Abstract
The aim of this investigation was to evaluate how parental anxiety predicted change in pediatric anxiety symptoms across four different interventions: cognitive-behavioral therapy (CBT), medication (sertraline; SRT), their combination (COMB), and pill placebo. Participants were 488 youths (ages 7-17) with separation anxiety disorder, generalized anxiety disorder, and/or social phobia and their primary caregivers. Latent growth curve modeling assessed how pre-treatment parental trait anxiety symptoms predicted trajectories of youth anxiety symptom change across 12 weeks of treatment at four time points. Interactions between parental anxiety and treatment condition were tested. Parental anxiety was not associated with youth’s pre-treatment anxiety symptom severity. Controlling for parental trait anxiety, youth depressive symptoms, and youth age, youths who received COMB benefitted most. Counter to expectations, parental anxiety influenced youth anxiety symptom trajectory only within the SRT condition, whereas parental anxiety was not significantly associated with youth anxiety trajectories in the other treatment conditions. Specifically, within the SRT condition, higher levels of parental anxiety predicted a faster and greater reduction in youth anxiety over the acute treatment period compared to youths in the SRT condition whose parents had lower anxiety levels. While all active treatments produced favorable outcomes, results provide insight regarding the treatment-specific influence of parental anxiety on the time course of symptom change. (ClinicalTrials.gov number NCT00052078.)
Keywords: children, adolescents, parent, anxiety, treatment
Considerable evidence supports the efficacy of both psychosocial and medication treatments for pediatric anxiety [1, 2, 3, 4], yet little is known about the timing of symptom change over the course of an acute intervention period and a sizeable proportion of youths who receive these interventions remain symptomatic after treatment. In the Child/Adolescent Anxiety Multimodal Study (CAMS) [4], a multi-site, randomized controlled trial comparing cognitive behavior therapy (CBT), sertraline (SRT), combined treatment of both CBT and sertraline (COMB), and pill placebo (PBO), 40% of youth failed to respond to CBT and 45% failed to respond to SRT monotherapy. Rates of remission, defined as loss of diagnoses, are even more concerning [5]. Although youths receiving COMB fared somewhat better (19% nonresponders), these rates underscore the need to better understand which youths might benefit from which particular treatments. Further, beyond treatment response, a clearer understanding of the time course of symptom change in different intervention modalities, and factors that influence symptom trajectories and speed of response is necessary to improving the efficiency of services.
Across several psychotherapy intervention trials, parental anxiety has been implicated in diminishing treatment response for pediatric anxiety [6-11]. However, study results have been inconsistent and several have not found this association [12-15]. Given that anxiety aggregates in families [16] and that parents of anxious youths are also likely to experience anxiety [17, 18], understanding the manner in which parental anxiety influences treatment outcome is of paramount importance.
By and large, extant research concerning the role of parental anxiety on pediatric anxiety treatment outcome has focused on cognitive behavioral therapies (CBT). The prior focus on CBT is reasonable given that parental anxiety may place higher demands on therapists and interfere with completion of behavioral exposures [19]. However, the impact of parental anxiety may differ in pediatric anxiety medication trials where treatment does not directly target change in the social environment. Unfortunately, research addressing this question is lacking. Indeed, data on predictors and moderators of medication treatment response for pediatric anxiety disorders are overall limited [20, 21] though there is critical need for these data given that medication use in children and adolescents has significantly expanded over the past decade [22-24]. Thus, impact of parental anxiety on youth medication treatment response remains an open, yet highly relevant question.
Two studies have examined the impact of lifetime family history of anxiety on medication response for anxious youth. Birmaher and colleagues [25] found that a positive family history of anxiety did not significantly predict response to fluoxetine in youth with generalized anxiety, separation anxiety, and/or social phobia. Similarly, in the Pediatric OCD Treatment Study, monotherapy sertraline and combination treatment effect sizes were generally robust to family history of OCD [26]. However, a positive family history of OCD was associated with a 6-fold decrease in effect size for monotherapy CBT compared to families with no family history of OCD. Although potentially providing insight into underlying biological vulnerabilities that may contribute to treatment response, these findings do not directly address the impact of current parental context on treatment course and outcome. Targeted examination of treatment-specific links between current parental anxiety and youth symptom change is needed to address this gap.
Using data from CAMS [4], the largest randomized trial for pediatric anxiety to date, this investigation builds upon the extant literature of the negative influence of parental anxiety on child psychotherapy outcomes, and the relative lack of such studies for medication treatments, to further probe the influence of parental anxiety across different treatment modalities. Current advances in statistical procedures enable us to move beyond static comparison of endpoints on clinical outcomes to examine the influence of selected variables on symptom trajectories across acute treatment (i.e., predictors of change/growth). Here, we use a longitudinal approach to model expected symptom trajectories based on individual patterns of change. Specifically, we aimed to examine the impact of parental anxiety on the trajectory of symptom decrease in each treatment condition while taking into consideration individual differences in other variables that may influence initial levels of youth anxiety (e.g., depression symptoms, age, gender). In addition, given that existing literature suggests that parental anxiety may interact most with individual child CBT, we made the a priori decision to use the CBT-only treatment condition as a clinically meaningful reference group in analyses in order to allow direct comparison of parental anxiety in each of the monotherapies. Because parental anxiety may interfere with specific process in CBT (e.g., parents may be resistant to supporting youth exposure exercises), we anticipated that parental anxiety would have more of an influence on the anxiety symptom trajectories of youths receiving CBT than for youths receiving SRT treatment, which may be less likely to interact with behaviors of anxious parents that might interfere with treatment processes.
Method
Participants
Participants were 488 youth, ages 7-17 years (mean age 10.7, SD=2.8 years; 49.6% girls, 78.9% Non-Hispanic White), and their primary caregivers (88.1% mothers, 8.8% fathers; herein referred to as parents) who enrolled across six geographically-diverse sites in the Child/Adolescent Anxiety Multimodal Treatment Study (CAMS) [4]. To be eligible for study entry, youth were required to meet DSM-IV criteria for a primary diagnosis of separation anxiety disorder, generalized anxiety disorder, and/or social phobia. Exclusionary criteria included comorbid mood, psychotic, or pervasive developmental disorders, and either one failed prior CBT trial or two failed SSRI trials for anxiety. Additional details on participating youth may be found elsewhere [4, 27].
