Abstract
Objectives
Recent studies of margin-related recurrence have raised questions on the necessity of ensuring wide resection margins in the resection of colorectal liver metastases. The aim of the current study was to determine whether resection margins of 10 mm provide a survival benefit over narrower resection margins.
Methods
A total of 425 laparoscopic liver resections were carried out in 351 procedures performed in 317 patients between August 1998 and April 2012. Primary laparoscopic liver resections for colorectal metastases were included in the study. Two-stage resections, procedures accompanied by concomitant liver ablations and one case of perioperative mortality were excluded. A total of 155 eligible patients were classified into four groups according to resection margin width: Group 1, margins of < 1 mm [n = 33, including 17 patients with positive margins (Group 1a)]; Group 2, margins of 1 mm to < 3 mm (n = 31); Group 3, margins of ≥ 3 mm to < 10 mm (n = 55), and Group 4, margins of ≥ 10 mm (n = 36). Perioperative and survival data were compared across the groups. Median follow-up was 31 months (range: 2–136 months).
Results
Perioperative outcomes were similar in all groups. Unfavourable intraoperative incidents occurred in 9.7% of procedures (including 3.2% of conversions). Postoperative complications developed in 11.0% of patients. Recurrence in the resection bed developed in three (1.9%) patients, including two (6.1%) patients in Group 1. Rates of actuarial 5-year overall, disease-free and recurrence-free survival were 49%, 41% and 33%, respectively. Median survival was 65 months. Margin status had no significant impact on patient survival. The Basingstoke Predictive Index (BPI) generally underestimated survival. This underestimation was especially marked in Group 1 when postoperative BPI was applied.
Conclusions
Patients with margins of < 1 mm achieved survival comparable with that in patients with margins of ≥ 10 mm. When modern surgical equipment that generates an additional coagulation zone is applied, the association between resection margin and survival may not be apparent. Further studies in this field are required. Postoperative BPI, which includes margin status among the core factors predicting postoperative survival, seems to be less precise than preoperative BPI.
Introduction
Colorectal carcinoma (CRC) is one of the most common of malignant tumours and accounts for at least one million new cases worldwide each year. Haematogenous spread to the liver occurs in 40–60% of CRC patients.1 Liver resection is generally accepted as the standard of care and may cure patients with colorectal metastases, resulting in 5-year survival rates of up to 58%.2 At present, the use of a laparoscopic approach to liver resection is increasing.1,3–8
The introduction of laparoscopic surgery has been accompanied by the rapid adoption of new dissection equipment for parenchymal liver transection.9 Concerns related to margin status have emerged with particular relation to the widespread increase in the use of parenchyma-sparing techniques.10
The present report describes a large-scale retrospective study that aimed to determine whether the achievement of a wide resection margin of 10 mm provides a survival benefit over narrower resection margins.
Materials and methods
Patients and data collection
Between 18 August 1998 and 27 April 2012, 425 laparoscopic liver resections were performed in 317 patients during 351 procedures at the Intervention Centre and Department of Hepatopancreatobiliary Surgery, Oslo University Hospital (Rikshospitalet, Oslo, Norway). In this series, 267 laparoscopic liver resections were performed for colorectal metastases in 186 patients during 205 procedures. Repeat resections (n = 36), liver resections combined with radiofrequency ablation or cryoablation (n = 11), two-stage resections (n = 2) and occurrences of perioperative mortality (n = 1) were excluded from the research analysis. A total of 155 patients were eligible for inclusion in the study. The data were obtained from a prospectively collected database and confirmed by medical records and patient reviews.
Patients were classified into four groups according to resection margin width: Group 1, margins of < 1 mm [n = 33, including 17 patients with positive margins (Group 1a)]; Group 2, margins of ≥ 1 mm to < 3 mm (n = 31); Group 3, margins of ≥ 3 mm to < 10 mm (n = 55), and Group 4, margins of ≥ 10 mm (n = 36).
Patients were staged preoperatively using computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen, as well as chest X-rays or CT. In the latter period of the series, selected patients underwent positron emission tomography (PET) scanning to rule out extrahepatic disease. Patients were defined as having synchronous metastases if they presented with secondaries at the same time or within 6 months of the detection of their primary tumour. The presence of extrahepatic disease amenable to radical surgery was not considered a contraindication to resection.
