Abstract
The senescence-accelerated mouse prone 8 (SAMP8) is considered a useful non-transgenic model for studying aspects of aging. Using SAM resistant 1 (SAMR1) as controls, the long-term effects of wheel running on skeletal muscle adaptations and behavioral traits were evaluated in senescent (P8) and resistant (R1) male and female mice. Long-term wheel running (WR) led to increases in locomotor activity, benefits in sensorimotor function, and changes in body weight in a gender-dependent manner. WR increased body weight and baseline levels of locomotor activity in female mice and improved balance and strength in male mice, compared to sedentary-control mice. WR resulted in key metabolic adaptations in skeletal muscle, associated with an increased activity of the sirtuin 1–AMP-activated protein kinase (AMPK)–PGC-1 alpha axis and changes in vascular endothelial growth factor A (Vegfa), glucose transporter type 4 (Glut4), and Cluster of Differentiation 36 (Cd36) gene expression. Overall, our data indicate that activity, balance, and strength decrease with age and that long-term WR may significantly improve the motor function in a mouse model of senescence in a gender-dependent manner.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9697-1) contains supplementary material, which is available to authorized users.
Keywords: Senescence-accelerated mice, Voluntary wheel running, Sarcopenia, Motor activity, Metabolism, Gender
Introduction
Motor performance alterations, metabolic dysfunction, and muscle atrophy (Clark and Fielding 2012; Crane et al. 2013; Lauretani et al. 2003) contribute to sarcopenia (Rolland et al. 2008), a condition associated with normal aging (Rolland et al. 2008). In the elderly person, failure to achieve functional motor skills represents a risk for frailty (Hausdorff et al. 1997), disability, and dependency (White et al. 2009), thereby affecting the individual’s quality of life.
Physical exercise has recently emerged as a potential treatment for sarcopenia (Montero-Fernandez and Serra-Rexach 2013; Crane et al. 2013), associated with benefits in musculoskeletal systems (Manabe et al. 2013) and metabolic function (Stephens et al. 2002). In mice, spontaneous wheel running may provide an excellent model to counteract the deterioration of motor skills and skeletal function associated with aging due to the fact that it is a voluntary rhythmic behavior, generally performed with a high degree of consistency and coordination.
Previous research has focused on describing molecular mechanisms behind the exercise-induced muscle adaptations, and the sirtuin 1–AMP-activated protein kinase (AMPK)–PGC-1 alpha axis appears to be one major contributor (Duan 2013). We previously reported that long-term exercise increased both sirtuin 1 protein content and activity and PGC-1α protein abundance (Bayod et al. 2012; Duan 2013). However, the role of these markers in improving skeletal muscle function and their contribution to motor function and baseline levels of physical activity in senescent mouse models are not yet completely understood.
The senescence-accelerated mouse (Samorajski et al. 1987) is a murine model increasingly used to investigate the complex physiological and pathological responses that occur during aging. Established through phenotypic selection from a common genetic pool of AKR/J strain, senescence-accelerated mouse (SAM) were noticed to become senile at an early age and had a short life span, with a median survival time of 9.7 months (Takeda et al. 1981). The SAM prone (SAMP) series includes nine sub-strains. Among these, SAMP8 (P8) mice exhibit characteristic disorders that correspond to pathophysiological states found in aged humans (Takeda et al. 1997), including a moderate to severe degree of activity loss (Flood and Morley 1998; Takeda 2009) and decreased motor coordination (McAuley et al. 2004). SAMP8 suffers from neuropathological changes frequently observed with advancing age in humans such as axonal dystrophy, astrogliosis, or reduction of spine density (see Takeda 2009 for a review), accompanied by molecular features of AD such as overproduction of amyloid-beta protein, increased tau phosphorylation, and increased oxidative stress (Takeda 2009; del Valle et al. 2011; Morley et al. 2012). With a similar genetic background to P8 but with 40 % longer survival time (16.3 months), resistance 1 (R1) mice show normal aging characteristics and are therefore frequently used as an appropriate control model.
