Abstract
Objective
The aim of this study was to identify prognostic factors, particularly pathological variables, that influence disease-free and overall survival following resection for colorectal liver metastases (CRLM).
Methods
Patients undergoing CRLM resection from January 2005 to December 2011 were included. Data analysed included information on demographics, laboratory results, operative findings, histopathological features and survival.
Results
A total of 259 patients were included. Of these, 138 (53.3%) patients developed recurrent disease, of which 95 died. The median length of follow-up in the remaining patients was 28 months (range: 12–96 months). There were significant associations between recurrence and higher tumour number (P = 0.002), presence of perineural invasion (P = 0.009) and positive margin (R1) resection (P = 0.002). Multivariate analysis showed all three prognostic factors to be independent predictors of disease-free survival. Significantly poorer overall survival after hepatic resection for CRLM was observed in patients undergoing hemi-hepatectomy or more radical resection (P = 0.021), patients with a higher number of tumours (P = 0.024) and patients with perineural invasion (P < 0.001). Multivariate analysis showed perineural invasion to be the only independent predictor of overall survival.
Conclusions
The presence of perineural invasion, multiple tumours and an R1 margin were associated with recurrent disease. Perineural invasion was also an independent prognostic factor with respect to overall survival.
Introduction
Hepatic resection has become the treatment of choice in resectable colorectal liver metastases (CRLM) and is associated with longterm survival in these patients. Around 25% of patients have synchronous liver metastases at presentation and a further 20% subsequently develop metachronous liver disease, usually within 2 years of the resection of the primary tumour.1,2 Despite the variability in the selection criteria of patients with CRLM for hepatic resection, 5-year survival rates of up to 58% have been reported.1,3,4
Several clinicopathological features have been identified as prognostic factors. These include the size of the largest hepatic metastasis, the number of hepatic metastases, the distribution of hepatic tumours, the extent of hepatic resection, preoperative expression of C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR), and the status of the resection margin.5–9 Various hepatobiliary units have proposed prognostic scoring systems to stratify patients into risk categories for clinical management.5,10 In patients with primary liver tumours such as those of cholangiocarcinoma and hepatocellular carcinoma, lymphatic, vascular and perineural invasion are well-established pathological variables that influence overall survival following hepatic resection.11–13 To date, data on the prognostic implications of vascular, biliary, perineural and lymphatic invasion in patients with CRLM are limited. In addition to clinical scoring systems, these histopathological features may potentially be useful in selecting patients for adjuvant therapy and clinical trials.
The aim of the current study was to analyse the impact of pathological variables, in particular biliary, vascular, perineural and lymphatic invasion, on outcomes in patients after potentially curative hepatic resection for CRLM.
Materials and methods
Patients
Patients with CRLM undergoing hepatic resection at Nottingham University Hospitals National Health Service (NHS) Trust, Nottingham, UK, during the 7-year period from January 2005 to December 2011 were identified from a prospectively maintained database. All patients who underwent primary hepatic resection with curative intent were included in the analysis. Prior to any treatment, patients were discussed at a specialist multidisciplinary (MDT) meeting that included hepatobiliary surgeons, hepatologists, oncologists, radiologists and pathologists. Preoperative radiological assessment included a computed tomography (CT) scans of the thorax, abdomen and pelvis and magnetic resonance imaging (MRI) of the liver. Patients considered for neoadjuvant chemotherapy and patients with indeterminate lesions, in particular lung nodules, underwent positron emission tomography (PET).
A subgroup of patients were given neoadjuvant oxaliplatin-based chemotherapy prior to liver resection. If this was unsuitable, an irinotecan-based regimen was administered. Following this, patients underwent reassessment prior to resection. According to the unit's protocol, all patients were offered adjuvant chemotherapy following liver resection unless they had undergone chemotherapy adjuvant to bowel resection within 12 months of the primary hepatic resection.
Collated data included information on patient demographics, laboratory analyses, type of surgical resection, histopathology analysis and clinical outcomes.
