Abstract
Objectives
Total pancreatectomy (TP) is associated with significant morbidity and mortality. The severity of postoperative diabetes and existence of ‘brittle diabetes’ are unclear. This study sought to identify quality of life (QoL) and diabetes-specific outcomes after TP.
Methods
Patients who underwent TP were matched for age, sex and duration of diabetes with patients with type 1 diabetes. General QoL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) core quality of life questionnaire QLQ-C30 and the PAN26 tool. Diabetes-specific outcomes were assessed using the Problem Areas in Diabetes (PAID) tool and an assessment of diabetes-specific complications and outcomes.
Results
A total of 123 patients underwent TP; 88 died (none of diabetic complications) and two were lost to follow-up. Of the remaining 33 patients, 28 returned questionnaires. Fourteen general and pancreas-specific QoL measurements were all significantly worse amongst the TP cohort (QLQ-C30 + PAN26). However, when diabetes-specific outcomes were compared using the PAID tool, only one of 20 was significantly worse. HbA1c values were comparable (P = 0.299), as were diabetes-related complications such as hypoglycaemic attacks and organ dysfunction.
Conclusions
Total pancreatectomy is associated with impaired QoL on general measures compared with that in type 1 diabetes patients. Importantly, however, there was almost no significant difference in diabetes-specific outcomes as assessed by a diabetes-specific questionnaire, or in diabetes control. This study does not support the existence of ‘brittle diabetes’ after TP.
Introduction
Total pancreatectomy (TP) is an uncommon operation, although the present indications for this procedure may bring about an increase in its use. One of the main barriers to performing TP is the morbidity inherently related to the procedure. Of particular relevance are concerns regarding postoperative diabetes, which some authors believe is so severe that it warrants the designation ‘brittle diabetes’ in reference to its erratic blood sugar levels and the difficulty of controlling them.1,2
Total pancreatectomy was initially performed in 19443 and gained popularity in the 1970s as a result of concerns related to the occurrence of pancreatic fistula following partial pancreatectomy, multicentric carcinoma and potential benefits to be derived from extended lymphadenectomy.4–6 However, because it is now recognized that local recurrence after partial pancreatic resection does not reflect multicentric disease, and because partial pancreatectomy is associated with improved survival and morbidity over TP, the latter is now seldom performed.7 To compound the high rates of perioperative morbidity and mortality previously associated with TP,8–10 the metabolic consequences of exocrine and endocrine insufficiency were severe and were compromised by the medical therapy available at the time.1,11 However, the treatment of pancreatic exocrine and endocrine insufficiency is presently well understood and medical management has improved.11–13
Thus there remains a role for TP in selected circumstances. It is likely that its use will increase in some, if not all, pancreatic centres as understanding of pancreatic disease and of what is considered achievable by surgical means evolves. Firstly, intraductal papillary mucinous neoplasia (IPMN) has been identified and with it the recognition that main duct type can be associated with malignant transformation throughout the pancreas gland.14–16 Other clinical scenarios in which TP is required include multifocal disease such as renal cell metastasis.17,18 The procedure can also be used as a strategy of debridement and sepsis control following serious complications of partial pancreatectomy.18 Post-pancreatectomy haemorrhage is a life-threatening complication following pancreatic surgery and almost exclusively occurs in the setting of postoperative pancreatic fistula (POPF).19 Arterial resection during pancreatectomy is uncommon, but one strategy for decreasing perioperative morbidity is to perform TP in this setting, thus avoiding the dreaded complication of POPF in the presence of an arterial anastomosis.20
The decision to undertake TP is made in the knowledge of the associated risks for morbidity and mortality. In some clinical scenarios, such as in completion pancreatectomy in the setting of complicated POPF, in IPMN without multifocal carcinoma and in the context of a decision on whether to perform arterial resection, there are alternative treatment options. Thus the decision to undertake TP may be affected by an understanding of the patient's post-pancreatectomy state. One of the most important aspects, if not the most important, is the severity of post-TP diabetes.
Thus the aim of this study was to perform an analysis of quality of life (QoL) and diabetes-specific outcomes in patients submitted to TP in order to clarify the severity of post-TP diabetes and to verify the existence of the entity described as ‘brittle diabetes’.
Materials and methods
Patients who had undergone TP at the University of Hospitals Birmingham National Health Service (NHS) Trust were identified from a prospectively maintained database. Both patients who had undergone planned TP and those with a subsequent completion pancreatectomy were included. The study period ran from 1 January 1988 to 1 June 2012; this end date permitted a minimum length of follow-up of 12 months.