Here, we evaluate the potential of long-term wheel running (WR), as a model to voluntarily increase physical activity, to counteract some of the characteristic features associated with aging in P8 mice. Precisely, we monitored WR of male and female R1 and P8 mice and investigated the ability of a 6-month WR intervention to affect body weight, to modulate the debilitating age-dependent locomotive deficit, and to improve skeletal muscle metabolism. We describe robust sex differences in the patterns of voluntary WR. Exercise increased overall activity and led to skeletal muscle adaptations in the P8 female mice; in P8 male mice, exercise attenuated the age-related reductions in balance and muscle strength.
Methods
Animals
One hundred naive, male, and female R1/P8 mice, purchased by El Parc Tecnològic (Barcelona, Spain) and weighing 26.45 ± 0.375 g at the time of delivery, were acclimatized for a week before starting the experimental procedure. The mice were maintained under standard conditions (temperature 23 ± 1 °C, humidity 50–60 %, 12:12-h light–dark cycle, lights on at 8 a.m.), with food (A04, Harlan, Spain) and tap water available ad libitum throughout the study. They were housed in groups, two to six same-sex mice per cage (except during wheel running sessions), in plastic Makrolon colony boxes (15 cm high × 27 cm wide × 27 cm deep) with a sawdust floor. Body weight (g) was monitored weekly.
All experimental procedures were approved by the Ethics Committee of the University Autonomous of Barcelona (Comissió Ètica d’Experimentació Animal i Humana, CEEAH, UAB), following the “Principles of laboratory animal care”, and were carried out in accordance with the European Communities Council Directive (86/609/EEC).
Voluntary wheel-running paradigm
The running wheels (ENV-044 Mouse Low-Profile Wireless Running Wheel, Med Associates Inc.; 15.5-cm circumference; 25° from horizontal plane) were located in the animal colony room inside cages that were 19 cm high × 27 cm wide × 40 cm deep. WR activity was monitored through a wireless transmitter system by using a hub (13.70 × 15.25 cm2) located in the same animal colony room. The hub was connected to a PC, and the number of rotations performed each minute was recorded.
As previously described (Álvarez-López et al. 2013), all mice in the WR condition were individually accommodated in cages (15 cm high × 27 cm wide × 27 cm deep) containing clean sawdust and a running wheel. Sedentary-control (CON) mice were equally single-housed as the WR mice but accommodated in cages (same size) containing only clean sawdust. WR/sedentary sessions were conducted three alternate days a week for 24 weeks (except for the testing period). We employed an alternate procedure to avoid long-term isolation, which is known to produce detrimental behavioral effects in rodents (Fone and Porkess 2008). WR/sedentary sessions started between 1 and 2 p.m. and lasted 24 h. At the end of each session, mice were returned to their home cage with their companions.
Behavioral tests
Procedures
Three-month-old R1 and P8 mice were randomly divided in four groups of males and four groups of females in a 2 (strains: P8 and R1) × 2 (gender: male and female) × 2 (intervention: CON and WR) factorial design (n = 12–14/group). The WR/sedentary sessions were initiated when the animals were approximately 12 weeks old and continued for 24 weeks until the end of the experiment. Behavioral tests started when the animals were 9 months old, 20 weeks after WR was initiated, lasted 4 weeks, and were administered in the order listed in Fig. 1. Prior or during testing days, WR sessions were not performed. At the end of the testing period, animals were sacrificed by beheading, and blood and gastrocnemius muscle tissue samples were collected. Serum was obtained by centrifugation at 4 °C and 2,000 × g for 15 min and stored at −80 °C until further use.
Fig. 1.
