.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–25. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 36.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital & Health Statistics - Series 11: Data From the National Health Survey. 2002;(246):1–190. [PubMed] [Google Scholar]
- 37.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132(2):204–10. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 38.Crystal DS, Chen C, Fuligni AJ, Stevenson HW, Hsu CC, Ko HJ, et al. Psychological maladjustment and academic achievement: a cross-cultural study of Japanese, Chinese, and American high school students. Child Dev. 1994;65(3):738–53. [PubMed] [Google Scholar]
- 39.Newcomb MD, Huba GJ, Bentler PM. A multidimensional assessment of stressful life events among adolescents: Derivation and correlations. Journal of Health and Social Behavior. 1981;22:400–415. [Google Scholar]
- 40.Herman AI, Kaiss KM, Ma R, Philbeck JW, Hasan A, Dasti H, et al. Serotonin transporter promoter polymorphism and monoamine oxidase type A VNTR allelic variants together influence alcohol binge drinking risk in young women. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):74–8. doi: 10.1002/ajmg.b.30135. [DOI] [PubMed] [Google Scholar]
- 41.Afghani A, Xie B, Wiswell RA, Gong J, Li Y, Anderson Johnson C. Bone mass of asian adolescents in China: influence of physical activity and smoking. Med Sci Sports Exerc. 2003;35(5):720–9. doi: 10.1249/01.MSS.0000064940.76574.BD. [DOI] [PubMed] [Google Scholar]
- 42.Xie B, Chou C, Spruijt-Metz D, Liu C, Xia J, Gong J, Li Y, Johnson CA. Effects of Perceived Peer Isolation and Social Support availability on the Relationship between Relative Body Mass Index and Depressive Symptoms. Int J Obes. 2005;29:1137–1143. doi: 10.1038/sj.ijo.0803006. [DOI] [PubMed] [Google Scholar]
- 43.Lung FW, Tzeng DS, Huang MF, Lee MB. Association of the MAOA promoter uVNTR polymorphism with suicide attempts in patients with major depressive disorder. BMC Med Genet. 2011;12:74. doi: 10.1186/1471-2350-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze TG, Muller DJ, Krauss H, Scherk H, Ohlraun S, Syagailo YV, et al. Association between a functional polymorphism in the monoamine oxidase A gene promoter and major depressive disorder. Am J Med Genet. 2000;96(6):801–3. [PubMed] [Google Scholar]
- 45.Yu YW, Yang CW, Wu HC, Tsai SJ, Hong CJ, Chen MC, Chen TJ. Association study of a functional MAOA-uVNTR gene polymorphism and personality traits in Chinese young females. Neuropsychobiology. 2005;52(3):118–21. doi: 10.1159/000087556. [DOI] [PubMed] [Google Scholar]
- 46.Huang SY, Lin M, Lin WW, Huang CC, Shy MJ. Association of monoamine oxidase A (MAOA) polymorphisms and clinical subgroups of major depressive disorders in the Han Chinese population. World J Biol Psychiatry. 2009;10(4 pt 2):544–51. doi: 10.1080/15622970701816506. [DOI] [PubMed] [Google Scholar]
- 47.Williams RB. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annu Rev Clin Psychol. 2008;4:349–65. doi: 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- 48.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 49.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 50.Price RA, Li WD, Kilker R. An X-chromosome scan reveals a locus for fat distribution in chromosome region Xp21-22. Diabetes. 2002;51(6):1989–91. doi: 10.2337/diabetes.51.6.1989. [DOI] [PubMed] [Google Scholar]
- 51.Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 2002;160(3):271–82. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- 52.Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–45. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- 53.Hodge S, Bunting BP, Carr E, Strain JJ, Stewart-Knox BJ. Obesity, whole blood serotonin and sex differences in healthy volunteers. Obes Facts. 2012;5(3):399–407. doi: 10.1159/000339981. [DOI] [PubMed] [Google Scholar]
- 54.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 55.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61(7):738–44. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 56.Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29(8):1498–505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]