Study patients were matched with a cohort of patients with type 1 diabetes in an attempt to identify whether the diabetic state associated with TP is indeed worse than or comparable with that in patients with type 1 diabetes. These patients were selected as the ideal control group because their diabetic state and treatment options are clearly understood, and the disease is common. Thus this group provides a natural comparison sample with which to compare diabetes-related outcomes and complications. Patients with type 2 diabetes were excluded, even if they were insulin-dependent, because this disease state reflects peripheral insulin resistance. Patients with type 1 diabetes were identified from a separate prospectively maintained database. Patients were matched on duration of insulin dependence (within 3 years of each other), by age (within 5 years of each other) and sex. For most patients in the TP cohort, the duration of diabetes corresponded to time since TP, although some patients had pre-existing insulin-treated diabetes (i.e. those with chronic pancreatitis). In this scenario, the duration of diabetes was taken from the time of diagnosis of insulin dependence.
Generic QoL was assessed using the European Organization for the Research and Treatment of Cancer (EORTC) core quality of life questionnaire QLQ-C3021 (Version 3, with permission from the EORTC data centre). Scores were analysed according to the scoring manual provided by the EORTC. The additional EORTC pancreatic model, PAN26, which has been validated in both chronic pancreatitis and malignant disease settings, was also used with the agreement of the EORTC.
A diabetes-specific questionnaire using the Problem Areas in Diabetes (PAID) scale was used to assess diabetes-specific outcomes.22,23 HbA1c levels are reported in mmol/mol.
A final set of specific questions was constructed to assess for evidence of diabetes control and end organ damage related to diabetes.
All patients were sent a postal request with copies of these questionnaires. Additional information relating to the medical outcomes of diabetes, such as HbA1c levels, were sought from general practitioners and hospital records. Any non-responders were re-sent the questionnaire.
The related-samples Wilcoxon signed rank and McNemar chi-squared tests were used to compare outcomes from matched subjects. Bonferroni correction was performed in the analyses of multiple responses to the QLQ-C30, PAN26 and PAID questionnaires. Non-matched data were compared using the Mann–Whitney and chi-squared tests. Data are expressed as the median [interquartile range (IQR)]. All analyses were performed using IBM spss Statistics Version 21 (IBM Corp., Armonk, NY, USA). A medical statistician provided advice on data analysis and interpretation.
Results
A total of 123 patients underwent TP over the study period of 24 years. There was an increase in the number of procedures over time: in the first, second, third and fourth quartiles of the study period, TP was performed in 14, 23, 44 and 42 patients, respectively.
The setting of TP (i.e. as an elective planned procedure or as an emergency completion pancreatectomy) and outcomes at last follow-up are summarized in Fig 1.
Figure 1.
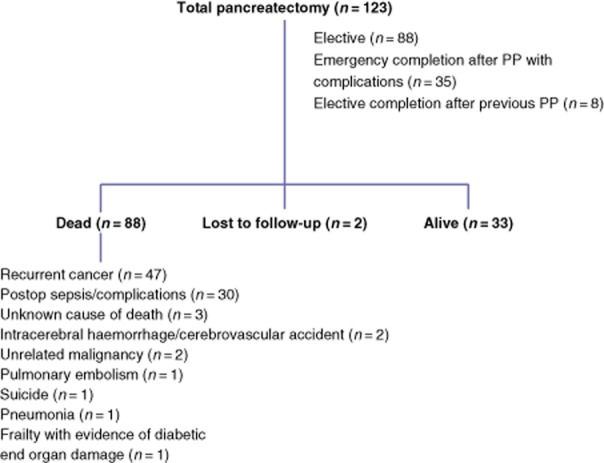
Summary of data in the entire cohort of patients undergoing total pancreatectomy (TP) and outcomes at last follow-up. No patient died as a direct result of diabetes-related complications; one patient died with evidence of diabetic organ damage. PP, partial pancreatectomy
A total of 88 patients (71.5%) had died and two were lost to follow-up. Causes of death are summarized in Fig 1; importantly, no patient died as a direct result of diabetes-related complications such as hypoglycaemia or ketoacidosis. One patient died with a history of diabetic nephropathy, below-knee amputation for peripheral vascular disease and general frailty. The median survival of the whole cohort was 2.0 years (IQR: 0.3–5.6 years) and median potential follow-up was 9.9 years (IQR: 6.1–14.6 years).
