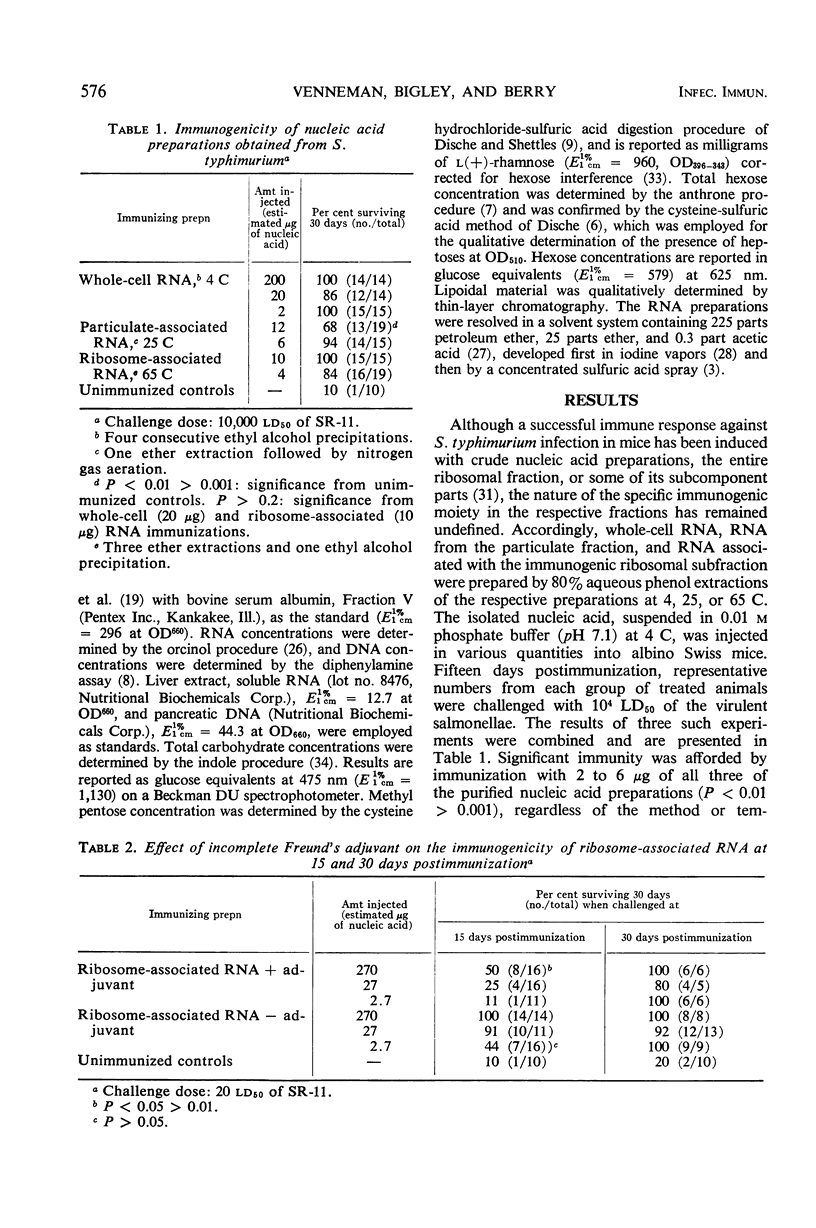
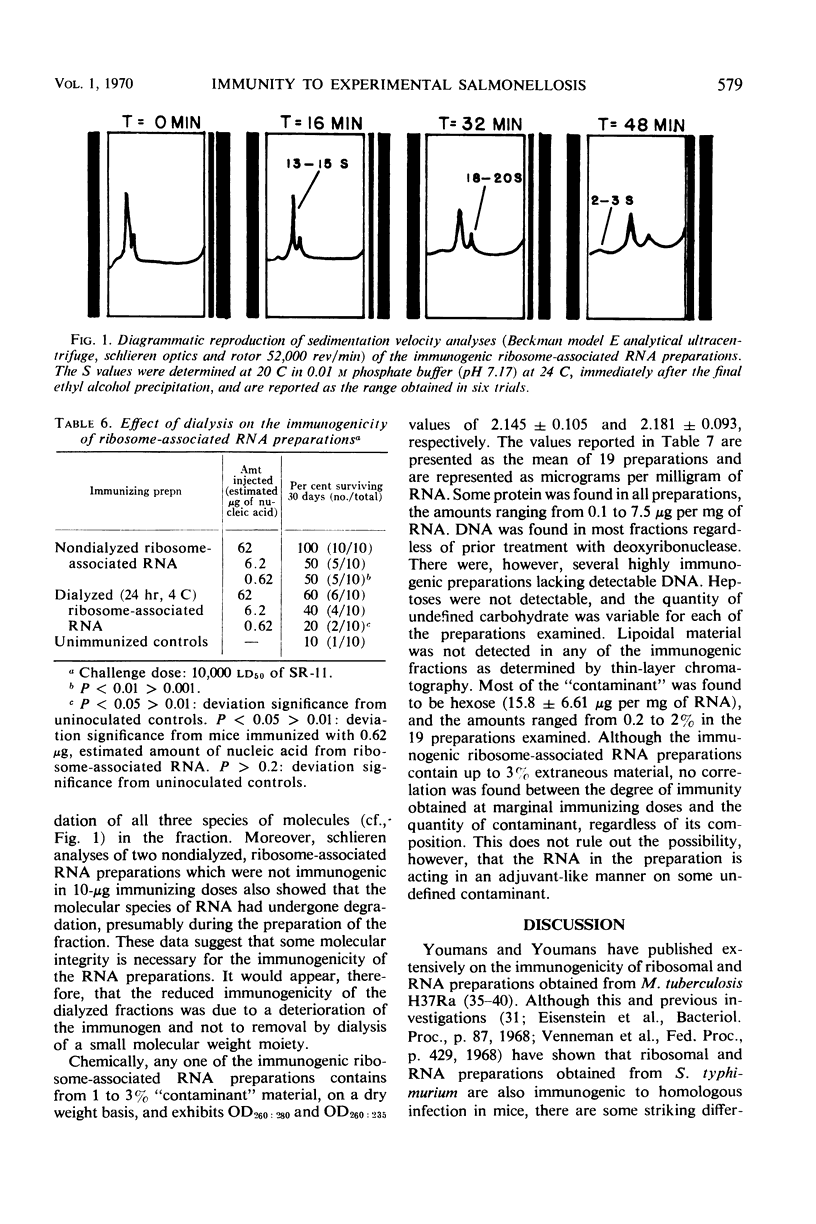
Abstract
Mice immunized with purified whole-cell ribonucleic acid (RNA), RNA from the bacterial “particulate” fraction, and ribosome-associated RNA obtained from Salmonella typhimurium were found to be resistant to subsequent challenge infection with virulent salmonellae. Chemically, the immunogenic nucleic acid fractions contained from 1 to 3% “contaminant” material defined (based on the mean of 19 different preparations) as protein (0.24%), deoxyribonucleic acid (0.43%), methyl pentose (0.64%), hexose (1.58%), and undefined carbohydrate (0.76%). Heptoses and lipoidal material were not detectable in any of the immunogenic preparations examined. Physically, the nucleic acid preparations, after analytical ultracentrifugation, exhibited three boundaries similar to those reported elsewhere in comparable systems: 4 to 5S, 16S, and 23S. An evaluation of the immunity induced by the ribosome-associated RNA established that the immune response was (i) comparable to that induced 15 days postimmunization with live salmonellae and by ribosomal vaccines, but greater at 30 days postimmunization than that in mice immunized with attenuated salmonellae; (ii) dependent on the quantity of immunogen administered; (iii) dependent on the size of the infective inocula; (iv) inhibited at 15 but not at 30 days postimmunization when the immunogenic nucleic acid preparations were incorporated into Freund's incomplete adjuvant, (v) reduced or lost by dialysis in relatively high or low immunizing doses, respectively; and (vi) unaffected by enzymatic treatment of the preparations with trypsin, deoxyribonuclease, Pronase plus pancreatic ribonuclease, or pancreatic ribonuclease alone. The possible mode of action of ribosome-associated RNA in inducing an immune response to subsequent challenge infection with the homologous organism is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., KOCH G., MOUNTAIN I. M., VAN DAMME O. Infectivity of ribonucleic acid from poliovirus in human cell monolayers. J Exp Med. 1958 Oct 1;108(4):493–506. doi: 10.1084/jem.108.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARONSON A. I., SPIEGELMAN S. On the use of chloramphenicol-inhibited systems for investigating RNA and protein synthesis. Biochim Biophys Acta. 1958 Jul;29(1):214–215. doi: 10.1016/0006-3002(58)90170-7. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- FISHMAN M., ADLER F. L. Antibody formation initiated in vitro. II. Antibody synthesis in x-irradiated recipients of diffusion chambers containing nucleic acid derived from macrophages incubated with antigen. J Exp Med. 1963 Apr 1;117:595–602. doi: 10.1084/jem.117.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L. The effect of ribonuclease on polysomes and ribosomes of bacteria and animal cells. Biochem J. 1968 Apr;107(4):481–489. doi: 10.1042/bj1070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., CONNOLLY J. H., GUPTA S. Blood nucleases and infectious viral nucleic acids. Nature. 1961 Mar 11;189:817–820. doi: 10.1038/189817a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. VI. ENHANCEMENT OF ANTIBODY FORMATION BY NUCLEIC ACIDS. J Immunol. 1965 Mar;94:416–422. [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Mar 25;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The dependence of nucleic acid synthesis on the presence of amino acids in Escherichia coli. J Bacteriol. 1956 Jun;71(6):677–683. doi: 10.1128/jb.71.6.677-683.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J. S., MILSTEIN S. W. Ribonuclease. I. A new assay method with P32 labeled yeast ribonucleic acid. J Biol Chem. 1952 May;196(2):489–498. [PubMed] [Google Scholar]
- Ushiba D., Nakae T., Akiyama T., Kishimoto Y. Characterization of "clearance" factor and "cell-bound" antibody in experimental typhoid. J Bacteriol. 1966 May;91(5):1705–1712. doi: 10.1128/jb.91.5.1705-1712.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., MILLMAN I., YOUMANS A. S. The immunizing activity against tuberculous infection in mice of enzymatically active particles isolated from extracts of Mycobacterium tuberculosis. J Bacteriol. 1955 Nov;70(5):557–562. doi: 10.1128/jb.70.5.557-562.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. doi: 10.1128/jb.91.6.2139-2145.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Ribonucleic acid, deoxyribonucleic acid, and protein content of cells of different ages of Mycobacterium tuberculosis and the ralationship to immunogenicity. J Bacteriol. 1968 Feb;95(2):272–279. doi: 10.1128/jb.95.2.272-279.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]