To assess postoperative survival probabilities, patients were scored using the Basingstoke Predictive Index (BPI), which represents a multifactorial 30-grade scoring system.11 One of the advantages of this scoring system is that it enables both preoperative and postoperative score calculation. The absence or presence of margin status as a scoring factor (for which the redistribution of score points in other cofactors compensates) accounts for differences in pre- and postoperative score calculations.
Unfavourable intraoperative incidents were graded on the basis of the Satava approach to surgical error evaluation.12,13 Postoperative complications were graded in agreement with the Accordion classification (Clavien–Dindo–Strasberg classification).14
Neoadjuvant or adjuvant chemotherapy was applied according to current guidelines. Palliative chemotherapy was offered to patients with inoperable disease during follow-up at the discretion of the oncologist.
Mortality was verified using the Norwegian National Population Register (Folkeregisteret).
Technique
The surgical technique used in the study centre has been described in earlier publications.3,15 Intraoperative ultrasonography was used to determine the final type of liver resection, as well as to mark resection lines and identify additional lesions.
Parenchymal transection was most frequently performed using a bipolar coagulator (LigaSure®; Covidien, Inc., Norwalk, CT, USA) or an ultrasonic dissector [AutoSonix® (Covidien, Inc.); SonoSurg® (Olympus Corp., Tokyo, Japan); Harmonic® (Ethicon Endosurgery, Inc., Blue Ash, OH, USA)] in combination with an ultrasonic surgical aspirator (SonoSurg Aspirator® or CUSA®/Selector®; Integra LifeSciences Corp., Plainsboro, NJ, USA). Ultrasonic dissectors were mostly used to achieve a superficial parenchymal transection. Surgical clips (EndoClip®; Covidien, Inc.) and the LigaSure® device were used in small and medium-sized vessel transections, whereas the Endo-GIA® (Covidien, Inc.) was applied in the transection of major vessels.
Pathologic examination and follow-up
Resection margins were reported macroscopically by the operating surgeon and confirmed by the pathologist after fixation in formaldehyde. Microscopic evaluation was carried out by the pathologist. The distance from the tumour to the closest resection margin was measured in millimetres. In cases of multiple concomitant liver resections, the shortest margin to any of the resected specimens was recorded as the final margin.
Patients were followed through outpatient appointments in which they submitted to clinical examinations, CT of the abdomen, CT of the thorax (or chest X-ray) and serum carcinoembryonic antigen (CEA) assay every 4 months to 1 year post-surgery, and every 6 months thereafter to 5 years or later if necessary.
Patient follow-up data were updated and all research data were ready for research analysis in July 2012.
Statistical analysis
Statistical analyses were carried out using spss Version 18 (SPSS, Inc., Chicago, IL, USA). Data are given as the median (range) and number (percentage) for perioperative parameters. Comparisons among the groups were made using the chi-squared test for categorical data and the Kruskal–Wallis test for continuous clinical outcomes. In the event of a verified statistical difference between groups, the Dunn method was applied for all pairwise multiple comparisons. Rates of 1-, 3- and 5-year survival were analysed using the life tables method. The Kaplan–Meier method was applied to calculate mean survival values and to construct survival curves. The log-rank test was used to compare survival among the groups.
Results
Preoperative patient demographics and clinical features were similar in all four groups (Table 1). The origin of the primary CRC was the colon in 97 (62.6%) patients and the rectum in 58 (37.4%) patients. Six (3.9%) patients had CRC of Dukes tumour stage A, 34 (21.9%) of Dukes stage B, 64 (41.3%) of Dukes stage C and 39 (25.2%) of Dukes stage D. In 12 (7.7%) patients, the stage of the primary tumour was unknown. Liver metastases were diagnosed synchronously in 91 (58.7%) patients. The groups differed significantly in preoperative BPI (Table 1).
Table 1.
