Abstract
Objectives
This study was conducted to compare 10-year survivors with patients who survived <10 years in a large Western series of patients submitted to hepatectomy for hepatocellular carcinoma (HCC).
Methods
A retrospective review of a series of hepatic resections conducted in a referral centre for HCC between January 1987 and October 2002 was conducted.
Results
A total of 176 patients were analysed. Twenty-eight patients survived ≥ 10 years (Group A) and were compared with the 148 patients who did not (Group B). Group A had smaller tumours (5.7 cm versus 8.2 cm; P = 0.001) and a lower incidence of microvascular invasion (18.5% versus 37.1%; P = 0.004). Recurrence did not differ significantly (Group A 18/28, 64.3% versus Group B 94/148, 63.5%). Median time to recurrence was longer in Group A (70 months versus 15 months; P < 0.0001), and more patients in Group A were able to undergo curative treatment for recurrence (88.8% versus 40.4%; P < 0.0001). Multivariate analysis showed that lack of vascular invasion (P = 0.020), absence of perioperative transfusion (P = 0.014), and recurrence at >2 years after primary resection (P = 0.045) were significantly associated with 10-year survival.
Conclusions
Ten-year survival after liver resection for HCC can be expected in approximately 15% of patients. Recurrence does not preclude longterm survival. Recurrence at >2 years after resection, absence of vascular invasion, and absence of perioperative transfusion are independently associated with 10-year survival.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most frequent cause of cancer-related deaths worldwide.1 Hepatic resection remains the treatment of choice for single HCC in patients with preserved liver function.2 Clinically significant portal hypertension and high total bilirubin (≥1.0 mg/dl) are generally regarded as contraindications for resection.3 According to these criteria, only about 20% of patients with HCC would be considered ideal candidates for resection.
Despite a large number of series reporting outcomes of hepatic resection for HCC, actual 10-year follow-up and survival data are seldom reported.4–7 A recent meta-analysis of 14 retrospective series found that only 303 actual 10-year survivors had been reported in the world literature over a 20-year span. This translated into an actual 10-year survival rate of only 7%.8
A high rate of recurrence after resection is thought to be the major impediment to longterm survival. In large series of resections performed for HCC, recurrence is generally reported to exceed 60% at 5 years and survival at 5 years is reported to be between 40–50%.4–7 Recurrence most commonly occurs during the first 2 years and has been shown to be mostly attributable to true metastases from the resected tumour during this early period.9,10 De novo tumours, accounting for the majority of late recurrences, arise in patients with underlying liver disease and their incidence increases with time.10,11
The aim of this study was to identify and characterize patients who have survived for ≥10 years after primary hepatic resection for HCC and to identify the variables associated with 10-year survival.
Materials and methods
This study was approved by the study centre's institutional review board. Subsequently, data for all primary liver resections performed for HCC from January 1987 to October 2002 were collected from a prospectively maintained database and reviewed retrospectively. Follow-up was complete until October 2012, allowing for a 10-year follow-up of all survivors. Patients lost to follow-up were excluded from the study. Patients were divided into two groups; Group A included only patients who survived ≥10 years after resection and Group B comprised those who died at < 10 years. The terms ‘10-year survivors’ and ‘longterm survivors’ will be used interchangeably.
Criteria for primary resection
Patients were required to have a single tumour on imaging and the absence of extrahepatic spread. Inclusion criteria for resection also required patients to demonstrate Child–Pugh class A liver function. Prior to 1995, a few patients with Child–Pugh class B liver function were offered resection. Evidence of portal hypertension on imaging or a platelet count of < 100 000/μl was not a contraindication for resection prior to 2002. A transjugular portocaval gradient of >10 mmHg was considered to contraindicate surgery after 2002 and was measured in patients with low platelet counts and no radiographic signs of portal hypertension (splenomegaly or variceal veins) and in those with previous splenectomy in whom platelet count cannot be used as a surrogate for portal hypertension. Gross vascular invasion was not considered a contraindication to resection as long as the main portal vein and the branch to the remaining portion of the liver were patent. Barcelona Clinic Liver Cancer (BCLC) staging was used to guide decision making, but resection was not limited to class A patients because segmental vascular involvement and tumours measuring >5 cm were not considered to contraindicate surgery.12 Patients were discussed weekly in a multidisciplinary conference as suggested by American Association for the Study of Liver Disease (AASLD) guidelines.3
Follow-up protocol
Patients were followed with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and non-contrast CT of the chest every 3 months during the first year after resection, every 4 months during the second year, and every 6 months subsequently. Serum alpha-fetoprotein (AFP) was measured at the same intervals.
