Abstract
Background
The effect of early organ dysfunction on long-term survival in acute pancreatitis (AP) patients is unknown.
Objective
The aim of this study was to ascertain whether early organ dysfunction impacts on long-term survival after an episode of AP.
Methods
A retrospective analysis was performed using survival data sourced from a prospectively maintained database of patients with AP admitted to the Royal Infirmary of Edinburgh during a 5-year period commencing January 2000. A multiple organ dysfunction syndrome (MODS) score of ≥ 2 during the first week of admission was used to define early organ dysfunction. After accounting for in-hospital deaths, long-term survival probabilities were estimated using the Kaplan–Meier test. The prognostic significance of patient characteristics was assessed by univariate and multivariate analyses using Cox's proportional hazards methods.
Results
A total of 694 patients were studied (median follow-up: 8.8 years). Patients with early organ dysfunction (MODS group) were found to have died prematurely [mean survival: 10.0 years, 95% confidence interval (CI) 9.4–10.6 years] in comparison with the non-MODS group (mean survival: 11.6 years, 95% CI 11.2–11.9 years) (log-rank test, P = 0.001) after the exclusion of in-hospital deaths. Multivariate analysis confirmed MODS as an independent predictor of long-term survival [hazard ratio (HR): 1.528, 95% CI 1.72–2.176; P = 0.019] along with age (HR: 1.062; P < 0.001), alcohol-related aetiology (HR: 2.027; P = 0.001) and idiopathic aetiology (HR: 1.548; P = 0.048).
Conclusions
Early organ dysfunction in AP is an independent predictor of long-term survival even when in-hospital deaths are accounted for. Negative predictors also include age, and idiopathic and alcohol-related aetiologies.
Introduction
Acute pancreatitis (AP) is a common disease characterized by acute inflammation of the pancreas, usually triggered by the passage of gallstones or excessive alcohol consumption.1,2 Usually an episode of AP will resolve with supportive measures, but the disease may also be fatal and results in reported mortality of 6–9% in the UK.3–6
Many systems have been devised to stratify the subgroup of patients who present with, or are predicted to develop, the severe form of AP, but these systems focus on the severity of the index episode. According to the widely accepted and updated definition of severe acute pancreatitis (SAP),7 which incorporates evidence of persistent organ failure, approximately one in four patients with AP will experience a severe episode.5,8,9 Unsurprisingly, mortality rates in SAP are considerably higher than in mild disease and range from 14% to 30%.5,10,11 It is well established that the majority of deaths occur early in the disease course; up to 60% of all AP deaths occur during the first 7 days of hospital admission and are mostly attributable to deteriorating organ function.3,4,6,12–14 It is understandable, therefore, that clinical research in AP to date has largely focused on improving early outcomes; relatively little is known about late deaths amongst hospital survivors.
Few contemporary studies have investigated long-term survival and the relevant prognostic factors in AP.15,16 Long-term studies have for the most part examined morbidity outcomes, such as progression to chronic pancreatitis, or have focused upon a severe pancreatitis subgroup. Those that do report survival suggest that SAP hospital survivors may carry a reduced life expectancy.17 After the exclusion of inpatient mortality, death rates amongst AP patients are reported to be in the order of one death in every four to eight cases, over 5–8 years.17,18 Late deaths seem to be most commonly attributed to cardiovascular disease or malignancy,15,16 but the factors that reduce long-term survival are likely to be complex.
The aim of this study was to determine whether early acute organ dysfunction is associated with altered long-term survival in hospital survivors of AP.
Materials and methods
Study approval
Caldicott Guardian approval was obtained to allow the use of confidential patient data. The study was assessed by the University of Edinburgh/National Health Service (NHS) Lothian ACCORD Research and Development Office and the South East Scotland Research Ethics Service and was declared exempt from requirements for formal research ethics committee review as a clinical audit.