Demographic and clinical features of patients submitted to laparoscopic liver resection for colorectal metastasis
| Parameters | Group 1 (margin: < 1 mm) (n = 33) | Group 2 (margin: ≥ 1 mm to < 3 mm) (n = 31) | Group 3 (margin: ≥ 3 mm to < 10 mm (n = 55) | Group 4 (margin: ≥ 10 mm) (n = 36) | P-value | Total (n = 155) |
|---|---|---|---|---|---|---|
| Age, years, median (range) | 66 (37–84) | 65 (43–84) | 64 (35–81) | 69 (44–82) | 0.689 | 66 (35–84) |
| Female/male, n | 15/18 | 16/15 | 24/31 | 13/23 | 0.644 | 68/87 |
| ASA score, median (range) | 3 (1–3) | 2 (1–3) | 2 (2–4) | 2 (2–3) | 0.906 | 2 (1–4) |
| Preoperative BPIa, median (range) | 7 (2–14) | 5 (3–16) | 8 (3–17) | 11 (5–23) | < 0.001 | 7 (2–23) |
| Metachronous/synchronous metastases, n | 15/18 | 12/19 | 20/35 | 17/19 | 0.707 | 64/91 |
| Dukes stage of primary tumour, A/B/C/D, n | 1/6/15/6 | 1/10/8/9 | 2/11/28/11 | 2/7/13/13 | 0.383 | 6/34/64/39 |
| (Not assessable or unknown) | (5) | (3) | (3) | (1) | 12 | |
| Differentiation of primary tumour, good/moderate/poor, n | 2/24/0 | 1/19/0 | 1/38/4 | 4/30/0 | 0.200 | 8/111/4 |
| (Not assessable or unknown) | (7) | (11) | (13) | (2) | 33 | |
| Number of tumours in the liver | ||||||
| 1, n (%) | 19 (58%) | 20 (65%) | 40 (73%) | 30 (83%) | 0.105 | 109 (70%) |
| ≥ 2, n (%) | 14 (42%) | 11 (35%) | 15 (27%) | 6 (17%) | 46 (30%) | |
| Preoperative CEA, median (range) | 16 (1–82) | 15 (1–408) | 18 (1–908) | 13 (1–498) | 0.956 | 15 (1–908) |
| Follow-up period, months, median (range) | 22 (2–86) | 33 (4–115) | 33 (3–132) | 45 (4–136) | 0.150 | 31 (2–136) |
The Dunn method verified a significant difference between Groups 2 and 4, and a significant difference between Groups 2 and 3.
ASA, American Society of Anesthesiologists; BPI, Basingstoke Predictive Index; CEA, carcinoembryonic antigen.
Table 2 shows the operative variables and perioperative outcomes in the four groups of patients. Five (3.2%) of the 155 laparoscopic procedures were converted to open surgery because of intra-abdominal adhesions (n = 2) or haemorrhage (n = 3).
Table 2.
Operative variables and perioperative outcomes in patients submitted to laparoscopic liver resection for colorectal metastasis
| Parameters | Group 1 (margin: < 1 mm) (n = 33) | Group 2 (margin: ≥ 1 mm to < 3 mm) (n = 31) | Group 3 (margin: ≥ 3 mm to < 10 mm (n = 55) | Group 4 (margin: ≥ 10 mm) (n = 36) | P-value | Total (n = 155) |
|---|---|---|---|---|---|---|
| Operative time, min, median (range) | 165 (60–357) | 126 (29–390) | 135 (41–488) | 171 (43–488) | 0.170 | 152 (29–488) |
| Intraoperative blood loss, ml, median (range) | 300 (0–2000) | 300 (0–3000) | 200 (0–4000) | 275 (0–4000) | 0.580 | 250 (0–4000) |
| Unfavourable intraoperative events, n (%) | 4 (12.1%) | 2 (6.5%) | 4 (7.3%) | 5 (13.9%) | 0.639 | 15 (9.7%) |
| Including Grade I | 2 | 2 | 2 | 2 | 8 (5.2%) | |
| Grade II | 2 | – | 2 | 3 | 7 (4.5%) | |
| Grade III | – | – | – | – | – | |
| Postoperative complications, n (%) | 3 (9.1%) | 2 (6.5%) | 8 (14.5%) | 4 (7.3%) | 0.685 | 17 (11.0%) |
| Including Grade I | 1 | 1 | 1 | 1 | 4 (2.6%) | |
| Grade II | – | – | 2 | – | 2 (1.3%) | |
| Grade III | 1 | 1 | 3 | 2 | 7 (4.5%) | |
| Grade IV | 1 | – | 2 | 1 | 4 (2.6%) | |
| Grade V | – | – | – | – | – | |
| Grade VI | – | – | – | – | – | |
| Formal hemi-hepatectomy, n | 1 | 1 | 3 | 2 | 0.921 | 7 (4.5%) |
| Size of largest tumour, cm, median (range) | 2.5 (1.0–8.7) | 2.5 (0.4–12.0) | 2.5 (0.6–8.0) | 3.4 (6.0–7.5) | 0.400 | 2.9 (4.0−12.0) |
| Conversion, n (%) | 2 (6%) | 0 | 2 (3.6%) | 1 (2.8%) | 0.616 | 5 (3.2%) |
| Postoperative hospital stay, days, median (range) | 2 (2.0–3.5) | 3 (2.0–3.5) | 3 (2.8–4.5) | 3 (2.0–3.0) | 0.622 | 3 (3.0–4.0) |
| Postoperative BPIa, median (range) | 14 (2–19) | 4.9 (2–12) | 6 (2–13) | 7 (4–18) | < 0.001 | 6 (2–19) |
The Dunn method verified a significance difference between Group 1 and all other groups, and a significant difference between Groups 2 and 4.