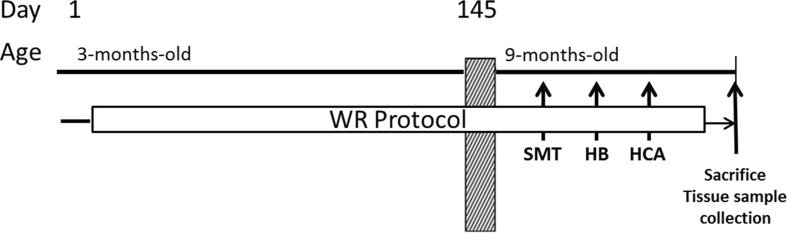
Experimental design, timeline of procedures, and age of the animals. WR mice were allowed to run in a running wheel three times per week; CON mice followed the same procedure without the running wheel. After 20 weeks of wheel running activity, the behavioral testing was initiated in the following order: sensorimotor tasks (SMT), hole board (HB), home cage activity (HCA), and sacrifice. Skeletal muscle samples were collected for gene expression and Western blot analysis
Sensorimotor tasks
Mice were checked for different sensorimotor tasks (Fernandez-Fernandez et al. 2012) in order to assess visual placing reflex, balance, and prehensile reflex. (a) For the visual placing reflex assessment, the animal was gently grasped from the tail, 35 cm above from a black arena, and moved toward the surface for three consecutive trials. One point was given if the animal did extend its forepaws in each trial (maximum score = 3). (b) For balance assessment, the animal was placed in the center of an elevated (36 cm high) flat wooden rod (40 cm long, 9-mm width; trials 1 and 2) and of an elevated cylindrical wire rod (40 cm long, 1 cm-diameter, trials 3–4), and the distance travelled (MacMaster et al. 2014) was recorded for each trial (total of four 20-s trial). (c) To evaluate muscle functional improvements from the WR intervention, we conducted the hanging test and measured muscle strength as the ability of the mice to remain suspended by the forepaws grasped around an elevated cylindrical wire (2-mm diameter) in two consecutive trials of 10 s. If a mouse fell, the latency to fall was recorded (Klein et al. 2012). If a mouse did not fall, a score of 10 s was given.
Exploratory activity in the “hole-board test” (HB)
A 32 × 32-cm white plastic arena with 30-cm-high walls was used to analyze spontaneous exploratory behavior (File and Wardill 1975). The floor of the apparatus contained four equidistant holes (3.7-cm diameter) and was divided into 16 equal squares (8 cm2 each, 3-cm diameter). A video camera was placed 245 cm above the arena to record each animal for 5 min. The number of ambulations (line crossings), number of head dips, and the time spent head dipping on each hole were measured.
Home cage motor activity
Motor activity was measured by individually placing the animals in home activity cages (25 × 18.5 × 15 cm) made of transparent Plexiglas (Med Associates, Inc.), during the dark phase of the light–dark cycle (5 p.m.–10 a.m.). Four home activity cages were placed into a sound-proof chamber with an infrared video camera placed in the upper side 43 cm above the cage covers. The chambers were also equipped with a temporizing illumination system that switched on the chamber light (8:00 a.m. to 8:00 p.m.), as what corresponded with the light–dark cycle of the animals’ room. The video camera was connected to a PC with a software system that provided measurements of the distance travelled in the cage across the day.
Total RNA extraction and real-time quantitative PCR
Total RNA was extracted from frozen gastrocnemius muscles using a mirVana™ RNA Isolation Kit (Applied Biosystems) following the manufacturer’s instructions. The yield, purity, and quality of RNA were determined spectrophotometrically (NanoDrop, USA) and using the Bioanalyzer 2100 capillary electrophoresis. RNAs with 260/280 ratios and RIN higher than 1.9 and 7.5, respectively, were selected.
Random-primed complementary DNA (cDNA) synthesis was performed at 37 °C starting with 0.3 μg of RNA, using the High-Capacity cDNA Archive kit (Applied Biosystems). Gene expression was measured in an ABI Prism 7900HT Real-Time PCR system using TaqMan FAM-labeled specific probes (Applied Biosystems, see Online Resource 1). Results were normalized to Tbp gene expression, a housekeeping gene that displays very low variability across samples (Life Technologies Application Note: Using TaqMan® Endogenous Control Assays to select an endogenous control for experimental studies).