Data collection and inclusion criteria
In 2013, a retrospective analysis was performed using long-term survival data sourced from a prospectively maintained database of patients with AP admitted to the Royal Infirmary of Edinburgh during the 5-year period between January 2000 and December 2004. This historical cohort was originally identified from the Lothian Surgical Audit database,19 and its clinical details and immediate outcomes have been previously reported.20 For this cohort, existing data on age, gender, organ dysfunction, aetiology of pancreatitis, necrosectomy and disease severity were supplemented with survival data. Patients for whom data on these characteristics were unavailable were excluded from subsequent analysis. For those patients with recurrent attacks of AP, the earliest episode during the study period was taken as the index episode for the purposes of survival analysis.
Patient inclusion in the original patient cohort was based on the presence of clinical features compatible with AP, supported by the finding of elevated serum amylase (three times higher than the upper limit of the laboratory reference range). In instances in which a strong clinical suspicion of AP in the context of a non-diagnostic amylase result existed, radiological evidence of AP by means of computed tomography or the finding of pancreatitis at laparotomy was required for inclusion. Patients admitted with chronic pancreatitis were excluded.
Definitions: severity, aetiology and organ dysfunction
Severity stratification was performed in accordance with the original version of the Atlanta consensus definition,21 which was the classification system in use at the time when patients in the study cohort presented with AP. Patients were not retrospectively reclassified according to the revised 2012 Atlanta criteria7 in order to avoid introducing error. Patients with an admission APACHE II (Acute Physiology and Chronic Health Evaluation) score of ≥ 8 were classified as predicted severe.
Gallstones were considered to be the precipitating cause of pancreatitis when gallbladder or bile duct calculi were detected by any imaging procedure. In the absence of gallstones and when excessive consumption of alcohol was reported by the patient or the patient's family, the pancreatitis was classified as alcohol-induced. When no definite cause was identified, the disease was characterized as idiopathic. Rarer established causes were collated under the category ‘other’.
Organ dysfunction scores were calculated for all patients at 24 h, 48 h and 7 days, based on the most extreme laboratory values or clinical measurements during each 24-h period, for five (respiratory, cardiovascular, renal, haematological, central nervous system) of six organ systems that constitute the multiple organ dysfunction score (MODS).22 Hepatic dysfunction (serum bilirubin) was excluded in order to avoid the confounding effects of biliary obstruction. A MODS score of ≥ 2 (based on dysfunction of a single or more than one organ system) at one or more time-points was considered to define organ dysfunction. The duration and persistence of organ dysfunction could not be retrospectively ascertained from the existing database; attempts to retrieve archived primary records to gather these data were not successful.
Follow-up and mortality
In-hospital mortality, overall mortality and long-term survival were the main outcome measures of the present study. NHS Lothian electronic patient records were individually reviewed; for surviving patients, the date of the most recent general practitioner and/or hospital visit, outpatient clinic attendance or hospital discharge were defined as the point of last known contact. Dates of death were retrieved and recorded for non-survivors.
Statistical analysis
Data for continuous variables are presented as the mean ± standard deviation (SD) or as the median and interquartile range (IQR). Categorical variables are presented as absolute and relative frequencies. Comparisons between groups were performed using Student's t-test, the Mann–Whitney U-test, the Kolmogorov–Smirnov test and the chi-squared test, as appropriate.
The Kaplan–Meier method was used to estimate survival probabilities. The generalized Wilcoxon test was used to detect early death rate differences between groups and the log-rank test was applied to detect differences manifesting throughout the entire follow-up period. Survival times were calculated from the date of admission to the date of death from any cause or date of last contact in survivors. Patients lost from follow-up were censored at the date of confirmed last contact. The prognostic impact of patient characteristics on survival was assessed by univariate and multivariate Cox's proportional hazards regression. For univariate analyses, the proportional hazards assumption was examined graphically with the use of pairwise Cox log(-log) plots, revealing approximately parallel curves between groups for each of the categorical covariates MODS, gender, severity and aetiology. Linear regression was implemented to test for goodness-of-fit of Schoenfeld partial residuals23 against natural logarithmic survival times for each of the aforementioned covariates; no violation of the proportional hazards assumption was revealed. The linearity assumption for age was confirmed graphically by examining the respective Cox log(-log) plots, after transformation to a categorical variable with six strata. Similar procedures were used for multivariate analyses.