Procedure
All participants and at least one parent/legal guardian provided informed consent/assent. The institutional review board at each site approved and monitored the protocol. Safety monitoring was performed quarterly by a NIMH Data Safety and Monitoring Board. Eligible youth were randomized to twelve weeks of CBT (n=139), SRT (n=133), COMB (n=140), or PBO (n=76). SRT and PBO were double-blind conditions, and COMB and CBT were masked to independent evaluators (IEs) but not to patients and therapists. All youth were assessed by IEs blind to study condition at weeks 0 (baseline), 4, 8, and 12 (post-acute treatment). Evaluations included collection of demographic data, anxiety and comorbid symptomatology, and psychosocial functioning measured via IEs and parent and child self-report. Efforts were made to maintain IE continuity over time and the blind was rigorously enforced.
Study treatment conditions
The CAMS CBT intervention was the Coping Cat Program [28, 29], which involved 14 sessions over 12 weeks. Two of these sessions were parent-only. Coping Cat includes skills training in anxiety management followed by practice (i.e., exposure tasks) in anxiety-provoking situations tailored to the participant’s needs. The SRT condition involved eight 30-60 minute medication management sessions plus bi-weekly telephone check-ins that included discussion of anxiety symptom severity, treatment response, and adverse events. Psychiatrists or nurse practitioners certified in the study pharmacotherapy protocol administered SRT, and pill counts and medication diaries were used to monitor adherence. Sertraline and matching placebo were delivered using a “fixed-flexible” dosing schedule tied to clinical response and tolerability with a maximum daily dose of 200 mg. Additional details are included in a previous report [4]. The 12-week COMB condition included both CBT and SRT, with sessions typically scheduled in the same place on the same day.
Measures
The Anxiety Disorders Interview Schedule for DSM-IV-TR, Child Version (ADIS-IV) [30] was used to determine diagnostic eligibility. The ADIS-IV is a semi-structured interview that assesses the major DSM-IV anxiety, mood, and externalizing disorders experienced by youth. In addition to generating DSMIV diagnoses, interviewers assign a clinical severity rating (CSR), based on a 9-point scale to each diagnosis. CSRs of 4 or above indicate the presence of diagnosis. Inter-rater reliability for the ADIS was excellent (r= .98 for the parent interview and r= .93 for the child) [31].
Pediatric Anxiety Rating Scale (PARS) [20], is a measure of overall anxiety symptom severity that was employed as the primary continuous outcome. The PARS is an interviewer-rated measure of anxiety that integrates youth and parent reports of youth anxiety. Total scores were obtained by summing six items assessing anxiety severity, frequency, distress, avoidance, and interference over the previous week. Total scores range from 0-30, with scores above 13 indicating clinically meaningful anxiety. The PARS has acceptable internal consistency (alpha = 0.64), strong inter-rater reliability (r = 0.97), and moderate retest reliability (r = 0.55).
The Mood and Feelings Questionnaire, Child- and Parent-reports (MFQ-C/MFQ-P) [32] were used to assess youth depressive symptoms. The MFQ is a 33-item youth- and parent-report inventory of depressive symptomatology in children and adolescents with sound psychometric properties. In this study, MFQ scores were used as covariates in analyses. In this sample, the MFQ-C and MFQ-P had excellent internal consistency with Cronbach α = .92 and α = .91, respectively.
The State-Trait Anxiety Inventory-Trait Scale (STAI-Trait) [33] is a 20-item self-report measure used to assess parental anxiety. The total score measures “trait” (versus state) anxiety with higher scores reflecting higher levels of anxiety. The psychometric properties of the STAI are well-documented [34, 35]. Internal consistency in this sample was excellent, with Cronbach α = .90.
Data Analytic Plan
Analyses used the CAMS intent-to-treat sample with established multiple imputation procedures for missing data. Latent Growth Curve Modeling (LGCM) (conducted using MPlus Version 7) [36] was used to estimate trajectories of child anxiety symptoms (as measured with the PARS) based on level of pre-treatment parental anxiety (STAI-Trait), and to evaluate how the role of parental anxiety on symptom trajectory varied by treatment condition. LGCM falls within the framework of Structural Equation Modeling and is a powerful approach to modeling individual differences in change on a designated outcome over time [37, 38]. To accomplish this, an intercept (mean initial level of the dependent variable) and a slope (mean rate of growth/change) are represented as latent variables, and time scores (i.e., factor loadings) represent growth. In a linear model with four time points, time scores would be fixed to 0, 1, 2, and 3. However, we allowed time scores to freely estimate to assess whether symptom growth deviates from strict linearity (e.g., non-linear growth with decelerating trajectories). Unconditional models only have time as a predictor of change, whereas conditional models include predictor variables.
In this study, a model with parental anxiety, treatment condition, and their multiplicative interaction was tested to predict youth anxiety intercept and slope at four time points across 12 weeks of treatment (PARS at Weeks 0, 4, 8, and 12). Because LGCM assesses change across the course of treatment, the interaction between parental anxiety and treatment condition represents a three-way interaction between parental anxiety, treatment condition, and time. The four-level treatment condition variable was recoded into three dummy variables with CBT as the referent treatment condition. This decision was based on our specific a priori goal of comparing the influence of parental anxiety on psychotherapy treatment to medication treatment. Model fit was evaluated using conventional criteria based on descriptive fit indices: comparative fit index (CFI) and Tucker Lewis Index (TLI) >.95 [39] indicated good fit and >.90 indicated acceptable fit; root mean square error of approximation (RMSEA) values of .05 or less indicate good or close model fit, whereas values within the range of .05 to .08 indicated an acceptable or fair model fit [40, 41]. For well-fitting models, we probed significant interaction terms by examining simple slopes. All continuous predictors and covariates were mean centered.