Inmunodetection by Western blot analysis
Tissue samples were homogenized in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA, 1 % Triton X-100, pH 7.4) containing complete, Mini, EDTA-free Protease Inhibitor Cocktail (Roche, Mannheim, Germany) and Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich, St. Louis, MO, USA). The protein concentration was determined by the Bradford method. Protein (20 μg) was separated by SDS-PAGE (5–15 %) and transferred to PVDF membranes (Millipore). The membranes were blocked in 5 % non-fat milk in Tris-buffered saline (TBS) containing 0.1 % Tween 20 (TBS-T) for 1 h at room temperature, followed by overnight incubation at 4 °C with antibodies diluted in TBS-T and 5 % BSA sirtuin 1 (1:1,000; Millipore), AMPKα (1:500; Cell Signaling) and p-AMPKα Thr172 (1:500, Cell Signaling), PGC-1α (1:500; Cayman Chemical), BDNF, and GADPH (1:2,000; Millipore). Membranes were then washed and incubated with secondary antibodies [(donkey ECL anti-rabbit IgG, horseradish peroxidase (HRP)-linked (NA934V 1,000 GE Healthcare, UK) or goat anti-mouse HRP conjugate (-5047 1,000 Bio-Rad, Hercules, CA, USA)] for 1 h at room temperature. Protein bands were quantified by a chemiluminescence detection kit (Amersham Biosciences). Band intensities were quantified by densitometric analysis, and values were normalized to GAPDH expression.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 17.0). To examine WR patterns across the experiment, a two-way analysis of variance (ANOVA; strain × gender) for repeated measures over two blocks of 10-week periods as a within subjects factor was applied. Due to the divergent body weight and wheel-running phenotype among males and females, body weight gain and average of the distance travelled in the wheels were analyzed separately for males and females by a two-way ANOVA (intervention × strain). Pearson correlation coefficients were used to highlight relationships between the body weight gain and wheel running activity within each gender. A three-way ANOVA (strain × gender × intervention) was applied to behavioral variables from sensorimotor and hole-board tests. Bonferroni post hoc test was used to determine differences among the groups after significant ANOVA. Those behavioral variables which did not meet criteria of normality or homogeneity of variances, such as the latency to fall for assessing muscle strength, and the distance travelled in the HCA test were analyzed with the Kruskal–Wallis nonparametric tests followed by the Mann–Whitney U test for comparisons between independent groups. Gene and protein data analysis was performed by one-way ANOVA followed by the Student’s t test, when appropriate. Statistical significance was set at p < 0.05 for all tests. Data are expressed as mean ± SEM.
Results
Voluntary wheel-running activity in SAMP8 and SAMR1 male and female mice
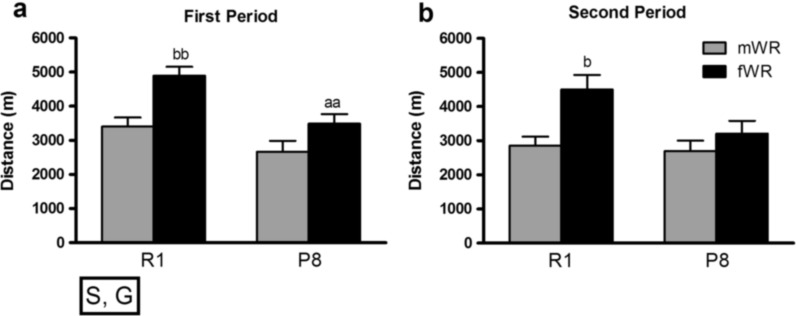
We found a significant reduction in the average distance travelled over the two periods of WR intervention (Fig. 2). Overall, R1 mice ran significantly further distances in comparison to P8 mice, consistent with the accelerated aging phenotype (McAuley et al. 2004). Female mice travelled further distances in comparison to male mice, and post hoc comparisons revealed gender differences in R1 mice but not in P8, not during the first or second period of WR activity. A closer inspection indicated that P8 female mice only differed from R1 females in the amount of distance travelled in the first period, but those differences were abolished in the second period (Fig. 2). In addition, R1 females travelled further distances than R1 males, and they were the group displaying the highest levels of WR activity.
Fig. 2.