Surgery
Parenchymal transection was performed using the Cavi-Pulse Ultrasonic Surgical Aspirator (CUSA). Intraoperative ultrasound was performed to confirm the findings of preoperative imaging and to assist in surgical planning. The number of hepatic (Couinaud14) segments resected was determined by the procedure to be performed according to the Brisbane nomenclature.15 The type of surgical procedure was selected with the aim of achieving the resection of all macroscopic disease, clear resection margins and the preservation of sufficient remnant liver. The extent of hepatic resection was used to classify study patients into two groups according to whether the resection represented a lesser procedure than hemi-hepatectomy, or a hemi-hepatectomy or more radical resection.
Follow-up
Patients were followed up in specialist hepatobiliary clinics. Following the initial postoperative review at 1 month, all patients were examined in the outpatient clinic at 3, 6, 12, 18 and 24 months and annually thereafter. At each clinical review, carcinoembryonic antigen (CEA) levels were measured. All patients in this study underwent a minimum follow-up of 1 year following hepatic resection for CRLM.
Surveillance imaging included CT of the thorax, abdomen and pelvis. Patients underwent 6-monthly CT scans during the first 2 years postoperatively, followed by annual CT scans thereafter. Liver MRI was used to characterize suspicious hepatic lesions demonstrated on CT. The development of symptoms suspicious of recurrence at any time-point prompted an earlier than scheduled review.
Following the detection of recurrence on surveillance imaging, patients with unresectable disease were referred to the oncologist and patients who were suitable for further surgery were submitted to liver and/or lung resection within an average of 4 weeks. Overall and disease-free survival data were recorded; disease-free survival was defined as the time from primary hepatic resection to the first documented disease recurrence on imaging. Overall survival was defined as the time between the dates of primary hepatic resection and death or most recent follow-up if the patient was still alive.
Histopathological analysis
Histopathological data for the resected liver specimen were collated. These included: tumour size (maximum diameter); tumour number, and the status of the resection margin. A negative margin (R0) resection was defined by no microscopic evidence of tumour at or within 1 mm of the margin. In addition, lymphatic, perineural, biliary and vascular invasion were determined in haematoxylin and eosin (H&E)-stained sections (Fig. 1).
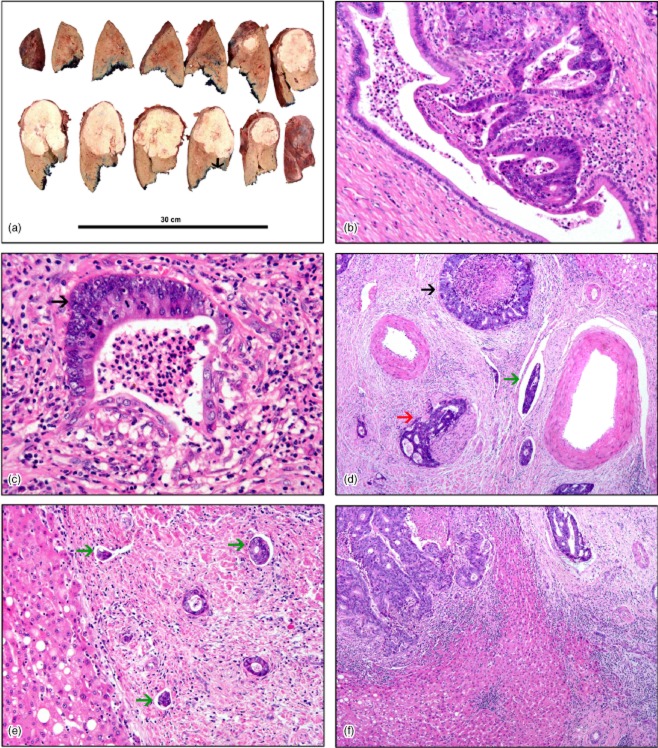
Figure 1.