Prior to testing the study hypotheses, we tested an unconditioned model of changes in anxiety symptoms across the acute treatment phase. This determined the rate at which symptoms changed, on average, across treatment as well as whether there was significant variability in initial levels of anxiety and the rate of change. We then tested a conditioned model in which potential covariates were tested as predictors of initial levels and the rate of change of anxiety symptoms. Nonsignificant covariates were dropped from subsequent analyses to preserve power needed to test for hypothesized interactions. A priori covariates included child gender, age, youth depressive symptoms, selected based on previous studies documenting their relevance to anxiety treatment response [42]. For all models, time scores for the first two time points were fixed at 0 and 1 and were freely estimated at Weeks 8 and 12 to allow for non-linear growth.
Results
There were no significant pre-treatment differences between the treatment conditions in parental anxiety (STAI-T, Mean = 38.68, SD = 9.50, range = 20-71), youth age (Mean = 11.87, SD = 2.81), youth anxiety (PARS, Mean = 19.18, SD = 4.21), or depressive symptoms (MFQ-C, Mean = 17.77, SD = 11.83). The mean parental anxiety total score in this sample was within one standard deviation above the mean for normal working adults in the validation sample [33]. Parental anxiety was positively correlated with youth depressive symptoms at baseline per youth-report (r=.12, p=.007) and parent-report (r=.33, p<.001), but was not significantly associated with youth age or gender (both p > .40). Further details on the pre-treatment clinical and demographic characteristics of youths in this sample can be found in previous publications [4, 27]. Mean IE-rated PARS scores for each time point, by treatment condition, are in Table 1.
Table 1.
Pediatric Anxiety Rating Scale (PARS) Scores Across Acute Treatment
| Week 0 - Baseline Mean (SD) |
Week 4 Mean (SD) |
Week 8 Mean (SD) |
Week 12 - Post-Treatment Mean (SD) |
|
|---|---|---|---|---|
| Total (N = 488) | 19.18 (4.21) | 14.47 (5.58) | 11.30 (6.02) | 9.70 (6.61) |
|
| ||||
| Sertraline + CBT (N = 140) | 19.54 (3.86) | 14.52 (5.71) | 9.93 (5.67) | 6.95 (5.17) |
| Sertraline (N = 133) | 18.76 (4.34) | 13.10 (5.63) | 10.98 (5.95) | 10.27 (7.16) |
| CBT (N = 139) | 19.08 (4.34) | 15.30 (5.35) | 12.37 (6.07) | 10.68 (6.58) |
| Placebo (N = 76) | 19.43 (4.39) | 15.25 (5.27) | 12.43 (6.19) | 12.01 (6.50) |
CBT = cognitive behavior therapy
Latent Growth Models
Unconditional model
The unconditional model was an acceptable fit: χ2 (3) = 11.04, p=.01; CFI=0.98, TLI=0.96, RMSEA=0.07. Time scores for this model were: Week 0=0, Week 4=1, Week 8=1.70, Week 12=2.01, indicating an average anxiety decrease with deceleration in symptom reduction over time (i.e., larger anxiety symptom decreases earlier in treatment), across the entire sample. There was significant variation around the intercept and the slope, and evaluation of conditional models with predictor variables was indicated.
Covariate model
The squared multiple correlation between youth’s and parent’s -report of the youth’s depressive symptoms was high at .70. Using the recommended cutoff of > .50 for exclusion of an independent variable [43], parent-reported youth depression was dropped from the analysis in order to increase power and decrease redundancy. Accordingly, youth age, gender, and youth-reported baseline depression symptoms were tested as simultaneous predictors of youth anxiety initial scores and treatment trajectory. The model was a good fit: χ2 (17) = 29.93, p=.03; CFI = 0.97, TLI = 0.95, RMSEA = 0.04. All covariates except for gender were significant; youth age and depressive symptoms at baseline significantly predicted the intercept, or initial levels of anxiety (b=0.16, p=.02, β=0.15, and b=0.07, p<.001, β=0.25, respectively). Based on these analyses, youth age and baseline depressive symptoms were included as covariates in the hypothesis-testing model.
Hypothesized model: Parental anxiety as moderator of symptom trajectory
The hypothesized moderation model was a good fit to the data: χ2 (21) = 43.00, p=.003; CFI = 0.96, TLI = 0.91, RMSEA = 0.046. There was a direct effect of youth age (b=0.15, p=.027, β=0.14) and baseline youth-reported depressive symptoms (b=0.06, p<.001, β=0.23) on intercept, indicating that these variables were both positively associated with initial levels of child anxiety. There was a main effect of COMB treatment (vs. CBT) on slope (b=−1.87, p<.001, β=−0.43), indicating that youths who received COMB treatment experienced a significantly greater decrease in symptoms (steeper slope, quicker symptom reduction) than children who received CBT (note: this replicated original findings [4]) regardless of parental anxiety. Controlling for other variables, there were no significant differences in the slope (i.e., rate and shape of change) between the CBT reference group and the PBO group. In addition, there was no significant main effect of parental anxiety on either initial levels or trajectory of youth anxiety symptoms (Table 2). Time scores for this model were 0, 1, 1.71, and 2.05, indicating that clinical trajectories did not follow strict linearity and that the rate of change decelerated later in treatment. Slope and intercept were not significantly correlated (r = .37, p =.71), indicating that initial levels of anxiety symptoms were not significantly associated with symptom trajectory when controlling for youth age, youth depressive symptoms, and parental anxiety symptoms.
Table 2.