Selection of variables for the multivariate Cox regression model was performed in accordance with the ‘purposeful selection’ algorithm,24,25 originally described by Hosmer and Lemeshow.26 Variables with a univariate P-value of > 0.25 were originally selected as candidates for multivariate analysis. Covariates were subsequently removed from the model if they were found to be non-significant at the α = 0.1 level and not to be confounders. Confounding was defined as a change of > 15% in any of the remaining covariate estimates. Variables that were not selected for the original model were returned one at a time, and any that were significant at the α = 0.15 level were retained.24 This procedure resulted in a multivariate model containing age, MODS, gender, severity and aetiology. The necrosectomy variable did not satisfy the proportional hazards assumption and was examined with the use of stratified Cox regression.
Results of the Cox models are presented as hazard ratios (HRs) with 95% confidence intervals (95% CIs) together with the P-values from Wald's tests. All statistical tests were based on a two-sided α-value of 0.05. Statistical analysis was performed using IBM spss Statistics Version 19.0 (IBM Corp., Armonk, NY, USA). Figures were designed using GraphPad Prism Version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). This double-arm cohort study followed the principles of the STROBE (strengthening the reporting of observational studies in epidemiology) statement.27
Results
Demographics
Unique patient identifiers with corresponding survival data and variables of interest were available for 694 of 759 (91.4%) AP patients in the database and were included in the present study. With regard to classification of disease severity according to APACHE II scores, 256 (36.9%) patients were predicted to have a severe attack, although ultimately 268 (38.6%) suffered from some degree of organ dysfunction during the first week of admission. A total of 235 (33.9%) patients had a MODS score of ≥ 2 at one or more time-points during the first week of their hospital stay and were classified as having organ dysfunction (denoted as the ‘MODS group’). Sixty-nine (9.9%) patients underwent one or more necrosectomy procedures during the study interval. These were performed predominantly as open procedures because minimally invasive retroperitoneal pancreatic (MIRP) necrosectomy was not routinely performed in the department during the study period. Patients’ demographic characteristics are summarized in Table 1.
Table 1.
Demographic characteristics of patients with acute pancreatitis
| Characteristics | Overall sample | Non-MODS groupa | MODS groupa | In-hospital deaths | Survivorsa | Non-survivorsa |
|---|---|---|---|---|---|---|
| Sample size, n (%) | 694 (100%) | 455 (69.1%) | 203 (30.9%) | 36 (5.2%) | 479 (72.8%) | 179 (27.2%) |
| Age, years, median (IQR) | 57.1 (43.2–71.2) | 54.3 (40.3–69.3) | 59.9 (47.7–74.0) | 70.9 (79.9–57.4) | 52.1 (39.0–65.0) | 66.7 (79.5–54.2) |
| Gender, n (%) | ||||||
| Male | 355 (51.2%) | 228 (50.1%) | 106 (52.2%) | 21 (58.3%) | 236 (49.3%) | 98 (54.7%) |
| Female | 339 (48.8%) | 227 (49.9%) | 97 (47.8%) | 15 (41.7%) | 243 (50.7%) | 81 (45.3%) |
| Aetiology of pancreatitis, n (%) | ||||||
| Gallstones | 337 (48.6%) | 223 (49.0%) | 104 (51.2%) | 10 (27.8%) | 237 (49.5%) | 90 (50.3%) |
| Alcohol | 223 (32.1%) | 159 (34.9%) | 54 (26.6%) | 10 (27.8%) | 158 (33.0%) | 55 (30.7%) |
| Idiopathic | 92 (13.3%) | 54 (11.9%) | 27 (13.3%) | 11 (30.6%) | 54(11.3%) | 27 (15.1%) |