The diagnosis of intrahepatic recurrence was based on imaging alone if the tumour displayed enhancement characteristics typical of HCC. Extrahepatic tumours and those with atypical imaging characteristics were biopsied to confirm HCC.
Variables studied
Twenty-two variables divided into four subgroups were identified. Preoperative variables included age, gender, BCLC classification, portal hypertension (on axial imaging and/or based on a platelet count of < 100 000/μl), AFP levels, and underlying liver disease [including hepatitis B and C viruses (HBV, HCV), alcoholic liver disease and other conditions (haemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis and cryptogenic cirrhosis)]. Perioperative variables included major resection (three or more segments), need for transfusion and portal thrombectomy. Pathological variables were identified through the systematic review of specimens for the presence and degree of fibrosis (Scheuer fibrosis stage), largest tumour diameter, tumour differentiation, degree of vascular invasion, presence of satellites, and positive margin status (defined as tumour cells on the cut surface of the resection). Postoperative variables were concentrated mainly in recurrence data and included the number of liver tumours at recurrence, AFP level and BCLC class at recurrence, site of recurrence, time to recurrence and treatment of recurrence. Treatment options after recurrence included potentially curative treatments, such as a second resection, liver transplant, ablation (radiofrequency or ethanol ablation), and non-curative treatments, including transarterial embolization (bland or chemoembolization) and other treatments (systemic chemotherapy and/or external beam radiation).
Statistical analysis
Descriptive statistics are reported as the percentage or as the mean ± standard deviation (SD). Categorical data were compared using the chi-squared test. Continuous data were analysed with t-tests. Survival was calculated in months from the time of initial hepatectomy until the last clinical or telephone encounter, or death. Clinically relevant variables that were significant on univariate analysis were entered into a multivariate binary logistic Cox regression model to identify independent predictors of outcome. Recurrence and time to recurrence were included to evaluate their impact on survival. Overall survival and recurrence curves were generated using the Kaplan–Meier method and compared using the log-rank test. Statistical analysis was performed using IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA). P-values of ≤0.05 were considered to indicate statistical significance.
Results
During the time period under study, 210 patients with HCC underwent hepatic resection as primary treatment, of whom 34 (16.2%) were lost to follow-up. The study population comprises the remaining 176 patients.
Of these 176 patients, 28 (15.9%) survived ≥10 years and were labelled as Group A. The remaining 148 (84.1%) patients survived for < 10 years and represent Group B. The mean length of follow-up in the entire series was 28 months. All survivors were followed for ≥10 years. Overall survival and recurrence curves are shown in Fig. 1.
Figure 1.
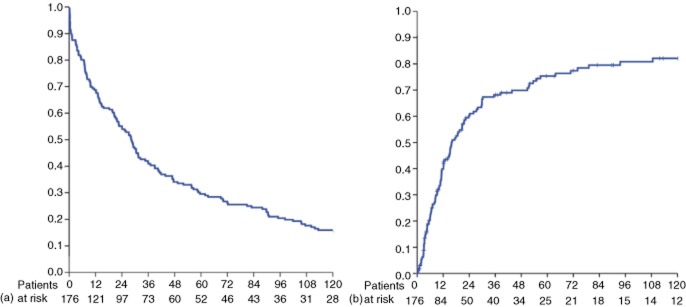
Kaplan–Meier curves showing (a) overall survival and (b) recurrence in the entire cohort of 176 patients submitted to hepatectomy for hepatocellular carcinoma
Baseline characteristics are displayed in Table 1. Perioperative variables and pathological data are shown in Table 2. Variables related to recurrence are recorded in Table 3. The absolute rates of recurrence were almost identical in the two groups [18 of 28 patients (64.3%) versus 94 of 148 patients (63.5%); P = 0.938]. However, Kaplan–Meier analysis and the log-rank test indicated that recurrence was significantly higher in Group B (P = 0.001) as shown in Fig. 2. Early recurrence was far more common in Group B. Median time to recurrence was 70.8 months in Group A and 12.1 months in Group B (P < 0.0001). Recurrence within 2 years was observed in six of 18 Group A patients and 80 of 94 Group B patients (P < 0.0001). Patients in Group A showed significantly better BCLC scores at the time of recurrence, which increased their eligibility for curative treatment. All 18 patients in Group A were treated for their recurrence as noted in Table 3, whereas 10 of the 94 patients in Group B received only supportive care.