Parameters from conditional latent growth curve model assessing trajectories of pediatric anxiety symptoms across treatment.
| Anxiety (PARS) Growth Parameters |
||||||
|---|---|---|---|---|---|---|
| Intercept Factor | Slope Factor | |||||
|
|
||||||
| b | SE | β | b | SE | β | |
| Main effects: | ||||||
| Youth age | 0.15* | 0.07 | 0.14 | 0.04 | 0.05 | 0.06 |
| Youth depression (MFQ-C) | 0.06** | 0.02 | 0.23 | 0-.02 | 0.01 | −0.10 |
| Parental anxiety (STAI-T) | 0.01 | 0.02 | 0.03 | −0.01 | 0.01 | −0.03 |
| Treatment (vs. CBT) | ||||||
| CBT + SRT | 0.56 | 0.48 | 0.09 | -1.87** | 0.35 | −0.43 |
| SRT | −0.68 | 0.49 | −0.10 | −0.33 | 0.35 | −0.08 |
| Placebo | 0.31 | 0.58 | 0.04 | 0.23 | 0.41 | 0.04 |
| Interactions | ||||||
| Parental anxiety (STAI-T) | ||||||
| X CBT + SRT (vs. CBT) | 0.05 | 0.05 | 0.08 | −0.04 | 0.04 | −0.09 |
| X SRT (vs. CBT) | 0.05 | 0.06 | 0.07 | −0.10* | 0.04 | −0.20 |
| X Placebo (vs. CBT) | 0.09 | 0.06 | 0.11 | −0.03 | 0.04 | −0.06 |
p<.05,
p<.001;
PARS = Pediatric Anxiety Rating Scale; b = unstandardized coefficient; SE = standard error; β = standardized coefficient; MFQ-C = Mood and Feelings Questionnaire – Child report; STAI-T = State-Trait Anxiety Inventory – Trait scale; CBT = cognitive behavior therapy; SRT = sertraline.
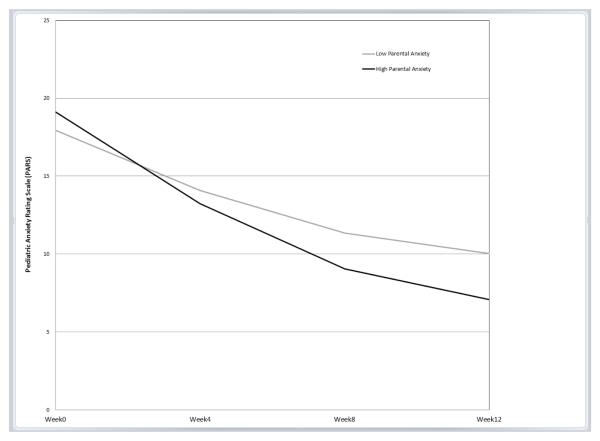
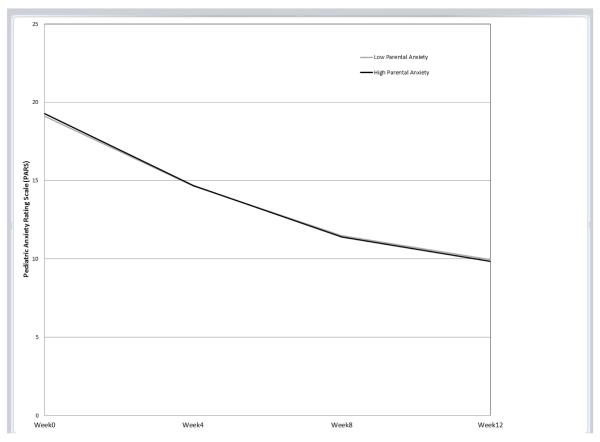
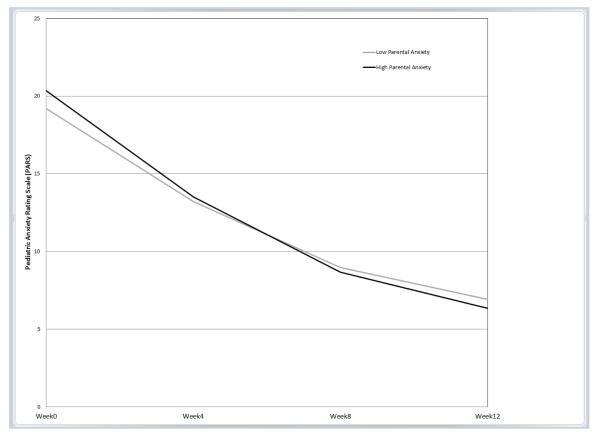
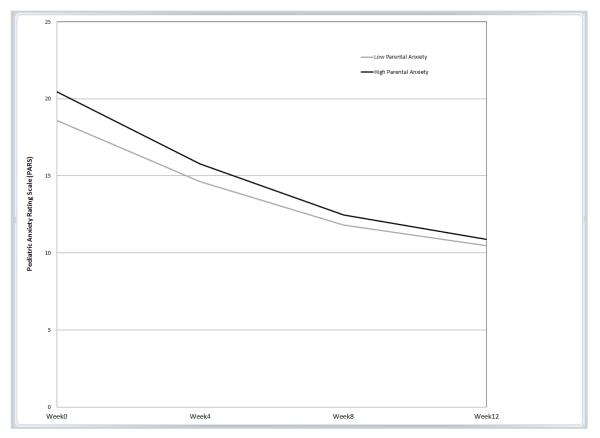
The only significant interaction between parental anxiety and treatment group was for SRT (b=−.10, p=.012, β=−.20; Table 2). This interaction indicated that the effect of parental anxiety on youth symptom trajectory for youths who received SRT was significantly different compared to the effect of parental anxiety on youth symptom trajectory for youths who received CBT; note that the lack of significant interactions for COMB or PBO indicate that the influence of parental anxiety of youth symptom trajectory in each of those two treatments did not differ from the influence of parental anxiety on CBT. Predicted symptom trajectories based on level of parental anxiety for each treatment group, controlling for the covariates, are displayed in Figures 1a-d.
Figure 1.