Study group
Of the remaining 33 patients, 28 were alive and responded to the questionnaire. These included 16 men. The median age of responders was 63 years. Of the TP procedures, 25 had been planned and three were completion pancreatectomies in the setting of POPF and sepsis. Nine procedures were performed for chronic pancreatitis, five for IPMN, three for renal cancer metastases, three for multifocal neuroendocrine tumours, two for pancreatic adenocarcinoma, one for necrotizing pancreatitis, one for solid pseudopapillary tumour and one for microcystic adenoma.
Matched analysis
Predominately because of age differences between patients in the two databases [young patients predominated in the control type 1 diabetes database (median age: 42 years; IQR: 29–54 years), whereas elderly patients predominated in the TP database (median age: 63 years; IQR: 47–69 years)], it was possible to match 23 patients. A comparison of matching variables between the control and TP cohorts, respectively, showed no differences in median age [53.0 years (IQR: 46.5–60.5 years) versus 58.0 years (IQR: 45.5–68.0 years); P = 0.169], median duration of diabetes [13.4 years (IQR: 5.9–16.9 years) versus 10.3 years (IQR: 7.6–12.4 years); P = 0.589] or male/female sex ratios (14/9 versus 14/9; P = 1). The median time since TP was 8.7 years (IQR: 5.6–11.6 years).
The median age of the remaining five unmatched TP patients was 69.0 years (IQR: 68.0–71.0 years). Their median duration of diabetes was short at 4.0 years (IQR: 3.5–5.7 years). All demographic variables, outcomes reported in the QLQ-C30, PAN26 and PAID questionnaires and assessments of diabetes-specific outcomes were compared between these five unmatched patients and the 23 matched patients. The only variable that differed significantly between these two groups was age (P = 0.041, Mann–Whitney U-test; remaining data not shown).
Quality of life outcomes
Quality of life outcomes as assessed by the QLQ-C30 were lower in the TP cohort than in the control cohort with reference to physical status, working ability, cognitive functioning, social functioning, fatigue, nausea and vomiting, and dyspnoea (Table 1). The difference between the control and TP cohorts in the score of patients reporting steatorrhoea almost reached significance (P = 0.050). The TP cohort also reported worse pancreas-specific pain symptoms, dietary restriction, hepatic symptoms, bloating, bad-tasting food, flatulence, weakness and treatment side-effects compared with the control diabetes cohort as assessed by the PAN26 tool (Table 1). After Bonferroni correction, only physical status, fatigue and bloating remained significant.
Table 1.
Responses of type 1 diabetes control patients and diabetes patients submitted to total pancreatectomy (TP) to general quality of life measures as assessed by the European Organization for the Research and Treatment of Cancer (EORTC) core quality of life questionnaire QLQ-C30 and pancreas-specific supplementary module PAN26
| Item | Cohort score, median (IQR) | P-value | |
|---|---|---|---|
| Control group | TP group | ||
| (n = 23) | (n = 23) | ||
| QLQ-C30 functional scales | |||
| Physical status | 100 (86.6–100) | 66.7 (43.3–86.6) | <0.001b |
| Working ability | 100 (91.7–100) | 66.7 (41.7–83.3) | 0.013a |
| Cognitive functioning | 100 (83.3–100) | 66.7 (33.3–91.7) | 0.009a |
| Emotional functioning | 83.3 (66.7–95.8) | 66.7 (50.0–95.8) | 0.204 |
| Social functioning | 100 (66.7–100) | 66.7 (33.3–100) | 0.019a |
| Global quality of life | 66.7 (50.0–83.3) | 58.3 (29.2–70.8) | 0.109 |
| QLQ-C30 symptom scales | |||
| Fatigue | 11.1 (0–27.8) | 44.4 (33.3–72.2) | 0.003b |
| Nausea and vomiting | 0 (0–0) | 0 (0–16.7) | 0.013a |
| Pain | 0 (0–25) | 16.7 (0–50.0) | 0.191 |
| Dyspnoea | 0 (0–16.7) | 0 (0–33.3) | 0.336 |
| Insomnia | 33.3 (0–33.3) | 66.7 (33.3–100) | 0.008a |
| Appetite loss | 0 (0–33.3) | 0 (0–66.7) | 0.219 |
| Constipation | 0 (0–0) | 0 (0–33.3) | 0.959 |
| Diarrhoea | 0 (0–0) | 0 (0–33.3) | 0.070 |
| Financial difficulties | 0 (0–16.7) | 33.3 (0–50.0) | 0.147 |