BPI, Basingstoke Predictive Index.
There were no significant differences in perioperative outcomes among the four groups. A total of 109 of 155 patients underwent resection for a solitary metastasis. A total of 211 resections were performed. There were 15 (9.7%) intraoperative incidents, of which eight (5.2%) were of Grade I severity and seven (4.5%) were of Grade II severity. There were no incidents of Grade III severity.
Postoperative complications were observed in 17 (11.0%) patients. These included four, two, seven and four instances of complications of Grades I, II, III and IV, respectively. No instances of Grade V or VI complications occurred (Table 2).
Three patients underwent synchronous resections for colorectal cancer and liver metastases. Liver resection was combined with cholecystectomy in 10 patients, adrenalectomy in two patients, resection of metastases in the left renal hilum in one patient, and left-sided salpingo-oophorectomy in one patient.
The median diameter of the resected metastases was 29 mm (range: 4–120 mm). Formal hemi-hepatectomies were performed in seven (4.5%) patients.
At final pathologic analysis, the resection margin was found to measure < 1 mm (Group 1) in 33 (21.3%) patients. These included 17 (11.0%) patients (Group 1a) with positive margins, all of whom were classified as having R1 resections. Margins measured ≥ 1 mm and < 3 mm (Group 2) in 31 (20.0%) patients, ≥ 3 mm and < 10 mm (Group 3) in 55 (35.5%) patients and ≥ 10 mm (Group 4) in 36 (23.2%) patients. The median margin width was 4 mm (range: 0–50 mm).
The median hospital stay was 3 days (range: 1–33 days).
After a median follow-up of 31 months (range: 2–136 months), 48 (31.0%) patients had died. Of the 17 patients with positive margins, two (11.8%) patients died at 2 months and 13 months after surgery, from diabetic nephropathy and multiple tumour recurrence, respectively. Mean postoperative survival was 81 months. Actuarial 1-, 3- and 5-year overall survival was 84%, 64% and 49%, respectively (Fig. 1). Actuarial 1-, 3- and 5-year disease-free survival was 61%, 45% and 41%, respectively. Actuarial 1-, 3- and 5-year recurrence-free survival was 48%, 35% and 33%, respectively. There were no significant differences in survival among the groups. Survival data for the respective patient groups and a comparison between the observed actuarial survival values and the values predicted by the BPI scoring system are presented in Table 3.
Figure 1.
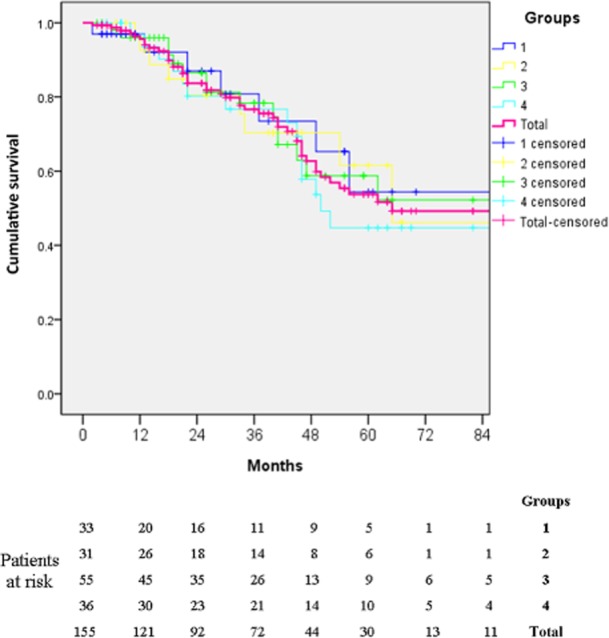
Kaplan–Meier curve for actuarial overall survival
Table 3.