Predicted trajectories of symptom change by level of parental anxiety (Mean STAI ± 1 SD).
a. Sertraline (SRT)
b. Cognitive Behavior Therapy (CBT)
c. Combination (COMB) Figure 1d. Placebo (PBO)
To follow up on the significant interaction, simple slopes for values of ±1 SD of the mean of parental anxiety were examined for the SRT and CBT treatment conditions using estimates from the full model with the entire sample derived from an online computational tool for graphing 3-way interactions in latent curve analysis [44]. In this sample, these values of ±1 SD corresponded to STAI-T scores of 29.37 and 48.17 for “low” and “high” anxious parents, respectively. Notably, the score representing “high” anxious parents is within the range of those previously reported for clinically anxious samples participating in adult anxiety treatment studies [e.g., 45, 46]. Contrary to our hypothesis, as seen in Figure 1a, for children who received SRT, high levels of parental anxiety predicted a greater decrease in youth anxiety symptoms over time compared to low levels of parental anxiety. Parental anxiety was not associated with the initial level or trajectory of anxiety symptoms for youths who received CBT, COMB, or PBO (as illustrated by near overlapping trajectories; Figure 1b, 1c, and 1d, respectively).
Post hoc analyses within SRT treatment condition
Given the unexpected interaction finding that higher parental anxiety predicted a more favorable youth response to SRT, we performed post hoc analyses in attempt to better understand the nature of this relationship. Potential moderating and mediating variables were probed within the SRT condition to examine whether baseline youth depression symptoms moderated the association between parental anxiety and youth anxiety symptom reduction, or whether a reduction in depressive symptoms mediated this relationship. In addition, we examined whether pharmacotherapist-rated medication compliance (1-7 scale, 1=Non-Compliant, 7=Completely Compliant; averaged across each study visit) mediated this relationship. Youth depression symptoms at baseline was not a significant moderator b=−.004, p=.30, β=−.13). Further, STAI was not significantly associated with youth depression symptoms at any subsequent time point, and parental anxiety was not significantly correlated with medication compliance. Accordingly, we ceased further analysis.
Clinical significance of predicted symptom change
Results were significant but modest, as indicated by rather small regression coefficients (<.30). Nevertheless, our full model results predict clinically meaningful changes in youth anxiety symptoms based on pre-treatment parental anxiety level, and provide insight into timing of meaningful symptom change. A recent study using CAMS data suggested that youths with acute post-treatment scores of PARS = 8-10 were most likely to experience symptom remission, defined as CGI-I of 1 or 2 and loss of all targeted diagnoses based on diagnostic interview [5, 47]. Using this criterion, for children in the SRT condition, level of parental anxiety predicted when the youth would reach this range for remission. Based on our model, children in the SRT condition whose parents scored 1 SD above the mean pre-treatment STAI score were expected to achieve a PARS score in the optimal range for remission by week 8 of treatment (estimated week 8 PARS score = 9.06), and those whose parents scored as little as ½ SD above the mean pre-treatment STAI score are predicted to have post-treatment anxiety scores in the optimal range of remission (i.e., by week 12). Conversely, children in the SRT condition whose parents scored 1 SD below the STAI mean were predicted to fall at the outer limit of this optimal range of remission at acute post-treatment (estimated post-treatment PARS score = 10.03), indicating a longer time until optimal response. Similarly, children who received CBT would be expected to reach the optimal range for remission by their acute post-treatment assessment, regardless of parent anxiety level. Thus, while children receiving CBT or SRT monotherapies would both be expected to have favorable acute treatment outcomes, youths receiving SRT who have a high anxious parent are estimated to have an even more favorable response and this response is estimated to occur four weeks earlier. For perspective, when a parent had a high level of anxiety, the modeled trajectories and symptom endpoints of youths in the SRT condition were in the range of youths who received COMB (figures 1a and 1c).
Discussion
The aim of this investigation was to examine the influence of parental anxiety symptoms on symptom trajectories of youths receiving 12 weeks of sertraline, CBT, their combination, or pill placebo. Latent growth curve analysis provided a model of expected symptom change across different treatment types based on individual differences, providing a more comprehensive picture of pediatric anxiety symptom trajectories than prior studies. Initial child anxiety levels were higher for youths who were older and had higher baseline depressive symptoms. Consistent with prior analyses of these data [4], estimated trajectories were most favorable for youths who received COMB treatment, regardless of parental anxiety level, and in general, symptom reduction occurred most rapidly the first eight weeks of treatment. However, contrary to hypotheses, parental anxiety was a significant moderator of SRT treatment, indicating that the influence of parental anxiety on youth symptom trajectory was significantly greater in the SRT condition compared to CBT. Specifically, for youths who received SRT monotherapy, higher levels of parental anxiety at baseline predicted quicker and greater symptom decreases compared to youths whose parents endorsed low levels of parental anxiety at baseline. Conversely, parental anxiety did not significantly influence the trajectories for youths who received CBT, COMB, or PBO. While all children receiving active treatment were predicted to demonstrate clinically meaningful symptom reductions, rates of improvement were superior for youths who received COMB treatment and for youths who received SRT and had a high anxious parent.
To our knowledge, this is the first study to examine the role of parental anxiety symptom levels on symptom trajectories in a pediatric anxiety medication trial and to directly compare the role of parental anxiety in medication and non-medication treatments. In previous analyses of the CAMS sample, parental anxiety did not emerge as a significant predictor or moderator of outcome among a comprehensive list of variables examined [48]. There was a trend demonstrating that parental anxiety moderated treatment outcome (raw p-value = .04), however, this effect did not meet statistical significance using conservative criteria adjusted for multiple comparisons (adjusted p-value=.13) [48]. In the present study, we utilized a latent growth modeling approach to estimate symptom trajectories, selected CBT as our treatment reference group, and controlled for relevant child clinical and demographic variables. Thus, the present significant finding must be considered in light of our analytic approach, which appropriately reflects the specific a priori aim of comparing how parental anxiety influenced youth symptom trajectories in the two monotherapies.