| PAN26 symptom scales | |||
| Pancreas-specific pain | 8.3 (4.2–26.7) | 33.3 (22.5–62.5) | 0.011a |
| Diet restriction | 16.7 (0–33.3) | 50 (25.0–66.7) | 0.007a |
| Jaundice and pruritus | 0 (0–16.7) | 16.7 (0–33.3) | 0.006a |
| Steatorrhoea | 16.7 (0–33.3) | 50 (33.3–66.7) | 0.05 |
| Poor body image | 16.7 (0–50.0) | 16.7 (0–58.3) | 0.716 |
| Sexual dysfunction | 33.3 (0–66.7) | 33.3 (0–100) | 0.322 |
| Dissatisfaction with health care | 66.7 (25–83.3) | 66.7 (41.7–100) | 0.392 |
| Bloating | 0 (0–0) | 33.3 (0–66.7) | 0.001b |
| Bad tasting food | 0 (0–0) | 0 (0–33.3) | 0.003b |
| Indigestion | 0 (0–0) | 0 (0–33.3) | 0.103 |
| Flatulence | 0 (0–33.3) | 33.3 (0–83.3) | 0.006a |
| Difficulty gaining weight | 0 (0–0) | 0 (0–16.7) | 0.327 |
| Weakness | 0 (0–33.3) | 33.3 (16.7–66.7) | 0.016a |
| Dry mouth | 0 (0–33.3) | 66.7 (16.7–66.7) | 0.008a |
| Treatment side-effects | 0 (0–33.3) | 33.3 (0–50.0) | 0.020a |
| Worry about health care in the future | 33.3 (16.7–66.7) | 33.3 (33.3–66.7) | 0.500 |
| Difficulty in planning future events | 0 (0–16.7) | 0 (0–33.3) | 0.264 |
On the functional scales, a higher value represents higher quality of life; on the symptom scales, a higher value represents worse symptoms (range: 0–100).
Significance at P < 0.05, Wilcoxon signed ranks test.
Significance with Bonferroni correction (P < 0.0033 for QLQ-C30 responses and P < 0.0029 for PAN26 responses).
IQR, interquartile range.
Diabetes-specific outcomes
There was almost no significant difference between the two cohorts. Scores on the PAID showed that the TP cohort reported statistically significantly more concern regarding hypoglycaemic reactions, although none of the other 19 questions elicited a significant difference in responses (Table 2). However, this difference became non-significant after Bonferroni correction.
Table 2.
Comparisons of scores on the Problem Areas in Diabetes (PAID) scale in type 1 diabetes control patients and diabetes patients submitted to total pancreatectomy (TP)
| Item | Cohort score, median (IQR) | P-value | |
|---|---|---|---|
| Control group | TP group | ||
| (n = 23) | (n = 23) | ||
| Not having clear and concrete goals for your diabetes care? | 0 (0–1) | 1 (0–2) | 0.113 |
| Feeling discouraged with your diabetes treatment plan? | 0 (0–1) | 0 (0–1.5) | 0.501 |
| Feeling scared when you think about living with diabetes? | 1 (0–2) | 1 (0–3) | 0.440 |
| Uncomfortable social situations related to your diabetes (e.g. people telling you what to eat)? | 0 (0–1) | 1 (0–2) | 0.402 |
| Feelings of deprivation regarding food and meals? | 1 (0–1) | 1 (0–2) | 0.204 |
| Feeling depressed when you think about living with diabetes? | 1 (0–2) | 1 (0–2.5) | 0.744 |
| Not knowing if your mood or feelings are related to your diabetes? | 1 (0–2) | 1 (0.5–2) | 0.220 |
| Feeling overwhelmed by your diabetes? | 1 (0–2) | 1 (0–2) | 0.698 |
| Worrying about low blood sugar reactions? | 1 (1–2) | 2 (1–3) | 0.014a |
| Feeling angry when you think about living with diabetes? | 1 (0–2) | 1 (0–2.5) | 0.299 |
| Feeling constantly concerned about food and eating? | 1 (0–1) | 2 (0–3) | 0.141 |
| Worrying about the future and the possibility of serious complications? | 2 (1–2.5) | 3 (0–4) | 0.127 |
| Feelings of guilt or anxiety when you get off track with your diabetes management? | 1 (1–2.5) | 1 (0–3) | 0.690 |
| Not ‘accepting’ your diabetes? | 0 (0–1) | 0 (0–1) | 1.000 |
| Feeling unsatisfied with your diabetes physician? | 0 (0–0) | 0 (0–0) | 0.461 |
| Feeling that diabetes is taking up too much of your mental and physical energy every day? | 1 (0–2) | 2 (0–3) | 0.086 |
| Feeling alone with your diabetes? | 0 (0–1) | 0 (0–1.5) | 0.543 |
| Feeling that your friends and family are not supportive of your management efforts? | 0 (0–1) | 0 (0–1) | 0.749 |
| Coping with the complications of diabetes? | 1 (0–2) | 2 (0–3) | 0.084 |
| Feeling ‘burned’ out by the constant effort needed to manage diabetes? | 1 (0–2) | 2 (0–3) | 0.338 |
Scores vary from 0, which represents ‘not a problem’, to 4, representing a ‘serious problem’.