Survival: observed actuarial and predicted survival in patients submitted to laparoscopic liver resection for colorectal metastasis
| Parameters | Group 1 (margin: < 1 mm) (n = 33) | Group 2 (margin: ≥ 1 mm to < 3 mm) (n = 31) | Group 3 (margin: ≥ 3 mm to < 10 mm (n = 55) | Group 4 (margin: ≥ 10 mm) (n = 36) | P-value | Total (n = 155) |
|---|---|---|---|---|---|---|
| Length of survival, months, mean (95% CI) | 63 (49–75) | 74 (54–95) | 81 (63–99) | 80 (61–100) | 0.988 | 81 (73–89) |
| Actuarial 1-year OSa | 86% | 80% | 87% | 80% | 84% | |
| Actuarial 3-year OSa | 72% | 71% | 61% | 58% | 64% | |
| Actuarial 5-year OSa | 54% | 46% | 53% | 45% | 49% | |
| Expected 5-year OS based on preoperative BPI | 42.4% | 46.5% | 37.2% | 33.6% | NA | 39.2% |
| Expected 5-year OS based on postoperative BPI | 30.9% | 49.3% | 44.8% | 41.2% | NA | 42.1% |
| Actuarial 5-year disease-free survivala | 36% | 39% | 49% | 37% | 0.978 | 41% |
| Actuarial 5-year recurrence-free survivala | 30% | 30% | 36% | 32% | 0.913 | 33% |
Retrieved from the life-tables.
95% CI, 95% confidence interval; OS, overall survival; BPI, Basingstoke Predictive Index; NA, not applicable.
Tumour recurrence developed in 73 (47.1%) patients after a median of 12 months (range: 2–44 months). Tumour recurrence in the liver occurred in 51 (32.9%) patients after a median of 9.5 months (range: 4–25 months), including three (1.9%) patients who experienced local recurrence in the resection bed. Two of the three instances of local recurrence were recorded in Group 1. Among the 17 patients with positive margins, tumour recurrence in the liver developed in eight (47.1%) patients, including one (5.9%) patient with local recurrences.
There were no statistical differences among the four groups of patients in rates of either overall or hepatic recurrence (P = 0.316 and P = 0.349, respectively). During follow-up, 36 patients were treated for liver disease recurrences. A total of 30 patients later underwent repeat liver resections of which 18 were performed by laparoscopy and 12 by laparotomy. Six patients underwent secondary radiofrequency ablation or cryoablation (Table 4).
Table 4.
Recurrence in the liver in patients submitted to laparoscopic liver resection for colorectal metastasis
| Parameters | Group 1 (n = 33) | Group 2 (margin: ≥ 1 mm to < 3 mm) (n = 31) | Group 3 (margin: ≥ 3 mm to < 10 mm (n = 55) | Group 4 (margin: ≥ 10 mm) (n = 36) | P-value | Total (n = 155) | |
|---|---|---|---|---|---|---|---|
| 1a (positive margin) (n = 17) | 1b (margin: > 0 mm to < 1 mm) (n = 16) | ||||||
| Overall recurrence, n (%) | 17 (51.5%) | 11 (35.5%) | 28 (50.9%) | 17 (47.2%) | 0.316 | 73 (47.1%) | |
| 8 (47.1%) | 9 (56.3%) | ||||||
| Recurrence in the liver, n (%) | 14 (42.4%) | 10 (32.3%) | 19 (34.5%) | 8 (22.2%) | 0.349 | 51 (32.9%) | |
| 7 (41.2%) | 7 (43.7%) | ||||||
| Recurrence including local (margin), n (%) | 2 (6.1%) | 0 | 1 (1.8%) | 0 | 0.232 | 3 (1.9%) | |
| 1 (5.9%) | 1 (6.3%) | ||||||
| Re-resection for recurrence in the liver, n (%) | 13 (92.9% of all recurrences in the liver) | 5 (50.0%) | 9 (47.4%) | 3 (37.5%) | 0.023 | 30 (58.8%) | |
| 6 (85.7%) | 7 (100%) | ||||||
| Ablation for recurrence in the liver, n (%) | 1 (7.1% of all recurrences in the liver) | 1 (10.0%) | 1 (5.3%) | 3 (37.5%) | 0.102 | 6 (11.8%) | |
Significant differences in the rate of re-resection of the liver were observed between Groups 1 and 3, and Groups 1 and 4 (P = 0.023). There were no significant differences among the groups in terms of later treatments by local ablation for tumour recurrences.