| Other | 42 (6.1%) | 19 (4.2%) | 18 (8.9%) | 5 (13.9%) | 30 (6.3%) | 7 (3.9%) |
| Severity, n (%) | ||||||
| Mild | 438 (63.1%) | 380 (83.5%) | 56 (27.6%) | 2 (5.6%) | 338 (70.6%) | 98 (54.7%) |
| Severe | 256 (36.9%) | 75 (16.5%) | 147 (72.4%) | 34 (94.4%) | 141 (29.4%) | 81 (45.3%) |
| Necrosectomy, n (%) | 69 (9.9%) | 17 (3.7%) | 42 (20.7%) | 10 (27.8%) | 43 (9.0%) | 16 (8.9%) |
| In-hospital deaths, n (%) | 36 (5.2%) | N/A | N/A | N/A | N/A | N/A |
| Post-discharge deaths, n (%) | 179 (25.8%) | 107 (59.8%) | 72 (40.2%) | N/A | N/A | 179 (100%) |
| MODS, n (%) | 235 (33.9%) | N/A | N/A | 32 (88.9%) | 131 (27.3%) | 72 (40.2%) |
After exclusion of in-hospital deaths.
MODS, multiple organ dysfunction syndrome; IQR, interquartile range; N/A, not available.
Study follow-up
The median follow-up in the whole patient series was 8.8 years. After excluding in-hospital deaths, median follow-up was 9.0 years. This period was marginally comparable between the MODS group (8.7 years, IQR: 4.7–10.5 years) and non-MODS group (9.1 years, IQR: 6.6–10.6 years) after the exclusion of in-hospital deaths (Mann–Whitney U-test, P = 0.046; Kolmogorov–Smirnov test, P = 0.105).
Overall survival
There were 36 (5.2%) in-hospital deaths in total, 35 of which occurred during the first 2.2 months. During the follow-up period, 179 patients died, giving an overall mortality rate of 31.0% (215 of 694 patients). Median survival in the overall patient sample remained undefined because more than 50% of patients were still alive at the end of follow-up. The overall mean survival was 10.6 years (95% CI 10.2–10.9 years); after the exclusion of in-hospital deaths this increased to 11.1 years (95% CI 10.8–11.5 years).
Organ failure and survival
Analysis of the overall patient sample demonstrated a clear difference in survival between the MODS and non-MODS groups (Fig. 1). Mean survival in the MODS group (8.6 years, 95% CI 7.9–9.3 years) was significantly lower than that in the non-MODS group (11.5 years, 95% CI 11.1–11.9 years) (Wilcoxon test, P < 0.001; log-rank test, P < 0.001). The highest frequency of death occurred during the in-hospital period; the majority of these patients had a MODS score of ≥ 2. Four (11.1%) of the 36 patients who died as inpatients had a MODS score of < 2.
Figure 1.
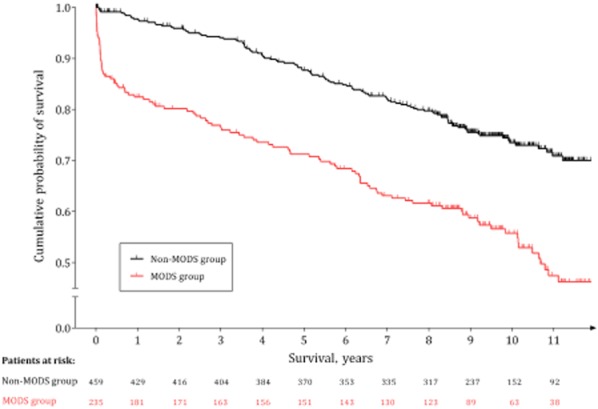
Kaplan–Meier survival plot by group of patients (non-MODS group, n = 459; MODS group, n = 235), representing cumulative percentages of surviving patients by time in years. A distinct, steep decrease is evident on the MODS curve during the first months of the study, corresponding to in-hospital deaths. A clear difference in survival is observed between the two groups. Vertical tick-marks represent right-censored patients. MODS, multiple organ dysfunction syndrome
To investigate long-term mortality, inpatient deaths were removed from the analysis. Interestingly and importantly, with this exclusion, mean survival in MODS group hospital survivors (10.0 years, 95% CI 9.4–10.6 years) remained significantly lower than that in non-MODS group hospital survivors (11.6 years, 95% CI 11.2–11.9 years) (Wilcoxon test, P = 0.002; log-rank test, P = 0.001). Furthermore, the survival curves continued to diverge several years after the index episode of AP (Fig. 2). Again, median survival was not defined; however the 75th percentiles of survival were 6.5 years and 9.9 years in the MODS and non-MODS groups, respectively. Survival rates in each group at 1, 3, 5 and 10 years are shown in Table 2.