Table 1.
Baseline characteristics of patients submitted to resection for hepatocellular carcinoma who did (Group A) and did not (Group B) survive for ≥10 years after resection (n = 176)
| Group A | Group B | ||
|---|---|---|---|
| Preoperative characteristics | (n = 28, 15.9%) | (n = 148, 84.1%) | P-value |
| Age, years, mean ± SD | 60.8 ± 12.1 | 57.5 ± 11.4 | 0.191 |
| Male gender, n (%) | 19 (67.9%) | 107 (72.3%) | 0.633 |
| Underlying liver disease, n (%) | 0.012 | ||
| Hepatitis B virus | 15 (53.6%) | 30 (20.3%) | |
| Hepatitis C virus | 9 (32.1%) | 59 (39.9%) | |
| Other | 1 (3.6%) | 23 (15.5%) | |
| None | 3 (10.7%) | 36 (24.3%) | |
| Stage 3–4 fibrosis, n (%) | 15 (53.6%) | 89 (60.1%) | 0.517 |
| Platelets <100 000/μl | 5 (21.0%) | 21 (15.2%) | 0.489 |
| (Missing data: 12, 6.8%) | |||
| AFP, mean ± SD | 7567 ± 33 070 | 6903 ± 60 350 | 0.926 |
| (Missing data: 28, 15.9%) | |||
| AFP of >400 ng/ml, n (%) | 7 (29.2%) | 40 (32.3%) | 0.766 |
| BCLC class at presentation, n (%) | 0.378 | ||
| (Missing data: 12, 6.8%) | |||
| A | 18 (66.7%) | 68 (48.9%) | |
| B | 3 (11.1%) | 26 (18.7%) | |
| C | 6 (22.2%) | 43 (30.9%) |
SD, standard deviation; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Table 2.
Perioperative and pathological factors affecting longterm survival in patients submitted to resection for hepatocellular carcinoma who did (Group A) and did not (Group B) survive for ≥10 years after resection (n = 176)
| Group A | Group B | ||
|---|---|---|---|
| Factors | (n = 28, 15.9%) | (n = 148, 84.1%) | P-value |
| Perioperative factors | |||
| Major resection (>3 segments), n (%) | 11 (39.3%) | 77 (52.0%) | 0.204 |
| Portal thrombectomy, n (%) | 1 (3.6%) | 19 (12.8%) | 0.154 |
| Required transfusion, n (%) | 8 (28.6%) | 82 (57.3%) | 0.005 |
| (Missing data: 5, 2.8%) | |||
| Pathological factors | |||
| Largest diameter, cm, mean ± SD | 5.7 ± 3.1 | 8.2 ± 5.2 | 0.001 |
| (Missing data: 6, 3.4%) | |||
| Largest diameter >5 cm, n (%) | 11 (40.7%) | 92 (64.3%) | 0.021 |
| Tumour differentiation, n (%) | 10 (38.5%)/12 (46.2%)/4 (15.4%) | 42 (33.6%)/59 (47.2%)/24 (19.2%) | 0.851 |
| (Good/moderate/poor) | |||
| (Missing data: 25, 14.2%) | |||
| Margin positive resection, n (%) | 1 (3.6%) | 9 (6.5%) | 0.581 |
| (Missing data: 9, 5.1%) | |||
| Satellites, n (%) | 8 (28.6%) | 68 (48.6%) | 0.075 |
| (Missing data: 8, 4.5%) | |||
| Vascular invasion, n (%) | 0.001 | ||
| (Missing data: 6, 3.4%) | |||
| None | 15 (55.6%) | 34 (23.8%) | |
| Micro | 5 (18.5%) | 53 (37.1%) | |
| Gross | 7 (25.9%) | 56 (39.2%) |
SD, standard deviation.
Table 3.