(a) Gross specimen of hepatic resection showing a single tumour nodule. The distance between the front of the tumour and the margin of excision is marked with an arrowhead. Histopathology shows: (b) early invasion of the biliary epithelium by metastatic adenocarcinoma; (c) invasion of the biliary epithelium of a medium-sized duct by metastatic adenocarcinoma (arrowhead); (d) complete replacement of the biliary epithelium by adenocarcinoma (black arrowhead) with perineural invasion (red arrowhead) and adenocarcinoma cells within vascular spaces (green arrowhead); (e) multiple lymphatic spaces containing metastatic adenocarcinoma cells (green arrowheads), and (f) metastatic adenocarcinoma within the lumen of a blood vessel (top right). [Haematoxylin and eosin stain; original magnification (b) ×20, (c) ×20, (d) ×10, (e) ×10, (f) ×10]
Biliary invasion was defined by the presence of adenocarcinoma cells infiltrating through part of or completely replacing the bile duct epithelium in the large, medium-sized or small intrahepatic bile ducts (Fig. 1b–d). Perineural invasion was defined as tumour cells within any layer of the nerve sheath or tumour in the perineural space (Fig. 1d). Lymphatic invasion was defined by the presence of adenocarcinoma cells within the lumen of the lymphatic space (Fig. 1e). The presence of vascular invasion was defined by adenocarcinoma cells within the lumen of the vascular channel (Fig. 1f).
In this study, all histopathological parameters in hepatic resection specimens of CRLM were reviewed by a specialist hepatobiliary histopathologist (AMZ).
Statistical analysis
Categorical data were presented as frequencies and proportions (%). The median and range were used to describe continuous data. Categorical data were analysed using Pearson's chi-squared test. The Kaplan–Meier method was used to assess actuarial and disease-free survival. Univariate analysis was performed to assess for any significant difference in clinicopathological characteristics that influenced disease recurrence and survival following hepatic resection. Multivariate analysis was performed using Cox regression (stepwise forward model) for variables significant on univariate analysis. Statistical analyses were performed using spss for Windows Version 16.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was set at the 5% level.
Results
Patient demographics, surgical procedures and pathological data
During the study period, 261 patients underwent primary hepatic resection for CRLM. Two patients died postoperatively. Data for these two patients were removed from further analyses. Eleven patients underwent induction chemotherapy prior to liver resection. Two patients submitted to staged liver resections and three patients underwent portal vein embolization. A total of 138 (53.3%) patients developed recurrent disease; 95 of these patients died. The median length of follow-up in the remaining patients was 28 months (range: 12–96 months).
Prognostic factors influencing disease recurrence and overall survival
Multivariate analysis identified three independent predictors of disease-free survival (Table 1): tumour number; perineural invasion, and resection margin. The presence of perineural invasion was the only independent predictor of poorer overall survival on multivariate analysis (Table 2).
Table 1.
Statistical analysis of prognostic factors with respect to disease-free survival
| Demographic, clinical and pathological factors | Survival, months, median (range) | Univariate analysis | Multivariate analysis | Risk ratio (95% CI) |
|---|---|---|---|---|
| Demographic factors | 0.059 | |||
| Age | ||||
| <65 years (n = 82) | 14 (3–82) | |||
| ≥65 years (n = 177) | 18 (3–96) | |||
| Gender | 0.771 | |||
| Male (n = 166) | 18 (3–96) | |||
| Female (n = 93) | 15 (3–84) | |||
| Presentation | 0.704 | |||
| Synchronous (n = 105) | 18 (3–79) | |||
| Metachronous (n = 154) | 16 (3–96) | |||
| Extent of surgery | ||||
| Less than hemi-hepatectomy (n = 136) | 18 (3–84) | 0.336 | ||
| Hemi-hepatectomy or more (n = 123) | 15 (3–96) | |||
| Pathological factors | ||||
| Largest tumour size | 0.110 | |||
| <5 cm (n = 173) | 18 (3–84) | |||
| ≥5 cm (n = 86) | 14 (3–96) | |||
| Number of metastases | 0.002 | 0.018 | 0.655 (0.461–0.930) | |
| Solitary (n = 127) | 21 (3–96) | |||
| Multiple (n = 132) | 12 (3–84) | |||
| Lymphatic invasion | 0.942 | |||
| Positive (n = 42) | 14 (3–96) | |||
| Negative (n = 217) | 17 (3–84) | |||
| Vascular invasion | 0.441 | |||
| Positive (n = 115) | 15 (3–96) | |||
| Negative (n = 144) | 18 (3–84) | |||
| Perineural invasion | 0.009 | 0.007 | 2.346 (1.256–4.382) | |
| Positive (n = 13) | 6 (3–52) | |||
| Negative (n = 246) | 18 (3–96) | |||
| Biliary invasion | 0.451 | |||
| Positive (n = 94) | 18 (3–96) | |||
| Negative (n = 165) | 16 (3–84) | |||
| Resection margin (R0) | 0.002 | 0.018 | 0.618 (0.414–0.922) | |
| R0 (n = 205) | 18 (3–96) | |||
| R1 (n = 54) | 10 (3–69) |
95% CI, 95% confidence interval
Table 2.