Significance at P < 0.05, Wilcoxon signed ranks test.
IQR, interquartile range.
There was no significant difference between the two groups in numbers of patients who had experienced hypoglycaemic attacks, hospital admissions or diabetic ketoacidotic episodes, or in the numbers of patients with documented evidence of peripheral vascular disease, cardiovascular disease, nephropathy, neuropathy or retinopathy. Median HbA1c was comparable between the control and TP groups (P = 0.299). Table 3 summarizes these data.
Table 3.
Comparison of diabetes-specific physiological outcome measures in type 1 diabetes control patients and diabetes patients submitted to total pancreatectomy (TP)
| Complication/outcome | Cohort | P-value | |
|---|---|---|---|
| Control group | TP group | ||
| (n = 23) | (n = 23) | ||
| Hypoglycaemic attack in the past month, n | 12 | 14 | 0.754 |
| Hypoglycaemia requiring hospital admission in past year, n | 0 | 5 | 0.5 |
| Previous episode of diabetic ketoacidosis, n | 2 | 0 | 0.5 |
| Peripheral vascular disease, n | 0 | 1 | 1 |
| Cardiovascular disease, n | 12 | 9 | 0.508 |
| Peripheral neuropathy, n | 3 | 5 | 0.727 |
| Autonomic neuropathy, n | 1 | 0 | 1 |
| Erectile dysfunction, n | 0 | 0 | 1 |
| Retinopathy, n | 5 | 3 | 0.688 |
| Nephropathy, n | 1 | 1 | 1 |
| Injection site complications, n | 0 | 0 | 1 |
| Has/under consideration for an insulin pump, n | 2 | 0 | 0.5 |
| HbA1c mmol/mol, median (IQR) | 74.5 (58.75–91) | 65 (56.25–80) | 0.299a |
McNemar's chi-squared test or
Wilcoxon signed rank test.
IQR, interquartile range.
Discussion
This study reports general QoL and diabetes-specific outcomes in patients submitted to TP compared with those in matched patients with type 1 diabetes with the specific aim of reviewing the diabetic state post-TP. Total pancreatectomy is associated with severe metabolic derangement1,8–11 and some authors consider the associated diabetic state, so-called ‘brittle diabetes’, to be particularly severe.1,2 The present study predominately used patient-reported outcomes obtained through validated questionnaires, together with objective evidence assessing the management of diabetes, such as occurrences of hypoglycaemic episodes and HbA1c values. The main findings concerned the significantly worse reported outcomes amongst the TP cohort, but these were centred around general QoL and non-diabetes outcomes. However, just one of 20 reported outcomes as assessed by the diabetes-specific PAID questionnaire showed a significant difference between the groups (although this significance was lost after Bonferroni correction) and, furthermore, no significant differences emerged in the incidence of diabetic complications or glucose control as assessed by HbA1c levels. One conclusion of this study is therefore that the post-TP diabetic state is similar to that in patients with type 1 diabetes and that there is no objective or subjective evidence to demonstrate that the post-TP diabetic state is more severe. The lack of any significant difference in organ-specific complications (such as nephropathy) may relate to the relatively short duration of the median follow-up of 8.7 years. However, the similarities between the groups in HbA1c levels and episodes of hypoglycaemia or ketoacidosis provide no evidence from short-term outcomes to support the suggestion that diabetes control is worse in TP patients. Longitudinal follow-up would be required to confidently compare organ-specific diabetes-related complications.