Given previous research [5, 7, 8, 9, 10, 12], we anticipated that parental anxiety would play a larger role in outcome for youths in the CBT condition compared to those in the medication condition. Our present finding that higher levels of parental anxiety predicted quicker and greater anxiety decreases within the medication-only treatment group, with no clinically meaningful influence on trajectories within the CBT-only treatment group (Figure 1b), was unexpected. This finding was not accounted for by medication adherence/compliance or medication-related decreases in depressive symptoms, although we note that the range of youth depression scores in this sample was limited and on average, subclinical. While we do not have data on the amount of direct contact between parents and pharmacotherapists, it is possible that parents had more regular contact with providers in the SRT condition than in the CBT condition. In CAMS, the majority of parents of children who received medication had regular weekly contact with the pharmacotherapist, and many parents were present for the entire appointment. Accordingly, it is plausible that regular clinical contact with anxious parents was associated with improved outcome for youths in the medication condition. Alternatively, anxiety in children of anxious parents may reflect underlying biological processes that are particularly responsive to medication.
The finding that parental anxiety was not associated with symptom trajectories in the CBT group was also unexpected. Notably, parents were involved as collaborators in CAMS CBT, not co-clients as may be the case in interventions with greater parental involvement [11]. There were two parent-only sessions in CAMS; exploratory post hoc analyses revealed that during the other 12 child-only sessions, parents spent an average of less than five minutes in session with therapists and parental anxiety was not significantly correlated with the amount of parent-therapist contact (average minutes per session as reported by therapists; r = .04, p = .11) . This may differ from practice in community settings where parental anxiety has been associated with increased collateral contact [49]. These study-specific CBT characteristics may simultaneously strengthen the internal validity of the individual child CBT condition while reducing the variability of parental impact that may occur in other treatments in which parents were more involved in child sessions. Indeed, several studies in which parental anxiety influenced child outcomes, after acute treatment or at later follow-up, had greater parental involvement. [7-12]. Notably, this may not extend to interventions that directly target parental anxiety and associated behaviors [50-52]. Thus, current findings may be most relevant for child anxiety treatments in which the parent has minimal involvement.
The present CAMS study is the largest child anxiety treatment study to date and utilized the most rigorous methodology across a geographically diverse sample. Nevertheless, results should be interpreted within the context of study limitations. The sample was ethnically homogenous, primarily non-Hispanic White, and it is unknown if current findings generalize to other ethnic groups. Further, while there were high levels of comorbidity within anxiety disorders, further work is needed to evaluate the applicability of these findings in more clinically diverse samples (e.g., comorbid depressive or externalizing disorders). For instance, youth depressive scores in this sample were subclinical on average and do not reflect the range and severity of comorbid depression that might be seen in public sectors of care [53]. Thus, present findings must be interpreted in light of the study-specific sample characteristics pending replication with broader clinical samples. In addition, Keeton and colleagues [54] found in the CAMS sample that a reduction in youth symptoms was associated with reduced Week 12 parental anxiety and psychological distress. Because we do not have data on parental anxiety in the intermediate weeks (weeks 4 and 8), we cannot rule out parental anxiety out as a mediator in the medication condition, though we do not view this as likely. In addition, our analyses included data from only one caregiver (88% mothers) and the influence of anxiety, either risk or protective, of a second parent on pediatric anxiety treatment remains an open question. While present results are clinically meaningful and statistically significant, they were modest (i.e., small regression coefficients), and it is possible that stronger effects would emerge in the presence of parent diagnostic data. Present results might also be attributed other variables not directly measured in this study, such as anxiety-enhancing parenting behaviors, parental depression, or family adversity factors [12, 15, 55, 56]. Genetic data are not available for the full sample and further work is needed to elucidate the interactive role of genes and family environment in response to medication compared to non-medication treatments.
This investigation extends previous work by examining the specific role of parental anxiety in both medication and non-medication treatments with direct comparison of a selective serotonin reuptake inhibitor (sertraline) and child-focused CBT (Coping Cat program), while considering individual factors that might influence initial levels and trajectories of symptom change. Knowledge regarding factors that influence speed of response may be critical for services in usual care settings where risk of attrition is high [57]. Importantly, results may inform clinical assessment and decision-making based on family characteristics and intervention time frame. Results underscore the importance of assessing current parent anxiety levels in addition to family history. Combined SRT and CBT may provide the most robust benefit to youths; however, present findings may help to guide treatment selection in situations when their combination is not a viable option. While CBT can be effective for youths regardless of parental anxiety levels, youths with highly anxious parents may experience greater and more immediate gains from SRT than individual, child-focused CBT.
Summary
Parental anxiety has been implicated in attenuating outcomes in cognitive-behavioral therapy (CBT) for pediatric anxiety but its influence on medication treatment for pediatric anxiety has received little research attention. The objective of this investigation was to evaluate associations between parental anxiety and pediatric anxiety symptom trajectories in four different interventions: cognitive-behavioral therapy (CBT), medication (sertraline; SRT), their combination, and pill placebo. Participants were 488 youth (ages 7-17 years) with separation anxiety disorder, generalized anxiety disorder, and/or social phobia enrolled in the Child/Adolescent Anxiety Multimodal Study (CAMS) [4] and their primary caregivers (88% mothers). Latent growth curve modeling assessed how pre-treatment parental trait anxiety symptoms predicted (a) initial levels and (b) trajectories of youth anxiety symptom change across 12 weeks of treatment. Youth anxiety was rated by blind independent evaluators on the Pediatric Anxiety Rating Scale at four time points. Interactions between parental anxiety and treatment condition were tested. Results indicated that parental anxiety was not associated with youth’s pre-treatment youth anxiety symptom severity. Controlling for parental trait anxiety, youth depressive symptoms, and youth age, there was a main effect of COMB treatment indicating that youth who received both medication and CBT benefitted most. In addition, there was an interaction between parental anxiety and SRT treatment. Counter to expectations, higher levels of parental anxiety predicted a more favorable symptom trajectory within the SRT condition leading to faster and greater reduction in youth anxiety compared to youths whose parents had lower levels of anxiety. Parental anxiety did not significantly influence youth anxiety trajectories in the other treatment conditions. Taken together, results indicate that reduction in youth anxiety symptoms were fastest and greatest when children (a) received COMB, regardless of parental anxiety level, and (b) received SRT and had a parent with high anxiety. These findings offer guidance for pediatric anxiety treatment selection based on parental anxiety levels.