Statistical analysis of prognostic factors with respect to overall survival
| Demographic, clinical and pathological factors | Survival, months, median (range) | Univariate analysis | Multivariate analysis | Risk ratio (95% CI) |
|---|---|---|---|---|
| Demographic factors | 0.718 | |||
| Age | ||||
| <65 years (n = 82) | 24 (5–82) | |||
| ≥65 years (n = 177) | 27 (4–96) | |||
| Gender | 0.709 | |||
| Male (n = 166) | 25 (4–96) | |||
| Female (n = 93) | 27 (4–84) | |||
| Presentation | 0.964 | |||
| Synchronous (n = 105) | 28 (7–79) | |||
| Metachronous (n = 154) | 25 (4–96) | |||
| Extent of surgery | ||||
| Less than hemi-hepatectomy (n = 136) | 24 (7–84) | 0.021 | 0.119 | 1.410 (0.916–2.170) |
| Hemi-hepatectomy or more (n = 123) | 27 (4–96) | |||
| Pathological factors | ||||
| Largest tumour size | 0.263 | |||
| <5 cm (n = 173) | 27 (9–84) | |||
| ≥5 cm (n = 86) | 24 (4–96) | |||
| Number of metastases | 0.024 | 0.103 | 0.705 (0.463–1.073) | |
| Solitary (n = 127) | 27 (4–96) | |||
| Multiple (n = 132) | 25 (6–84) | |||
| Lymphatic invasion | 0.730 | |||
| Positive (n = 42) | 24 (4–96) | |||
| Negative (n = 217) | 25 (4–84) | |||
| Vascular invasion | 0.612 | |||
| Positive (n = 115) | 24 (4–96) | |||
| Negative (n = 144) | 28 (5–84) | |||
| Perineural invasion | <0.001 | <0.001 | 3.152 (1.636–6.074) | |
| Positive (n = 13) | 19 (6–56) | |||
| Negative (n = 246) | 26 (4–96) | |||
| Biliary invasion | 0.901 | |||
| Positive (n = 94) | 24 (4–96) | |||
| Negative (n = 165) | 26 (4–84) | |||
| Resection margin (R0) | 0.087 | |||
| R0 (n = 205) | 27 (4–96) | |||
| R1 (n = 54) | 20 (4–69) |
95% CI, 95% confidence interval
Impact of perineural invasion on survival
Patients with perineural invasion were found to have a significantly higher likelihood of lymphatic (P < 0.001), vascular (P = 0.003) or biliary (P < 0.001) invasion compared with patients who did not exhibit perineural invasion (Table 3). Patients with perineural invasion were more likely to undergo a hemi-hepatectomy or more radical resection (P = 0.029).
Table 3.
Demographic, clinical and pathological factors in patients with and without perineural invasion
| Demographic, clinical and pathological factors | Patients with perineural invasion (n = 13), n | Patients without perineural invasion (n = 246), n | P-value |
|---|---|---|---|
| Demographic factors | |||
| Age ≥65 years | 6 | 171 | 0.078 |
| Male gender | 9 | 157 | 0.692 |
| Synchronous presentation | 3 | 102 | 0.188 |
| Extent of surgery | |||
| Hemi-hepatectomy or more | 10 | 113 | 0.029 |
| Pathological factors | |||
| Largest tumour size ≥5 cm | 7 | 79 | 0.105 |
| Solitary hepatic metastases | 4 | 123 | 0.172 |
| Lymphatic invasion | 7 | 35 | <0.001 |
| Vascular invasion | 11 | 104 | 0.003 |
| Biliary invasion | 11 | 83 | <0.001 |
| Resection margin | 12 | 193 | 0.231 |
Median disease-free and overall survival in patients with perineural invasion were 12 months and 21 months, respectively. Five-year disease-free survival in patients without perineural invasion was 43.1% (P = 0.009) (Fig. 2). Five-year overall survival in patients without perineural invasion was 48.0% (P < 0.001) (Fig. 3). None of the patients with perineural invasion survived to 5 years.