Figure 2.
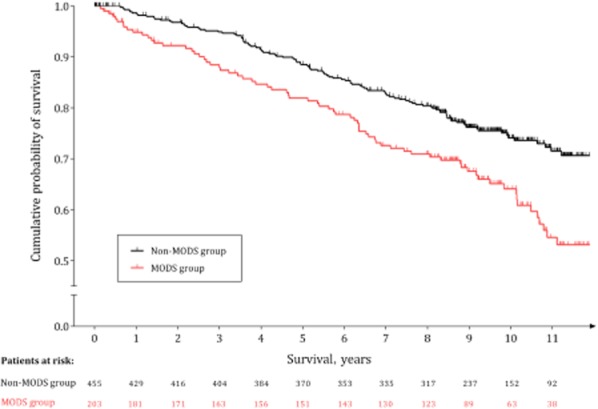
Kaplan–Meier survival plot by group of patients, after the exclusion of in-hospital deaths (non-MODS group, n = 455; MODS group, n = 203), representing the percentages of surviving patients by time in years. A clear, moderately increasing difference in survival is observed between the two groups. Vertical tick-marks represent right-censored patients. MODS, multiple organ dysfunction syndrome
Table 2.
Number of patients at risk and survival rates for each group at 1, 3, 5 and 10 years of follow-up, after exclusion of in-hospital deaths
| Follow-up | MODS group | Non-MODS group | ||||
|---|---|---|---|---|---|---|
| Patients at risk | Cumulative proportion of patients surviving | Standard error | Patients at risk | Cumulative proportion of patients surviving | Standard error | |
| 1 year | 181 | 0.92 | 0.02 | 429 | 0.97 | 0.01 |
| 3 years | 163 | 0.85 | 0.03 | 404 | 0.91 | 0.01 |
| 5 years | 151 | 0.79 | 0.03 | 370 | 0.86 | 0.02 |
| 10 years | 63 | 0.55 | 0.04 | 152 | 0.72 | 0.02 |
MODS, multiple organ dysfunction syndrome.
To account for the difference in mean ± SD age between the MODS (59.9 ± 17.1 years) and non-MODS (54.3 ± 17.9 years) groups (P < 0.001), and because other AP characteristics may impact on long-term survival, a multivariate analysis was performed. The MODS score was shown to be an independent predictor of patient survival, with the MODS group carrying a higher level of risk for post-discharge death than the non-MODS group in both univariate and multivariate analyses. Age and cause of pancreatitis were also established as individual predictors, whereas severity and gender were identified as confounding factors (Table 3). When necrosectomy was added by stratification on the multivariate Cox regression model, a negligible effect was observed. Specifically, the resulting HR for the MODS group was 1.573 (95% CI 1.102–2.246; P = 0.013).
Table 3.