Discussion
Liver resection is generally accepted as the standard of care and may provide a cure for patients with colorectal liver metastases.16 Several clinicopathologic factors have been established as important determinants of treatment failure in colorectal liver metastases,17 including the width of the resection margin.18,19 Most authors have reported that the resection margin is a significant factor that influences both overall and disease-free survival.17–20 In past decades, the risk for a resection margin of < 10 mm has to a certain extent been considered to contraindicate liver resection.21,22
Recent advances in surgical technique and anaesthesia have enabled more patients to undergo hepatic resection. Despite initial concerns about its oncologic adequacy, the laparoscopic approach to the surgical treatment of malignant diseases, particularly to colorectal metastases, has been found to achieve outcomes comparable with those of conventional open resection.6,23–25 Comparative studies have concluded that there are no differences in rates of margin-free resection or in the magnitude of resection margins between laparoscopic and open liver resections.26 Despite the routine use of intraoperative ultrasonography, the rate of positive resection margins still ranges from 5% to 13% and the width of the optimal liver resection margin is still controversial.18,21,27,28 At present, a tumour-free margin is considered to be adequate in the resection of colorectal metastases.16,17
A number of large series have analysed survival factors following liver resection for colorectal metastases. Indeed, earlier studies indicated that survival rates were significantly better in patients in whom resection margins of ≥ 10 mm were achieved than in those in whom margins of < 10 mm were obtained.2,28,29 However, the findings of many recent studies oppose this doctrine.16,17,19,20,28
The present study failed to demonstrate a beneficial effect of margins of ≥ 10 mm. Interestingly, in the current series, patients with resection margins of < 1 mm (Group 1) achieved a 5-year survival rate of 54%, whereas patients with resection margins of ≥ 10 mm (Group 4) obtained a 5-year survival rate of 45%. However, this difference was not significant. Patients with margins of intermediate width (Groups 2 and 3) did not demonstrate any differences with either of the other groups in terms of oncologic outcome. In the present study, patient survival corresponded with preoperative patient scoring rather than margin status or margin width.
The availability of modern, energy-based surgical instruments for parenchymal transection may represent a possible explanation. The use of electrosurgical instruments induces thermal damage to surrounding tissue, which may provide an additional zone of tissue necrosis adjacent to the specimen to be resected. The traditional Kelly clamp crushing technique identifies intraparenchymal structures to be divided without the same thermal tissue damage.25 Thus, as a result of the thermal damage that is likely to have occurred in this series, the true resection margins may be several millimetres wider than those estimated by the pathologist. Correspondingly, pathologists are at risk of reporting a radical resection margin as positive. This may partly explain why the present results seem to contradict the ‘1-cm rule’. Further indirect confirmation of this suggestion is provided by the growing number of publications in which average resection margins reported for both open and laparoscopic liver resection have decreased in recent years.17,19,20,22,29,30 This may primarily reflect a slight change in the attitudes of hepatopancreatobiliary surgeons, but it may also point to a histopathologic underestimation of the resection margins that result from the thermal damage discussed earlier.
Modern energy-based surgical instruments were introduced early in the development of laparoscopic liver resection and their use in open surgery may be less frequent.
In the present series, the frequency of local recurrence in the resection bed is too low to support any definite conclusions regarding potential differences between the subgroups; it is obvious that the numbers are small and a much larger study is required to verify the present results. According to the hypothesized thermal margin, the present authors can only speculate that the true resection margin in the majority of the current patients was actually wider than measured because it was adjusted by thermal injury caused by energy-based instruments.