It is not surprising that general QoL outcomes were worse in the TP cohort because the majority of these patients underwent TP for either malignant disease or pancreatitis. Both of these conditions are known to adversely affect QoL measurements.24–26 There is evidence that TP is related to a worse QoL than partial pancreatic resection. In a matched analysis of QoL outcomes assessed by the QLQ-C30 tool in patients submitted to TP and pylorus-preserving pancreatoduodenectomy, respectively, a worse functional scale, role function, social function and symptom scale were observed in the TP cohort.18
Further studies addressing QoL and outcomes following TP are required because information in this area is scant and there is growing re-interest in TP as an operative strategy. It is likely that surgeons will consider TP in more patients as a result of the recognition of diffuse parenchymal diseases such as IPMN,14–16 multifocal metastases17,18 and chronic pancreatitis.27–29 A further strategic use of TP is in the emergency setting as a way of controlling sepsis in a small proportion of patients with POPF after partial pancreatectomy.18 Hence, understanding of the postoperative outcomes following TP is important in order to allow patients and clinicians to make informed decisions on whether or not to proceed with TP. The few other studies to assess QoL after TP have tended to focus upon general outcomes, although there is evidence to support the observation of this study that post-TP diabetes may not be as severe as previously thought.1,2 The Heidelberg group reported a mean HbA1c of 7.8% in a cohort of TP patients, among whom no life-threatening complications attributable to diabetes occurred.18 Diabetes control in TP patients at the present study institution has been previously reviewed in a matched comparison with diabetes patient controls. Median HbA1c was found to be comparable between the TP and control groups (8.2% and 8.1%, respectively).30 However, no formal QoL or subjective diabetes assessment was performed. In an observational study of Italian TP patients, diabetes control was summarized as good: there was a low rate of readmissions for diabetes, no deaths attributable to diabetes and median HbA1c was near normal at 8%.31 In a non-matched comparison of TP patients with non-surgical diabetes patient controls, Billings et al. found no difference in outcomes reported using the diabetes-specific Audit of Diabetes Dependent QoL tool.32 They did, however, observe three late postoperative deaths caused by hypoglycaemia. Deaths attributable to complications of diabetes (hypoglycaemia, n = 1; ketoacidosis, n = 1) were also recently reported amongst 56 TP patients.33 In the present study, only one patient died with evidence of end organ damage related to diabetes. No patient died as a direct result of hypoglycaemia or ketoacidosis. One potential weakness of the present study is the median duration of follow-up as this limits an assessment of the longterm effects of diabetes. This duration, however, is longer than follow-up in other studies reviewing QoL and diabetes-related complications after TP.18,31–33
A further weakness of the present study refers to its failure to successfully match a small proportion of the TP patients included. Cross-analysis of the unmatched and matched TP patients, however, suggests that outcomes in both groups were comparable and that the only significant difference referred to age. It was this variable that made matching impossible for these patients. Thus, although these patients were not included in the matched analysis, it seems that their reported outcomes were comparable with those of the TP patients who were included in the matched analysis.
In conclusion, TP is associated with a significant risk for mortality and impaired general QoL in comparison with those in matched non-surgical controls with type 1 diabetes. However, the diabetic state that occurs following TP does not appear to differ significantly from that in the group of diabetes patient controls when assessed according to diabetes-specific outcomes.
Acknowledgments
The authors would like to acknowledge James Hodson, medical statistician, who advised on data analysis, Chris Coldham, who is responsible for prospective data collection within the unit, and Professor J. A. C. Buckels and Mr D. Mayer, retired consultant surgeons within the unit.
Conflicts of interest
None declared.