Acknowledgments
This research was supported by grants (U01MH064089, to Dr. Walkup; U01MH64092, to Dr. Albano; U01MH64003, to Dr. Birmaher; U01MH63747, to Dr. Kendall, U01MH64107, to Dr. March; U01MH64088, to Dr. Piacentini; U01MH064003, to Dr. Compton, and T32MH073517, supporting Dr. Gonzalez) from the National Institute of Mental Health. Sertraline and matching placebo were supplied free of charge by Pfizer. Views expressed within this article represent those of the authors, were not influenced by funding sources, and are not intended to represent the position of NIMH, NIH, or DHHS.
References
- 1.Compton SN, March JS, Brent D, Albano AM, Weersing VR, Curry J. Disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43(8):930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- 2.Silverman WK, Pina AA, Viswesvaran C. Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. J Clin Child Adolesc Psychol. 2008;37(1):105–130. doi: 10.1080/15374410701817907. [DOI] [PubMed] [Google Scholar]
- 3.Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527–539. doi: 10.1016/j.chc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Eng J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg GS, Kendall PC, Sakolsky D, Compton SN, Piacentini J, Albano AM, et al. Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J Consult Clin Psychol. 2011;79:806–813. doi: 10.1037/a0025933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman SL, Weems CF, Silverman WK, Kurtines WM. Predictors of outcome in exposure-based cognitive and behavioral treatments for phobic and anxiety disorders in children. Behav Ther. 2000;31(4):713–731. [Google Scholar]
- 7.Bodden DM, Bögels SM, Nauta MH, De Haan E, Ringrose J, Appelboom C, et al. Child versus family cognitive-behavioral therapy in clinically anxious youth: an efficacy and partial effectiveness study. J Am Acad Child Adolesc Psychiatry. 2008;47(12):1384–1394. doi: 10.1097/CHI.0b013e318189148e. [DOI] [PubMed] [Google Scholar]
- 8.Cobham VE, Dadds MR, Spence SH. The role of parental anxiety in the treatment of childhood anxiety. J Consult Clin Psychol. 1998;66:893–905. doi: 10.1037//0022-006x.66.6.893. [DOI] [PubMed] [Google Scholar]
- 9.Cooper PJ, Gallop C, Willetts L, Creswell C. Treatment response in child anxiety is differentially related to the form of maternal anxiety disorder. Behav Cogn Psychother. 2008;36(1):41–48. [Google Scholar]
- 10.Gar NS, Hudson JL. The association between maternal anxiety and treatment outcome for childhood anxiety disorders. Behav Change. 2009;26:1–15. [Google Scholar]
- 11.Kendall PC, Hudson JL, Gosch E, Flannery-Shroder E, Suveg C. Cognitive-behavioral therapy for anxiety disordered youth: a randomized clinical trial evaluating child and family modalities. J Consult Clin Psychol. 2008;76(2):282–297. doi: 10.1037/0022-006X.76.2.282. [DOI] [PubMed] [Google Scholar]
- 12.Crawford AM, Manassis K. Familial predictors of treatment outcome in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2001;40(10):1182–1189. doi: 10.1097/00004583-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Gordon M, Antshel KM, Lewandowski L. Predictors of treatment outcome in a child and adolescent psychiatry clinic: A naturalistic exploration. Child Youth Serv Rev. 2012;34:213–217. [Google Scholar]
- 14.Thienemann M, Moore PS, Tomkins K. A parent-only group intervention for children with anxiety disorders: pilot study. J Am Acad Child Adolesc Psychiatry. 2006;45(1):37–46. doi: 10.1097/01.chi.0000186404.90217.02. [DOI] [PubMed] [Google Scholar]
- 15.Victor AM, Bernat DH, Bernstein GA, Layne AE. Effects of parent and family characteristics on treatment outcome of anxious children. J Anxiety Disord. 2007;21(6):835–848. doi: 10.1016/j.janxdis.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirshfeld-Becker DR, Micco JA, Simoes N, Henin A. High risk studies and developmental antecedents of anxiety disorders. Am J Med Genet C Semin Med Genet. 2008;148C(2):99–117. doi: 10.1002/ajmg.c.30170. [DOI] [PubMed] [Google Scholar]
- 17.Last CG, Hersen M, Kazdin AE, Francis G, Grubb HJ. Pediatric illness in the mothers of anxious children. Am J Psychiatry. 1987;144:1580–1583. doi: 10.1176/ajp.144.12.1580. [DOI] [PubMed] [Google Scholar]
- 18.Lieb R, Wittchen HU, Hofler M, Fuetsch M, Stein MB, Merikangas KR. Parental psychopathology, parenting styles, and the risk of social phobia in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2000;57(9):859. doi: 10.1001/archpsyc.57.9.859. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg GS, Siqueland L, Masia-Warner C, Hedtke KA. Anxiety disorders in children: Family matters. Cogn Behav Pract. 2004;11:28–43. [Google Scholar]
- 20.RUPP Anxiety Study Group The Pediatric Anxiety Rating Scale (PARS: development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Rynn M, Puliafico A, Heleniak C, Rikhi P, Ghalib K, Vidair H. Advances in pharmacotherapy for pediatric anxiety disorders. Depress Anxiety. 2011;28(1):76–87. doi: 10.1002/da.20769. [DOI] [PubMed] [Google Scholar]
- 22.Katz LY, Kozyrskyj AL, Prior HJ, Enns MW, Cox BJ, Sareen J. Effect of regulatory warnings on antidepressant prescription rates, use of health services and outcomes among children, adolescents and young adults. Can Med Assoc J. 2008;178(8):1005–1011. doi: 10.1503/cmaj.071265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray ML, de Vries CS, Wong ICK. A drug utilisation study of antidepressants in children and adolescents using the General Practice Research Database. Arch Dis Child. 2004;89(12):1098–1102. doi: 10.1136/adc.2004.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitiello B, Zuvekas SH, Norquist GS. National estimates of antidepressant medication use among U.S. children, 1997-2002. J Am Acad Child Adolesc Psychiatry. 2006;45(3):271–279. doi: 10.1097/01.chi.0000192249.61271.81. [DOI] [PubMed] [Google Scholar]