It should be noted that patients in Group 1 were more likely to have two or more liver metastases than those in Group 4 (42.4% versus 16.7%). Several previous studies have shown that a high number of tumours correlates with reduced disease-free survival, liver recurrence-free survival and overall survival.31 This tendency may partly explain the higher rate of hepatic recurrence in Group 1 compared with Group 4 (42.4% versus 22.2%).
Interestingly, the rate of repeat liver resection was significantly higher in Group 1 than in Group 4. This correlates with the higher rate of hepatic recurrence in this group. After parenchyma-sparing liver surgery, repeat resections can be safely performed laparoscopically with good oncologic outcomes.23
Rates of local liver recurrence after the resection of colorectal liver metastases have not been reported in laparoscopic liver resection and have been poorly reported in the setting of open liver resection.32,33 Although the present sample numbers are small, the rate of local liver recurrence revealed in this series is lower than most rates reported for open liver resection.
Understanding the contemporary discordance between real margin status and that reported by the pathologist may have consequences for both surgeons and pathologists. This emphasizes the importance of collaboration between surgeons and pathologists in further studies on the topic. The survival rates observed in the present study were better than predicted by both the preoperative and postoperative BPIs. Although this underestimation of survival was more remarkable across the whole patient cohort for the preoperative (10%) rather than postoperative (7%) BPI, postoperative BPIs provided gross underestimations, particularly with regard to the group of patients with resection margins of < 1 mm (23%), which may reflect the fact that margin status is a major scoring factor in the postoperative BPI. Thus it would seem that in order to provide a more precise survival prognosis, preoperative BPI is preferable to postoperative BPI for routine use.
In a recently published series of laparoscopic liver resections, Buell et al. compared stapler resection and electrosurgical resection.34 They reported equivalent recurrence and survival rates in the two groups. By contrast with the present series, both groups in the earlier study34 demonstrated a wide median margin (10 mm and 15 mm, respectively). Further, local recurrences in the resection bed were not specifically addressed. Therefore, it was impossible to evaluate the significance of very narrow resection margins in this study.
Although the present study provides additional data on the topic, the impact of a narrow resection margin on patient survival after modern, energy-based liver resection is controversial. The sample size per group in the present study was small and thus the study carries a possibility of type II error. The low rate of local liver recurrences and the difference in the number of metastatic lesions may have influenced the study outcome. Local recurrences after parenchyma-sparing liver resections are frequently resectable. This may also contribute to the low impact of local recurrence on survival.
When modern surgical equipment which generates an additional zone of coagulation is applied, the association between resection margin and survival may not be apparent. This does not mean that surgeons should not try to avoid a positive or very narrow resection margin whenever possible. However, the present authors believe that the findings of this study support the performance of liver resection even in patients in whom the resection margin expected is < 10 mm in width.
Additional studies of both an experimental and a clinical nature are necessary to further define the role of the microscopically involved resection margin in oncologic outcomes after liver resection with modern parenchymal dissection techniques.
Conflicts of interest
None declared.