Association of patient characteristics with overall survival, after exclusion of in-hospital deaths
| Factors | Overall survival | |||||
|---|---|---|---|---|---|---|
| Univariate analyses | Multivariate analysis | |||||
| Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value | |
| MODS | ||||||
| Yes | 1.661 | 1.232–2.240 | 0.001 | 1.528 | 1.072–2.176 | 0.019 |
| No | 1 | 1 | ||||
| Gender | ||||||
| Male | 1.181 | 0.879–1.585 | 0.269 | 1.302 | 0.946–1.793 | 0.106 |
| Female | 1 | 1 | ||||
| Age | 1.050 | 1.040–1.061 | < 0.001 | 1.062 | 1.049–1.075 | < 0.001 |
| Severity | ||||||
| Severe | 1.862 | 1.387–2.499 | < 0.001 | 0.850 | 0.587–1.231 | 0.390 |
| Mild | 1 | 1 | ||||
| Cause | ||||||
| Gallstones | 1 | 1 | ||||
| Alcohol | 0.927 | 0.662–1.296 | 0.656 | 2.027 | 1.359–3.024 | 0.001 |
| Idiopathic | 1.350 | 0.878–2.076 | 0.171 | 1.548 | 1.004–2.387 | 0.048 |
| Other | 0.788 | 0.365–1.701 | 0.544 | 1.488 | 0.679–3.263 | 0.321 |
HR, hazard ratio; 95% CI, 95% confidence interval; MODS, multiple organ dysfunction syndrome.
A supplementary univariate Cox regression analysis was performed in the MODS group alone (after the exclusion of in-hospital deaths) in order to examine whether the magnitude of the MODS score (worst MODS score value of days 1, 2 and 7 of admission) or APACHE II score on the day of admission had any effect on long-term survival. No significant effects were revealed for either the MODS score (HR: 0.876, 95% CI 0.748–1.026; P = 0.102) or APACHE II score (HR: 1.019, 95% CI 0.954–1.088; P = 0.573).
Discussion
This study demonstrates that organ dysfunction has a lasting negative impact on long-term survival after an attack of AP. Importantly, this effect is seen after the exclusion of in-hospital deaths and is independent of age, aetiology and gender. In addition, age and an alcohol-related or idiopathic aetiology were also found to negatively influence long-term survival.
The extent to which the consequences of critical illness contribute to post-discharge mortality is poorly understood,28–31 especially in AP. No previous study has specifically investigated the impact of early organ dysfunction as a predictive marker for long-term mortality. Findings of the available long-term AP studies have been equivocal in associating multiple organ failure or various surrogate markers of organ dysfunction with long-term survival. Lund et al.32 found that an increased Ranson's score and length of stay in the intensive care department were associated with an increased long-term incidence of mortality in univariate but not multivariate analyses. Conversely, Halonen et al.17 reported that a smaller proportion of late-death patients had suffered multiple organ failure than had long-term survivors. There was no statistical analysis of this frequency as the study had other objectives pertaining to quality of life outcomes, but this is a surprising result and is difficult to rationalize. However, median follow-up in this study was limited to 61 months, the rate of capture of events (deaths) was subsequently low and the number of participants relatively small.17 Furthermore, acute organ dysfunction has been shown to be associated with reduced life expectancy amongst survivors of critical illness compared with matched controls in a number of other conditions.33,34
In addition to early organ dysfunction, increasing age was observed to be an independent predictor of reduced survival. It is important not to dismiss this finding as obvious, albeit expected, because the observation refers to more than just the fact that older people die sooner from all causes. Although age has been established as an independent predictor in the present and previous studies,35–37 it has been suggested that poorer outcomes in elderly patients may reflect residual functional disability and dysfunction of organ systems caused by critical illness.38 This may be supported by the findings of Lankisch et al., who reported that the most common causes of death were cardiovascular and cerebrovascular diseases.16 This finding was echoed by Nøjgaard et al. with reference to cardiovascular disease.39 The pathophysiological mechanisms and exact long-term confounding effects of critical illness on individual patient subgroups, particularly elderly patients, deserve further investigation.