References
- Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131–140. doi: 10.1097/00000658-199108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300:573–578. doi: 10.1056/NEJM197903153001101. [DOI] [PubMed] [Google Scholar]
- Priestley JT, Comfort MW, Radcliffe J. Total pancreatectomy for hyperinsulinism due to an islet-cell adenoma: survival and cure at sixteen months after operation presentation of metabolic studies. Ann Surg. 1944;119:211–221. doi: 10.1097/00000658-194402000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla and other related sites. Jpn J Surg. 1983;13:385–394. doi: 10.1007/BF02469723. [DOI] [PubMed] [Google Scholar]
- Ihse I, Anderson H, Andren S. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–293. doi: 10.1007/s002689900046. [DOI] [PubMed] [Google Scholar]
- van Heerden JA, ReMine WH, Weiland LH, McIlrath DC, Ilstrup DM. Total pancreatectomy for ductal adenocarcinoma of the pancreas. Mayo Clinic experience. Am J Surg. 1981;142:308–311. doi: 10.1016/0002-9610(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Buchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- Grace PA, Pitt HA, Tompkins RK, DenBesten L, Longmire WP., Jr Decreased morbidity and mortality after pancreatoduodenectomy. Am J Surg. 1986;151:141–149. doi: 10.1016/0002-9610(86)90024-3. [DOI] [PubMed] [Google Scholar]
- McAfee MK, van Heerden JA, Adson MA. Is proximal pancreatoduodenectomy with pyloric preservation superior to total pancreatectomy? Surgery. 1989;105:347–351. [PubMed] [Google Scholar]
- Sarr MG, Behrns KE, van Heerden JA. Total pancreatectomy. An objective analysis of its use in pancreatic cancer. Hepatogastroenterology. 1993;40:418–421. [PubMed] [Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of longterm complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME. The longterm effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care. 2013;36:3131–3138. doi: 10.2337/dc12-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC, Lehman GA, Vasileva G, Malecka-Panas E, Gubergrits N, Shen Y, et al. Pancrelipase delayed-release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: a double-blind randomized trial. Am J Gastroenterol. 2010;105:2276–2286. doi: 10.1038/ajg.2010.201. [DOI] [PubMed] [Google Scholar]
- Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente MN, Kleeff J, Esposito I, Hartel M, Muller MW, Frohlich BE, et al. Renal cancer cell metastasis into the pancreas: a single-centre experience and overview of the literature. Pancreas. 2005;30:218–222. doi: 10.1097/01.mpa.0000153337.58105.47. [DOI] [PubMed] [Google Scholar]
- Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, et al. Post-pancreatectomy haemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnis B, Lebeau R, Chopin-Laly X, Adham M. Post-pancreatectomy haemorrhage (PPH): predictors and management from a prospective database. Langenbecks Arch Surg. 2013;398:441–448. doi: 10.1007/s00423-013-1047-8. [DOI] [PubMed] [Google Scholar]
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, et al. Development of a disease-specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Mokrowiecka A, Pinkowski D, Malecka-Panas E, Johnson CD. Clinical, emotional and social factors associated with quality of life in chronic pancreatitis. Pancreatology. 2010;10:39–46. doi: 10.1159/000225920. [DOI] [PubMed] [Google Scholar]
- van der Gaag NA, van Gulik TM, Busch OR, Sprangers MA, Bruno MJ, Zevenbergen C, et al. Functional and medical outcomes after tailored surgery for pain due to chronic pancreatitis. Ann Surg. 2012;255:763–770. doi: 10.1097/SLA.0b013e31824b7697. [DOI] [PubMed] [Google Scholar]
- Alexakis N, Ghaneh P, Connor S, Raraty M, Sutton R, Neoptolemos JP. Duodenum- and spleen-preserving total pancreatectomy for end-stage chronic pancreatitis. Br J Surg. 2003;90:1401–1408. doi: 10.1002/bjs.4324. [DOI] [PubMed] [Google Scholar]
- Braasch JW, Vito L, Nugent FW. Total pancreatectomy of end-stage chronic pancreatitis. Ann Surg. 1978;188:317–322. doi: 10.1097/00000658-197809000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janot MS, Belyaev O, Kersting S, Chromik AM, Seelig MH, Sulberg D, et al. Indications and early outcomes for total pancreatectomy at a high-volume pancreas centre. HPB Surg. 2010;2010:686–702. doi: 10.1155/2010/686702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jethwa P, Sodergren M, Lala A, Webber J, Buckels JA, Bramhall SR, et al. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38:415–419. doi: 10.1016/j.dld.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D, et al. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg. 2010;62:41–46. doi: 10.1007/s13304-010-0005-z. [DOI] [PubMed] [Google Scholar]
- Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality-of-life after total pancreatectomy: is it really that bad on longterm follow-up? J Gastrointest Surg. 2005;9:1059–1066. doi: 10.1016/j.gassur.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P, et al. Impact of total pancreatectomy: short- and longterm assessment. HPB. 2013;15:882–892. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]