Long-term WR performance in P8 and R1 mice. Data are represented as mean ± SEM of the average running distance travelled per session in a the first period (weeks 1–10) and b the second period (weeks 11–20). Overall, animals decreased WR activity in the second period (period effects p < .05); R1 mice were more active than P8 (S: strain effect p < .05), and females were more active than males (G: gender effect p < .001). aa p < .01 versus the corresponding R1 group (same gender); b p < .05; bb p < .01 versus the corresponding male group (same strain)
Voluntary wheel running increases body weight of SAMR1 and SAMP8 female but not male mice
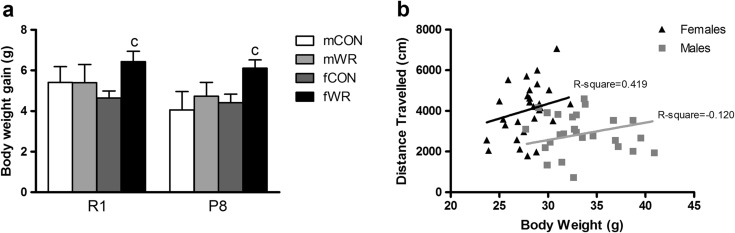
Weekly body weight changes were monitored throughout the experiment (see Online Resource 2). The analysis of the body weight gain revealed a higher increase in WR female mice in comparison to the CON female mice (intervention p < .001), whereas no significant effects were found in males (Fig. 3a). In addition, we found a significant positive correlation between WR, measured by the average of distance travelled per session during the 20-week period of intervention, and the final body weight in female mice, indicating that higher amounts of WR activity were associated with higher body weight in female but not in male mice (Fig. 3b).
Fig. 3.
Body weight change induced by voluntary WR in R1 and P8 male and female mice. a Mean ± SEM (n = 12–14) of the body weight gain from the time of arrival to the last week of the WR intervention is shown. c p < .05 versus the corresponding fCON group (same strain). b Scatter plot of body weight at week 20 and the averaged wheel-running activity measured as the mean of distance travelled per session during the 6 months of WR intervention for male (n = 26) and female (n = 28) R1 and P8 mice. Distance travelled significantly correlated with the body weight of R1 and P8 female (Pearson, R 2 = 0.419, p < .05) but not that of R1 and P8 male mice (R 2 = −0.120, p < .558)
Balance and grip strength improvement after long-term wheel running in male and female mice
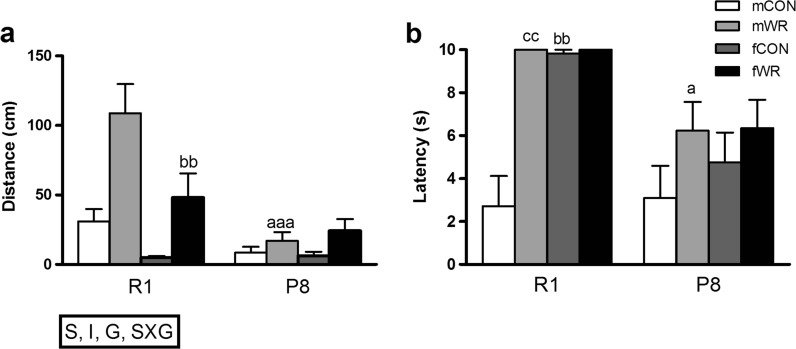
In the visual placing reflex, all groups reached the maximum score of 3, thus indicating no visual placing deficits in any group. In contrast, in the balance rod task, P8 male mice performed worse than R1 male mice (Fig. 4a), whereas WR performed better than CON mice. Overall, male mice had better balance than female mice, but this gender effect was more prominent in the R1 strain. Interestingly, post hoc comparisons revealed that mice from WR groups showed superior balance performance in comparison to mice from CON groups (mR1WR > fR1WR = mR1CON = mP8WR = fP8WR > fR1CON = fP8CON = mP8CON).
Fig. 4.