Postoperative factors affecting longterm survival in patients submitted to resection for hepatocellular carcinoma who did (Group A) and did not (Group B) survive for ≥10 years after resection (n = 176)
| Group A | Group B | ||
|---|---|---|---|
| Factors | (n = 28, 15.9%) | (n = 148, 84.1%) | P-value |
| Recurrence, n (%) | 18 (64.3%) | 94 (63.5%) | 0.938 |
| Intrahepatic/intra-extra/extrahepatic, n (Missing data: 1, 0.5%) | 16/2/0 | 54/26/13 | 0.049 |
| Single recurrence, n | 13/18 | 30/94 | 0.009 |
| (Missing data: 16, 14.3%) | |||
| Recurrence at < 2 years, n | 6/18 | 80/94 | <0.001 |
| BCLC class A/B/C/D, n | 12/0/2/0 | 27/9/39/4 | 0.006 |
| (Missing data: 19, 17.0%) | |||
| AFP at recurrence, ng/ml, mean ± SD | 7340 ± 26 219 | 4723 ± 21 964 | 0.702 |
| (Missing data: 28, 25.0%) | |||
| AFP >400 ng/ml, n (%) | 2 (15.4%) | 15 (21.1%) | 1.000 |
| Type of treatment after recurrence, n (%) | 18 (64.3%) | 94 (63.5%) | 0.010 |
| (Missing data: 11, 9.8%) | |||
| Re-resection | 5/18 | 8/94 | |
| Transplant | 8/18 | 14/94 | |
| Radiofrequency ablation | 3/18 | 16/94 | |
| Transarterial chemoembolization | 1/18 | 19/94 | |
| Other (chemotherapy and/or radiation) | 1/18 | 16/94 | |
| Supportive care | – | 10/94 |
BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; SD, standard deviation.
Figure 2.
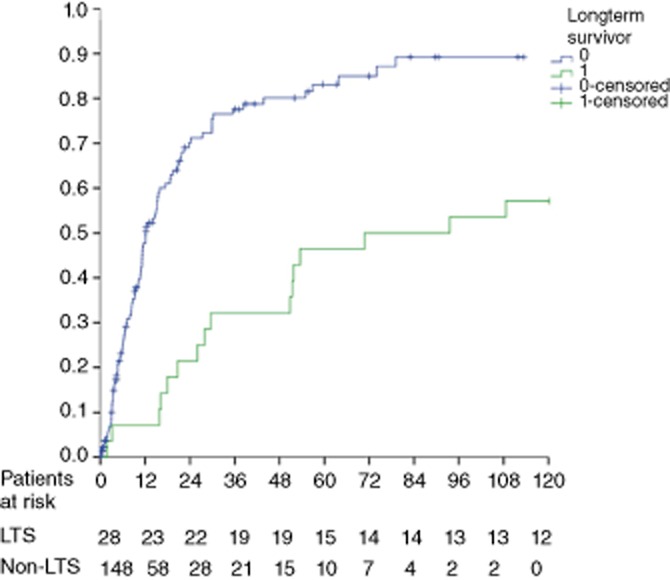
Kaplan–Meier curves for recurrence in patients who did and did not achieve 10-year survival after hepatectomy for hepatocellular carcinoma. LTS, longterm survivors
Multivariate analysis showed the absence of perioperative transfusion, the absence of vascular invasion and recurrence at >2 years to be independently associated with 10-year survival (Table 4).
Table 4.
Multivariate analysis of factors affecting longterm survival in all patients submitted to resection (n = 176)
| P-value | |
|---|---|
| Pathological factors, n (%) | |
| Vascular invasion, yes | 0.020 |
| Tumour diameter of >5 cm | 0.192 |
| Intraoperative factors, n (%) | |
| Blood transfusion (yes/no) | 0.014 |
| Recurrence-related factors, n (%) | |
| Recurrence in < 2 years | 0.045 |
Discussion
This study presents a large series of patients submitted to resection for HCC with actual 10-year survival data; these longterm survivors are the focus of the paper. Few series, particularly in the West, are large enough and have long enough follow-up to provide robust actuarial outcomes for HCC patients 10 years after resection, much less actual survival data. A recent meta-analysis found an actual 10-year survival rate of 7.2%.8
Although hepatic resection is considered a potentially curative treatment for HCC, approximately 70% of patients will develop recurrence within 5 years. The present study confirms this: Kaplan–Meier curves for the entire cohort show that 75.3% of patients experienced recurrence within 5 years and 82.1% did so within 10 years. Of note, recurrence was as common among 10-year survivors as it was in those who died within 10 years of resection in absolute numbers, but, when calculated using the Kaplan–Meier method, the rate of recurrence was statistically higher in Group B. This, however, represents an anomaly of the Kaplan–Meier method. The rate of recurrence is calculated based on the number of patients at risk at a certain time-point. Thus, as none of the patients in Group A died, the denominator in this group remained constant. As patients in Group B died, the denominator in this group became progressively smaller and the proportion of the remaining population at risk increased. Thus, although absolute rates of recurrence were the same in both groups, Kaplan–Meier analysis showed the percentage of patients experiencing recurrence to be higher in Group B.