- 25.Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 26.Garcia AM, Sapyta JJ, Moore PS, Freeman JB, Franklin ME, March JS, et al. Predictors and moderators of treatment outcome in the Pediatric Obsessive Compulsive Treatment Study (POTS I) J Am Acad Child Adolesc Psychiatry. 2010;49(10):1024–1033. doi: 10.1016/j.jaac.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall PC, Compton SN, Walkup JT, Birmaher B, Albano AM, Sherrill J, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall PC, Flannery-Schroeder E, Panichelli-Mindel SM, Southam-Gerow M, Henin A, Warman M. Therapy for youths with anxiety disorders: A second randomized clinical trial. J Consult Clin Psychol. 1997;65(3):366. doi: 10.1037//0022-006x.65.3.366. [DOI] [PubMed] [Google Scholar]
- 29.Kendall PC, Hedtke KA. Cognitive-behavioral therapy for anxious children: Therapist manual. Workbook Publishing; Ardmore, PA: 2006. [Google Scholar]
- 30.Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for Children for DSMIV: (Child and Parent Versions) Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 31.Silverman WK, Nelles WB. The anxiety disorders interview schedule for children. J Am Acad Child Adolesc Psychiatry. 1988;27(6):772–778. doi: 10.1097/00004583-198811000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Wood A, Kroll L, Moore A, Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: A research note. J Child Psychol Psychiatry. 1995;36:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 33.Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (Form Y) Mind Garden; Palo Alto, CA: 1983. [Google Scholar]
- 34.Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): comparison to the State-Trait Anxiety Inventory (STAI) Psychol Assess. 2007;19(4):369. doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- 35.Grös DF, Simms LJ, Antony MM. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA) in friendship dyads. Behav Ther. 2010;41(3):277–284. doi: 10.1016/j.beth.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- 37.Li F, Duncan TE, Acock A. Modeling interaction effects in latent growth curve models. Struct Equ Modeling. 2000;7:497–533. [Google Scholar]
- 38.Curran PJ, Hussong AM. The use of latent trajectory models in psychopathology research. J Abnorm Psychol. 2003;112(4):526. doi: 10.1037/0021-843X.112.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 40.Browne MYC, Cudeck RR. Alternative ways of assessing model fit. Testing Structural Equation Models. 1993:136–162. [Google Scholar]
- 41.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1(2):130. [Google Scholar]
- 42.Garber J, Weersing VR. Comorbidity of anxiety and depression in youth: Implications for treatment and prevention. Clin Psychol: Sci Prac. 2010;17(4):293–306. doi: 10.1111/j.1468-2850.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th Edition Pearson Education; 2006. [Google Scholar]
- 44.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 45.Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118(1):28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. J Abnorm Psychol. 2008;117(4):860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, et al. Defining treatment response and remission in child anxiety: signal detection analysis using the pediatric anxiety rating scale. J Am Acad Child Adolesc Psychiatry. 2013;52(1):57–67. doi: 10.1016/j.jaac.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Compton, et al. Predictors and Moderators of Treatment Response in Childhood Anxiety Disorders: Results from the CAMS Trial. J Consult Clin Psychol. doi: 10.1037/a0035458. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon M, Antshel KM, Lewandowski L. The cost of collaboration: Predictors of hours spent in collateral contacts. Psychiatr Serv. 2010;61(5):440–442. doi: 10.1176/ps.2010.61.5.440. [DOI] [PubMed] [Google Scholar]
- 50.Chavira DA, Garland A, Yeh M, McCabe K, Hough RL. Child anxiety disorders in public systems of care: Comorbidity and service utilization. J of Beh Health Serv & Res. 2009;36(4):492–504. doi: 10.1007/s11414-008-9139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett PM. Evaluation of cognitive-behavioral group treatments for childhood anxiety disorders. J Clin Child Psychol. 1998;27(4):459–468. doi: 10.1207/s15374424jccp2704_10. [DOI] [PubMed] [Google Scholar]
- 52.Cobham VE, Dadds MR, Spence SH, McDermott B. Parental anxiety in the treatment of childhood anxiety: A different story three years later. J Clin Child Adolesc Psychol. 2010;39(3):410–420. doi: 10.1080/15374411003691719. [DOI] [PubMed] [Google Scholar]
- 53.Wood JJ, Piacentini JC, Southam-Gerow M, Chu BC, Sigman M. Family cognitive behavioral therapy for child anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(3):314–321. doi: 10.1097/01.chi.0000196425.88341.b0. [DOI] [PubMed] [Google Scholar]
- 54.Keeton CP, Ginsburg GS, Drake KL, Sakolsky D, Kendall PC, Birmaher B, et al. Benefits of child-focused anxiety treatments for parents and family functioning. Depress Anxiety. 2013;30(9):865–872. doi: 10.1002/da.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breinholst S, Esbjørn BH, Reinholdt-Dunne ML, Stallard P. CBT for the treatment of child anxiety disorders: A review of why parental involvement has not enhanced outcomes. J Anxiety Disord. 2012;26(3):416–424. doi: 10.1016/j.janxdis.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 56.Simpson D, Suarez L, Connolly S. Treatment and outcomes for anxiety disorders among children and adolescents: A review of coping strategies and parental behaviors. Curr Psychiatry Rep. 2012;14(2):87–95. doi: 10.1007/s11920-012-0254-2. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez A, Weersing VR, Warnick EM, Scahill LD, Woolston JL. Predictors of treatment attrition among an outpatient clinic sample of youths with clinically significant anxiety. Adm Policy Ment Health. 2011;38:356–367. doi: 10.1007/s10488-010-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]