Evaluation of the sensorimotor status of R1 and P8 mice. a Overall, R1 mice showed better performance than P8 mice on the balance test (S: strain effect, p < .001; mean ± standard error of the mean (SEM) of the distance travelled on the flat and circular rod), WR mice travelling further distances in the rods (I: intervention effect, p < .001), male mice showing better balance than female mice (G: gender effect, p < .05), this effect being greater in R1 male mice (SXG: strain × gender interaction, p < 0.01). aaa p < .001 versus the corresponding R1 group (same gender and type of intervention); bb p < .01 versus the corresponding male group (same strain and type of intervention). b WR intervention improved muscular strength in R1 and P8 mice (mean ± SEM of the latency scored in the hanging test); a p < 0.05 versus the corresponding R1 group (same gender and type of intervention), bb p < .01 versus the corresponding male group (same strain and type of intervention), cc p < 0.01 versus the corresponding CON group (same strain and gender)
Additional group differences were also found in muscular strength (p < .001; Fig. 4b). Post hoc comparisons revealed that the order of the groups, from longer to shorter latency to fall down, i.e., from better to worse performance, was similar with the order of balance performance and is listed as follows: mR1WR = fR1CON = fR1WR > mP8WR = fP8CON = fP8WR > mR1CON, mP8CON.
Wheel running marginally increases locomotor activity but not exploratory behavior in SAMP8 female mice
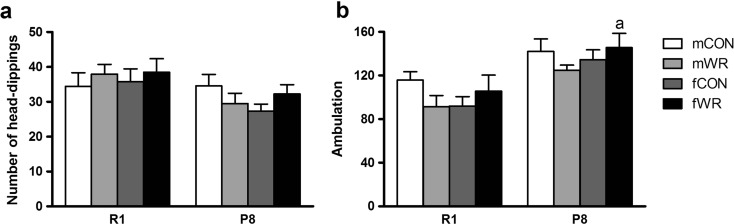
As previously reported (Chen et al. 2007), the head-dipping behavior was decreased in P8 mice in comparison to R1 (strain p < .05; Fig. 5a), whereas no other significant effects were observed for this measure. On the contrary, when analyzed for motor activity, ambulation was greater in P8 mice in comparison to R1 (strain p < .001), and a residual gender × intervention interaction (p = .062) revealed a tendency to display higher ambulation in fWR mice. No other significant effects appeared in this test.
Fig. 5.
Behavior of the SAMR1 and SAMP8 mice in the hole-board test. Data represent means ± SEM of a number of head dips and b ambulation. a p < .05 versus the corresponding R1 group (same gender and type of intervention)
Wheel running increases baseline activity in SAMP8 female mice
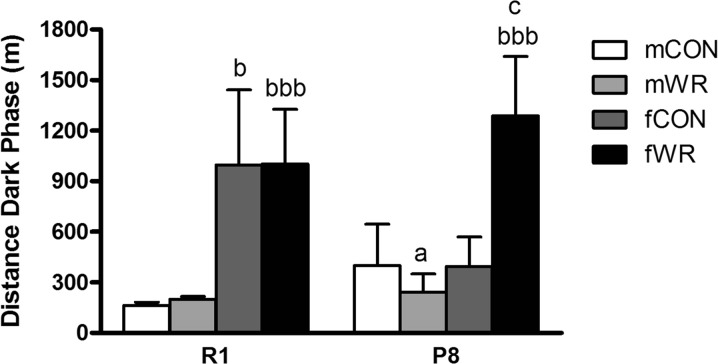
The overall analysis of the home cage activity test revealed significant differences among groups (p < .001; Fig. 6). Post hoc comparisons between pairs of groups indicated that fR1CON and fR1WR groups were significantly more active than the corresponding male mR1CON or mR1WR groups (p < .05 or p < .001, respectively), but only the fP8WR group was significantly more active than mP8WR (p < .001), indicating that WR affected P8 female and male mice differently and that those gender-specific effects were not observed in R1 mice. Indeed, WR significantly increased home cage activity during the dark phase in the fP8WR group compared with the corresponding fP8CON sedentary group (p < .05). However, WR did not affect home cage activity in male mice of either strain.
Fig. 6.
Home cage activity test. Data represent means ± SEM of total distance travelled in the cages. a p < .05 versus the corresponding R1 group (same gender and intervention), b p < .05, bbb p < 0.001 versus the corresponding male group (same strain and intervention), c p < .05 versus the corresponding control (CON) group (same strain and gender)
Impact of wheel running in the expression of skeletal muscle angiogenic and metabolic genes
The locomotor-activity improvement in the WR P8 females, and fP8WR group performing significantly more exercise than their corresponding male controls (Álvarez-López et al. 2013), led us to explore the impact of WR in the expression of genes that regulate skeletal muscle cell survival and metabolism.