It is known that the aetiology of AP can influence morbidity amongst hospital survivors, but the influence of aetiology on long-term mortality has been less well researched. Compared with biliary pancreatitis, alcohol seems to have greater deleterious effects in AP with regard to exocrine insufficiency and morphological changes to the pancreas16 and thus it may be unsurprising that patients with alcohol-related pancreatitis have a life expectancy lower than that reported for gallstone pancreatitis. Nøjgaard et al.39 reported significantly higher long-term mortality in patients with AP related to high alcohol consumption, in keeping with the results of the present study.
The longitudinal design of the present study, its long follow-up with a median of approximately 9 years, and the fact that it was conducted in a single centre and included consecutive AP patients regardless of disease severity or treatment are amongst its strengths. Potential weaknesses include the study's retrospective design, although a prospective long-term survival study of this duration would be a major undertaking. Secondly, there is little information regarding the mode of death or any subsequent comorbidity of survivors. The latter detail would certainly merit further investigation. Furthermore, it was not feasible to retrieve and include in the analysis data on the comorbid status of the present cohort. It is therefore possible, and indeed one might argue reasonable, that patients with comorbidities may have been at higher risk for organ dysfunction during AP, which as a consequence resulted in shorter survival. Future studies would benefit from including details on important confounders such as detailed patient comorbid status, the presence of pancreatic necrosis, occurrence of pancreatic insufficiency and other long-term sequelae, as well as specific organ system dysfunction duration and severity. Notwithstanding these weaknesses, the present study identifies an interesting and important long-term negative impact of organ dysfunction in AP, which extends beyond the injury seen in the critical care unit during the index episode. This work reinforces the importance of the early identification, stratification and correct management of extrapancreatic organ dysfunction in AP.
Early organ dysfunction in AP is associated with a shortened lifespan. It is hypothesized that patients who sustain organ dysfunction during their index episode, and who survive, accrue systemic changes which impact negatively on life expectancy in comparison with hospital survivors without multiple organ dysfunction. In AP, the effects of critical illness do not cease after the initial insult has subsided. This study reinforces the cardinal need to identify and pre-empt organ dysfunction, and introduces the need to consider interventions that impact on long-term outcomes in high-risk individuals.
Acknowledgments
DJM holds a Clinician Scientist Fellowship from the Health Foundation/Academy of Medical Sciences, the support of which is gratefully acknowledged.
Conflicts of interest
None declared.
References
- Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ. 2004;328:1466–1469. doi: 10.1136/bmj.328.7454.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg. 1999;86:1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- Toh SK, Phillips S, Johnson CD. A prospective audit against national standards of the presentation and management of acute pancreatitis in the south of England. Gut. 2000;46:239–243. doi: 10.1136/gut.46.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- Winslet M, Hall C, London NJ, Neoptolemos JP. Relation of diagnostic serum amylase levels to aetiology and severity of acute pancreatitis. Gut. 1992;33:982–986. doi: 10.1136/gut.33.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Kemppainen EA, Mayer JM, Fitzpatrick JM, Raraty MG, Slavin J, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955–1960. doi: 10.1016/s0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- Ashley SW, Perez A, Pierce EA, Brooks DC, Moore FD, Jr, Whang EE, et al. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg. 2001;234:572–579. doi: 10.1097/00000658-200110000-00016. ; discussion 579–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg. 1999;86:1020–1024. doi: 10.1046/j.1365-2168.1999.01176.x. [DOI] [PubMed] [Google Scholar]
- Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302. doi: 10.1046/j.0007-1323.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- Mole DJ, Olabi B, Robinson V, Garden OJ, Parks RW. Incidence of individual organ dysfunction in fatal acute pancreatitis: analysis of 1024 death records. HPB. 2009;11:166–170. doi: 10.1111/j.1477-2574.2009.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner IG, Savage WT, 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- Nøjgaard C. Prognosis of acute and chronic pancreatitis – a 30-year follow-up of a Danish cohort. Dan Med Bull. 2010;57:B4228. [PubMed] [Google Scholar]
- Lankisch PG, Breuer N, Bruns A, Weber-Dan B, Lowenfels AB, Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797–2805. doi: 10.1038/ajg.2009.405. ; quiz 806. [DOI] [PubMed] [Google Scholar]
- Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Long-term health-related quality of life in survivors of severe acute pancreatitis. Intensive Care Med. 2003;29:782–786. doi: 10.1007/s00134-003-1700-8. [DOI] [PubMed] [Google Scholar]
- Lankisch PG, Burchard-Reckert S, Petersen M, Lehnick D, Schirren CA, Stöckmann F, et al. Aetiology and age have only a limited influence on the course of acute pancreatitis. Pancreas. 1996;13:344–349. doi: 10.1097/00006676-199611000-00003. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Nixon SJ, Ruckley CV. Lothian surgical audit: a 15-year experience of improvement in surgical practice through regional computerized audit. Lancet. 1997;350:800–804. doi: 10.1016/s0140-6736(97)01021-0. [DOI] [PubMed] [Google Scholar]
- Mofidi R, Madhavan KK, Garden OJ, Parks RW. An audit of the management of patients with acute pancreatitis against national standards of practice. Br J Surg. 2007;94:844–848. doi: 10.1002/bjs.5670. [DOI] [PubMed] [Google Scholar]
- Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G. Medical Biostatistics 2. Available at http://www.academia.edu/2658157/Medical_Biostatistics_2 (last accessed 4 November 2013)
- Hosmer JDW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York, NY: Wiley; 2008. [Google Scholar]
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Keenan SP, Dodek P, Chan K, Hogg RS, Craib KJ, Anis AH, et al. Intensive care unit admission has minimal impact on long-term mortality. Crit Care Med. 2002;30:501–507. doi: 10.1097/00003246-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Kaplan V, Angus DC. Surviving intensive care. Crit Care Med. 2002;30:703–705. doi: 10.1097/00003246-200203000-00037. [DOI] [PubMed] [Google Scholar]
- Angus DC, Carlet J Brussels Roundtable Participants. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–377. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- Williams TA, Dobb GJ, Finn JC, Webb SA. Long-term survival from intensive care: a review. Intensive Care Med. 2005;31:1306–1315. doi: 10.1007/s00134-005-2744-8. [DOI] [PubMed] [Google Scholar]
- Lund H, Tønnesen H, Tønnesen MH, Olsen O. Long-term recurrence and death rates after acute pancreatitis. Scand J Gastroenterol. 2006;41:234–238. doi: 10.1080/00365520510024133. [DOI] [PubMed] [Google Scholar]
- Ulvik A, Kvåle R, Wentzel-Larsen T, Flaatten H. Multiple organ failure after trauma affects even long-term survival and functional status. Crit Care. 2007;11:R95. doi: 10.1186/cc6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: subgroup analysis and comparison with the general population. Anaesthesia. 2003;58:637–642. doi: 10.1046/j.1365-2044.2003.03205.x. [DOI] [PubMed] [Google Scholar]
- Niskanen M, Kari A, Halonen P. Five-year survival after intensive care – comparison of 12,180 patients with the general population. Finnish ICU Study Group. Crit Care Med. 1996;24:1962–1967. doi: 10.1097/00003246-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Thoner J. Outcome and costs of intensive care. A follow-up study on patients requiring prolonged mechanical ventilation. Acta Anaesthesiol Scand. 1987;31:693–698. doi: 10.1111/j.1399-6576.1987.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Dragsted L. Outcome from intensive care. A five-year study of 1308 patients. Dan Med Bull. 1991;38:365–374. [PubMed] [Google Scholar]
- Ridley S, Jackson R, Findlay J, Wallace P. Long-term survival after intensive care. BMJ. 1990;301:1127–1130. doi: 10.1136/bmj.301.6761.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøjgaard C, Matzen P, Bendtsen F, Andersen JR, Christensen E, Becker U. Factors associated with long-term mortality in acute pancreatitis. Scand J Gastroenterol. 2011;46:495–502. doi: 10.3109/00365521.2010.537686. [DOI] [PubMed] [Google Scholar]