The most substantial differences between longterm survivors and the rest of the HCC resection population pertained to the ‘when, where and how’ aspects of recurrence rather than the ‘if’. On univariate analysis, 10-year survivors were found to experience recurrence later, were more likely to have a single recurrence in the liver, had a better BCLC score at recurrence and received curative treatment for recurrence (second resection, transplant or radiofrequency ablation) more frequently.
Tumour size has been linked repeatedly to recurrence and survival across multiple studies. A direct relationship between the size and degree of vascular invasion is also well known, and vascular invasion is, by itself, a well-defined predictor of poor outcome.13–15 In this study, the mean tumour size in both groups was relatively large (>5 cm.). Longterm survivors in this study had significantly smaller tumours and were less likely to have vascular invasion on univariate analysis. Microvascular invasion was found to be significantly different in univariate analysis (P = 0.004), but remained independently associated with 10-year survival on multivariate analysis only when clustered together with gross vascular invasion. Another known predictor of poor outcome is need for transfusion; Group A had significantly less need for transfusion on univariate and multivariate analyses.4,16 Although it was not statistically significant, a trend toward higher percentages in Group B emerged in relation to thrombectomy and the presence of satellite nodules, both of which are regarded as ominous.17,18
The distribution of underlying liver disease in this series reflects the heterogeneity of the population. Univariate analysis showed that significantly more patients with HBV were longterm survivors in this series, suggesting that HCC in association with HBV may have a more favourable prognosis. Conflicting results have emerged in previous research pertaining to this association, including a large multicentre study by Pawlik et al.19–22 Other preoperative factors that have been reported to impact prognosis, such as the presence of stage III–IV fibrosis and low platelet count (a surrogate for severe fibrosis),23 did not differ between the groups in this study.
In this series only 35.7% (10 of 28) of longterm survivors remained recurrence-free at 10 years. A recent Japanese study showed that 14 of 74 (18.9%) patients who were disease-free at 10 years after resection for HCC eventually developed recurrence, prompting the authors to suggest lifelong follow-up.24 Delayed recurrence in longterm survivors is likely to represent de novo HCC rather than the progression of occult metastatic disease.10,11
Conclusions
Ten-year survival after liver resection for HCC is possible and can be expected in approximately 16% of patients. Recurrence alone does not preclude longterm survival. Lack of vascular invasion, absence of perioperative transfusions and time to recurrence of >2 years were independent factors associated with 10-year survival.
Conflicts of interest
None declared.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. American Association for the Study of Liver Disease. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–388. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and non-surgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–78. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluer AM, Cocco N, Laurence JM, Johnston ES, Hollands MJ, Pleass HC, et al. Systematic review of actual 10-year survival following resection for hepatocellular carcinoma. HPB. 2012;14:285–290. doi: 10.1111/j.1477-2574.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- Ko S, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Kin T, et al. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy. Result of multivariate analysis. Ann Surg. 1996;224:591–595. doi: 10.1097/00000658-199611000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumour size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western centre. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. ; discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicentre study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Poon RT, Abdalla EK, Sarmiento JM, Ikai I, Curley SA, et al. Hepatitis serology predicts tumour and liver-disease characteristics but not prognosis after resection of hepatocellular carcinoma. J Gastrointest Surg. 2004;8:794–804. doi: 10.1016/j.gassur.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Western experience. Ann Surg Oncol. 2000;7:764–770. doi: 10.1007/s10434-000-0764-8. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Tanaka T, Tanaka W, Yamanaka J, Yasui C, Kuroda N, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. doi: 10.1002/(sici)1097-0142(19970415)79:8<1509::aid-cncr10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Itamoto T, Amano H, Kohashi T, Ohdan H, Tashiro H, et al. Clinicopathologic features of hepatocellular carcinoma patients with compensated cirrhosis surviving more than 10 years after curative hepatectomy. World J Surg. 2007;31:345–352. doi: 10.1007/s00268-006-0513-7. [DOI] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Kaibori M, Kubo S, Nagano H, Hayashi M, Haji S, Nakai T, et al. Clinicopathological features of recurrence in patients after 10-year disease-free survival following curative hepatic resection of hepatocellular carcinoma. World J Surg. 2013;37:820–828. doi: 10.1007/s00268-013-1902-3. [DOI] [PubMed] [Google Scholar]