Figure 2.
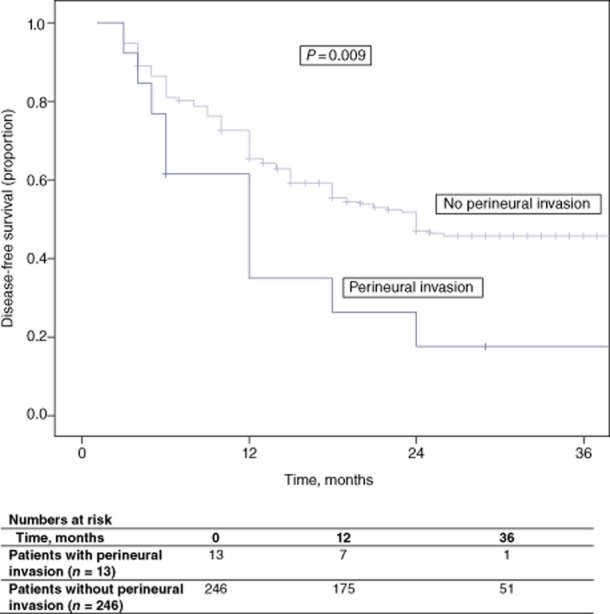
Disease-free survival in patients with and without perineural invasion
Figure 3.
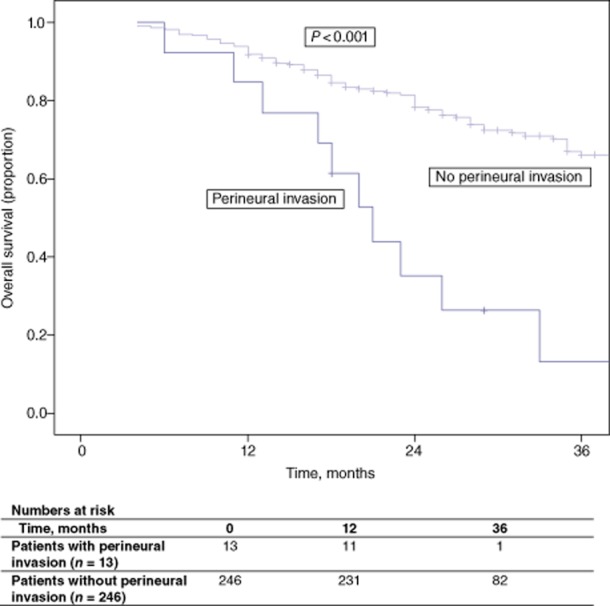
Overall survival in patients with and without perineural invasion
Discussion
Hepatic resection for CRLM has consistently achieved good longterm disease-free and overall survival based on absence of recurrence16,17 and thus offers outcomes that stand in stark contrast to those in patients with unresectable disease.18 The present series demonstrates that tumour number, resection margin and perineural invasion significantly influenced outcome.
Number of metastases
Various studies have shown tumour number to significantly influence survival outcomes,7,8,19 although other authors have not demonstrated this finding.1,5,7,20 In the present series, patients with multiple metastases had poorer disease-free survival, but this variable did not influence overall survival. The fact that tumour number has been inconsistently identified suggests that other factors, such as tumour biology, may influence outcomes in CRLM patients depending on their tumour burden at presentation.