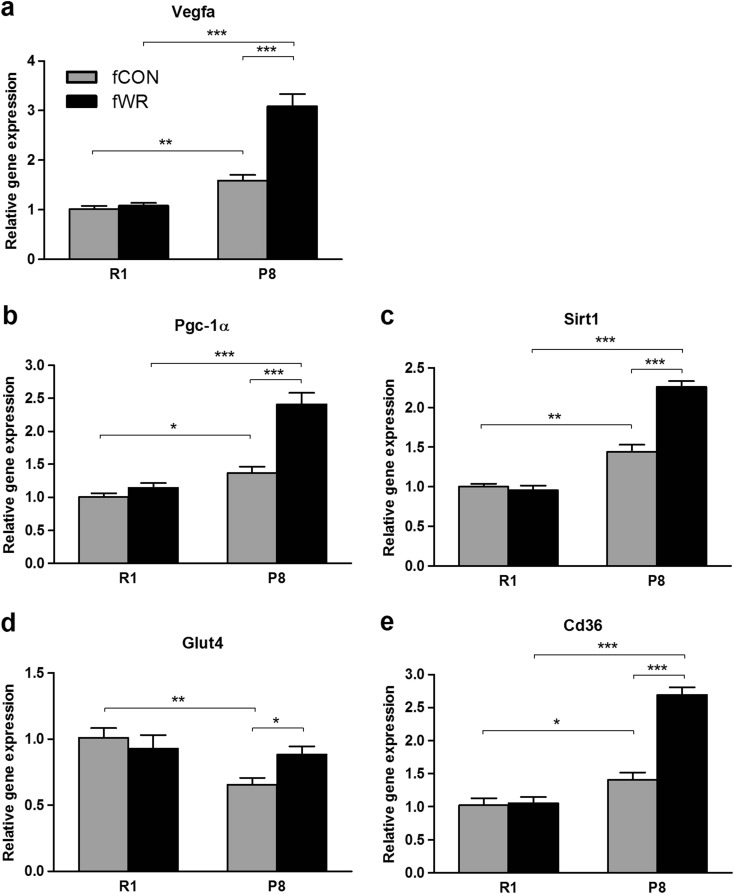
Angiogenesis is a key determinant of skeletal muscle function, and previous reports in animal models and humans have shown a beneficial effect of physical exercise on factors that sustain capillarization levels in skeletal muscles (Fernandes et al. 2012; Shibuya 2013). In this context, we found that the vascular endothelial growth factor A (Vegfa) was significantly increased by WR in fP8WR female mice, while no change was detected in fR1CON controls (Fig. 7a). The fP8WR group also exhibited a WR-dependent increase in the expression of peroxisome proliferator-activated receptor γ co-activator 1α and sirtuin 1 genes, known regulators of mitochondrial biogenesis, an essential process for cell metabolism and viability (Rodgers et al. 2005; Gerhart-Hines et al. 2007). These effects were not observed in fR1CON mice (Fig. 7b, c). Furthermore, we examined gene expression of glucose transporter type 4 (Glut4) and Cluster of Differentiation 36 (Cd36) antigen, both modulated by PGC-1α and involved in glucose and fatty acid metabolism, respectively (Calvo et al. 2008; Michael et al. 2001). We found lower Glut4 gene expression levels in fP8CON compared with fR1CON mice. After the WR intervention, Glut4 expression levels in P8 mice were restored to control levels (Fig. 7d). Finally, Cd36 antigen was also upregulated by WR in P8 but not in R1 mice (Fig. 7e).
Fig. 7.