References
- Punt CJ. New options and old dilemmas in the treatment of patients with advanced colorectal cancer. Ann Oncol. 2004;15:1453–1459. doi: 10.1093/annonc/mdh383. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. ; discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazaryan AM, Marangos IP, Røsok BI, Rosseland AR, Villanger O, Fosse E, et al. Laparoscopic resection of colorectal liver metastases: surgical and longterm oncologic outcome. Ann Surg. 2010;252:1005–1012. doi: 10.1097/SLA.0b013e3181f66954. [DOI] [PubMed] [Google Scholar]
- Kazaryan AM, Røsok BI, Edwin B. Laparoscopic and open liver resection for colorectal metastases: different indications? HPB. 2010;12:434. doi: 10.1111/j.1477-2574.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mala T, Edwin B. Role and limitations of laparoscopic liver resection of colorectal metastases. Dig Dis. 2005;23:142–150. doi: 10.1159/000088596. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Geller DA. Outcomes of laparoscopic hepatic resection for colorectal cancer metastases. J Surg Oncol. 2010;102:975–977. doi: 10.1002/jso.21655. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- Sarpel U, Ayo DM, Newman E. Choice of device for parenchymal transection in laparoscopic hepatectomy. Surg Technol Int. 2012;22:33–38. [PubMed] [Google Scholar]
- Viganò L, Ferrero A, Sgotto E, Polastri R, Muratore A, Capussotti L. [Parenchyma sparing: evolution of the resective surgical approach of hepatic metastasis from the colorectum.] Suppl Tumori. 2005;4:35. [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Satava RM. Identification and reduction of surgical error using simulation. Minim Invasive Ther Allied Technol. 2005;14:257–261. doi: 10.1080/13645700500274112. [DOI] [PubMed] [Google Scholar]
- Kazaryan AM, Røsok BI, Marangos IP, Rosseland AR, Edwin B. Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc. 2011;25:3881–3889. doi: 10.1007/s00464-011-1815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion severity grading system: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:286–298. doi: 10.1016/j.jamcollsurg.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Mala T, Edwin B, Rosseland AR, Gladhaug I, Fosse E, Mathisen O. Laparoscopic liver resection: experience of 53 procedures at a single centre. J Hepatobiliary Pancreat Surg. 2005;12:298–303. doi: 10.1007/s00534-005-0974-3. [DOI] [PubMed] [Google Scholar]
- Edwin B, Nordin A, Kazaryan AM. Laparoscopic liver surgery: new frontiers. Scand J Surg. 2011;100:54–65. doi: 10.1177/145749691110000110. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Vauthey J-N. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not millimetres defines outcome. Ann Surg Oncol. 2008;15:677–679. doi: 10.1245/s10434-007-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady ZZR, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JPA. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17:1324–1329. doi: 10.1245/s10434-009-0770-4. [DOI] [PubMed] [Google Scholar]
- Vandeweyer D, Neo EL, Chen JW, Maddern GJ, Wilson TG, Padbury RT. Influence of resection margin on survival in hepatic resections for colorectal liver metastases. HPB. 2009;11:499–504. doi: 10.1111/j.1477-2574.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaee Z, Kazaryan AM, Marvin MR, Cannon R, Buell JF, Edwin B, et al. Is laparoscopic repeat hepatectomy feasible? A tri-institutional analysis. J Am Coll Surg. 2011;212:171–179. doi: 10.1016/j.jamcollsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Geller DA. Laparoscopic liver resection – current update. Surg Clin North Am. 2010;90:749–760. doi: 10.1016/j.suc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Mala T, Edwin B, Gladhaug I, Fosse E, Søreide O, Bergan A, et al. A comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases. Surg Endosc. 2002;16:1059–1063. doi: 10.1007/s00464-001-9176-5. [DOI] [PubMed] [Google Scholar]
- Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centres. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008;15:2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- Welsh FK, Tekkis PP, O'Rourke T, John TG, Rees M. Quantification of risk of a positive (R1) resection margin following hepatic resection for metastatic colorectal cancer: an aid to clinical decision-making. Surg Oncol. 2008;17:3–13. doi: 10.1016/j.suronc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Elias D, Cavalcanti A, Sabourin JC, Pignon JP, Ducreux M, Lasser P. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. doi: 10.1002/(sici)1096-9098(199810)69:2<88::aid-jso8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cady B, Jenkins RL, Steele GD, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JS, Are C, Kornprat P, Jarnagin WR, Gönen M, Fong Y, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247:109–117. doi: 10.1097/SLA.0b013e3181557e47. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Hojo K, Moriya Y, Yamasaki S, Kosuge T, Takayama T. Pattern of recurrence after hepatic resection for colorectal metastases. Br J Surg. 1993;80:1032–1035. doi: 10.1002/bjs.1800800837. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Adachi E, Toh Y, Ohgaki K, Ikeda O, Oki E, et al. Risk factors for early recurrence after curative hepatectomy for colorectal liver metastases. Surg Today. 2011;41:526–532. doi: 10.1007/s00595-010-4471-1. [DOI] [PubMed] [Google Scholar]
- Buell JF, Gayet B, Han H-S, Wakabayashi G, Kim K-H, Belli G, et al. Evaluation of stapler hepatectomy during a laparoscopic liver resection. HPB. 2013;15:845–850. doi: 10.1111/hpb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]