Resection margin
Some authors have observed that resection margin and margin width did not correlate significantly with survival following resection for CRLM,21 whereas others have shown margin status to be a predictor of survival outcome.22,23 A recent meta-analysis24 demonstrated that a margin of ≥ 1 cm confers a survival benefit compared with a margin of < 1 cm. In the present cohort, tumour at the resection margin was a predictor of poorer disease-free survival, but not overall survival. These differences suggest that only a selected group of CRLM patients undergoing resection are influenced by an R0 margin, especially in the era of systemic chemotherapy, which represents an important avenue of treatment in the multimodal approach to therapy in these patients.25–27
Intrahepatic invasion
Few studies have investigated the incidence of intrahepatic invasion,28–34 and the exact definitions, types and methods of detection in CRLM specimens have not been described. Sasaki et al.32 defined portal vein, hepatic vein and bile duct invasion as cancer cells invading the lumen of an artery or vein or bile duct branches within the liver, and lymphatic invasion as cancer cells involving the luminal structures in the portal area lined by endothelial cells. Korita et al.34 defined lymphatic invasion as the presence of tumour cells within vessels that showed immunoreactivity for D2-40 monoclonal antibody, but did not define other forms of intrahepatic invasion. By contrast, the present study defined vascular, biliary, lymphatic and perineural invasion, as well as the methods used to detect the presence of tumour cells in their respective channels.
A limited number of studies have assessed the influence of vascular invasion on outcomes in patients with CRLM.27,29–31,34 Although improved survival has been observed in patients without portal vein invasion compared with patients with portal vein invasion,35 the sample sizes referred to elsewhere were small, leading to significant heterogeneity.27,29,31,32 Similarly, few studies have reported outcomes in patients with hepatic vein invasion in CRLM specimens,29,31,32 the influence of which remains to be determined.35 A recent meta-analysis of five studies that assessed outcomes in CRLM patients with biliary invasion29,31–33,36 showed no correlation between the presence of biliary invasion and overall survival.35 A meta-analysis of two studies evaluating the impact of lymphatic invasion on outcomes in CRLM patients32,34 demonstrated lymphatic invasion to be associated with significantly poorer survival.35 In the present study, vascular, lymphatic and biliary invasion in CRLM patients did not significantly influence survival outcome. Similarly, Bockhorn et al.37 observed that the presence of vascular invasion or lymphatic infiltration in isolation did not significantly influence survival, but the presence of both in combination resulted in significantly poorer survival than in patients without vascular and lymphatic invasion.
Perineural invasion
Leibig et al.38 showed perineural infiltration to be a predictor of survival in patients undergoing surgery for colorectal cancer. Although perineural invasion has been observed in 12–17% of patients undergoing hepatic resection for CRLM,28,29,33 it has been reported not to significantly influence survival.28,33 In the present cohort, 5% of patients had perineural invasion and achieved significantly poorer disease-free and overall survival. In addition, the presence of perineural invasion was significantly associated with lymphatic, vascular and biliary invasion. These results demonstrate that this subgroup of patients is more likely to have aggressive tumour biology resulting in intrahepatic invasion of the tumour. This may account for the significantly higher number of major liver resections required to achieve clear margins in this group of patients.
Although it was retrospective in nature, the present study defined each histopathological feature of intrahepatic invasion and the methods used for its detection. The presence of perineural invasion following resection of CRLM was an independent predictor of a poorer outcome. The association of perineural invasion with other features of intrahepatic invasion is likely to reflect more aggressive disease and hence a poorer outcome.
Conflicts of interest
None declared.
References
- Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, et al. Outcome of patients with colorectal liver metastasis: analysis of 1613 consecutive cases. Ann Surg Oncol. 2012;19:2860–2868. doi: 10.1245/s10434-012-2356-9. [DOI] [PubMed] [Google Scholar]
- Bockhorn M, Frilling A, Fruhauf NR, Neuhaus J, Molmenti E, Trarbach T, et al. Survival of patients with synchronous and metachronous colorectal liver metastases – is there a difference? J Gastrointest Surg. 2008;12:1399–1405. doi: 10.1007/s11605-008-0508-9. [DOI] [PubMed] [Google Scholar]
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. ; discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Sangha VK, Morris-Stiff G, Malik HZ, Guthrie AJ, Toogood GJ, et al. Outcomes of intensive surveillance after resection of hepatic colorectal metastases. Br J Surg. 2010;97:1552–1560. doi: 10.1002/bjs.7136. [DOI] [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, Prasad KR, et al. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer. 2007;96:222–225. doi: 10.1038/sj.bjc.6603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- Malik HZ, Gomez D, Wong V, Al-Mukthar A, Toogood GJ, Lodge JP, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007;33:1003–1009. doi: 10.1016/j.ejso.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. ; discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Yamazaki O, Kinoshita H. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:969–974. doi: 10.1046/j.0007-1323.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Kawarada Y, Yamagiwa K, Das BC. Analysis of the relationships between clinicopathologic factors and survival time in intrahepatic cholangiocarcinoma. Am J Surg. 2002;183:679–685. doi: 10.1016/s0002-9610(02)00853-x. [DOI] [PubMed] [Google Scholar]
- Couinaud C. Anatomic principles of left and right regulated hepatectomy: technics. Presse Med. 1954;62:709–712. [Google Scholar]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, et al. Longterm survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. doi: 10.1001/archsurg.140.2.183. [DOI] [PubMed] [Google Scholar]
- Lee WS, Kim MJ, Yun SH, Chun HK, Lee WY, Kim SJ, et al. Risk factor stratification after simultaneous liver and colorectal resection for synchronous colorectal metastasis. Langenbecks Arch Surg. 2008;393:13–19. doi: 10.1007/s00423-007-0231-0. [DOI] [PubMed] [Google Scholar]
- Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Interaction of tumour biology and tumour burden in determining outcome after hepatic resection for colorectal metastases. HPB. 2010;12:84–93. doi: 10.1111/j.1477-2574.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234–242. doi: 10.1097/SLA.0b013e318223c609. [DOI] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2012;19:1618–1627. doi: 10.1245/s10434-011-2114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2011;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Sugihara K, Kosuge T, Takayama T, Shimada K, Yamasaki S, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995;221:74–78. doi: 10.1097/00000658-199501000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86:332–337. doi: 10.1046/j.1365-2168.1999.01030.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shimada H, Kubota K, Ueda M, Endo I, Sekido H, et al. Effectiveness of prehepatectomy intra-arterial chemotherapy for multiple bilobar colorectal cancer metastases to the liver: a clinicopathologic study of peritumoral vasculobiliary invasion. Surgery. 2005;137:156–164. doi: 10.1016/j.surg.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- Sasaki A, Aramaki M, Kawano K, Yasuda K, Inomata M, Kitano S. Prognostic significance of intrahepatic lymphatic invasion in patients with hepatic resection due to metastases from colorectal carcinoma. Cancer. 2002;95:105–111. doi: 10.1002/cncr.10655. [DOI] [PubMed] [Google Scholar]
- Okano K, Yamamoto J, Moriya Y, Akasu T, Kosuge T, Sakamoto M, et al. Macroscopic intrabiliary growth of liver metastases from colorectal cancer. Surgery. 1999;126:829–834. [PubMed] [Google Scholar]
- Korita PV, Wakai T, Shirai Y, Sakata J, Takizawa K, Cruz PV, et al. Intrahepatic lymphatic invasion independently predicts poor survival and recurrences after hepatectomy in patients with colorectal carcinoma liver metastases. Ann Surg Oncol. 2007;14:3472–3480. doi: 10.1245/s10434-007-9594-2. [DOI] [PubMed] [Google Scholar]
- Knijn N, de Ridder JA, Punt CJ, de Wilt JH, Nagtegaal ID. Histopathological evaluation of resected colorectal cancer liver metastases: what should be done? Histopathology. 2013;63:149–156. doi: 10.1111/his.12124. [DOI] [PubMed] [Google Scholar]
- Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, et al. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol. 2003;82:28–33. doi: 10.1002/jso.10188. [DOI] [PubMed] [Google Scholar]
- Bockhorn M, Sotiropoulos G, Neuhaus J, Sgourakis G, Sheu SY, Molmenti E, et al. Prognostic impact of intrahepatic lymphatic and microvascular involvement in cases of colorectal liver metastases. Int J Colorectal Dis. 2009;24:845–850. doi: 10.1007/s00384-009-0674-6. [DOI] [PubMed] [Google Scholar]
- Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]