Impact of wheel running in the expression of skeletal muscle angiogenic and metabolic genes. a Vascular endothelial growth factor A (Vegfa), b peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), c deacetylase sirt1, d insulin-sensitive glucose transporter type 4 (Glut4), and e fatty acid translocase Cd36. Analysis was performed by quantitative real-time PCR from gastrocnemius muscle mRNA. Gene expression levels are expressed relative to housekeeping TATA-binding protein (TBP) gene. fCON sedentary-control female mice, fWR wheel-runner female mice (mean ± SEM; *p < .05; **p < .01; ***p < .001; n = 5 per group)
PGC-1α and AMPK, but not sirtuin 1, upregulation by wheel running in skeletal muscle of SAMP8 female mice
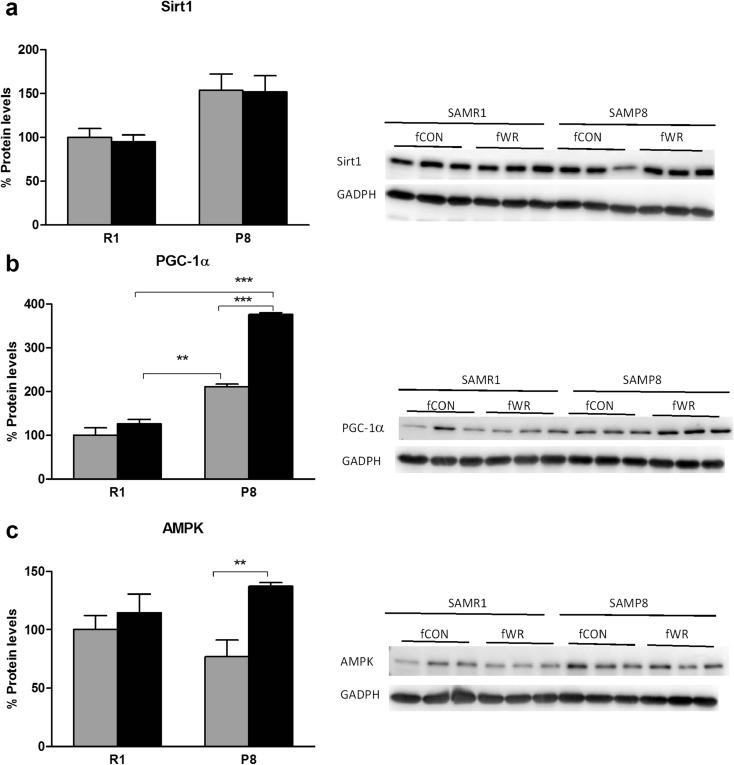
We identified the multiple cellular markers related to sirtuin 1, suggested as the key contributor of the WR-induced beneficial effect and master modulator of mitochondrial biogenesis. Sirtuin 1 protein levels were not modified by the WR intervention (Fig. 8a). However, to investigate whether sirtuin 1 was regulated under WR conditions, PGC-1α and AMPK expression, reported to be intrinsically related with the sirtuin 1 pathway (Longo 2009), was measured. PGC-1α protein expression of P8 female mice showed a significant increase in comparison to the CON P8 female group (Fig. 8b). Additionally, post hoc comparisons among WR groups indicated greater levels of PGC-1α in P8 mice in comparison to R1. This increase in PGC-1α in P8 resulting from WR was also accompanied by a significant increase in AMPK protein levels in P8 female mice, but not R1 female mice (Fig. 8b, c), overall revealing greater benefits of the WR intervention in the P8 strain.
Fig. 8.
Impact of wheel running in protein levels of skeletal muscle. a Sirtuin 1. b PGC-1α and c pAMPK levels in muscle for R1, P8, sedentary-control (CON), and wheel runner (WR) female mice. Images show representative Western blot and bar graph obtained from semi-quantitative image analysis (mean ± SEM; **p < .01; ***p < .001; n = 5–6 per group)
Discussion
In this study, we evaluated the long-term effects of wheel running activity on motor function and survival and metabolic signaling pathways in the skeletal muscle of senescence-accelerated mice. In a previous study (Álvarez-López et al. 2013), we demonstrated that exercise practice improved brain vascularization and alleviated phenotypic features associated with premature aging, such us prevention of deficient skin color, tremor, and lordokyphosis. The main results obtained here demonstrate that a long-term WR intervention improved balance and muscle strength performance both in R1 and P8 mice and increased motor activity in P8 female mice. We found an upregulation of the AMPK–sirtuin 1–PGC-1α pathway in skeletal muscle of exercised female P8 mice as well as increased Vegfa, Glut4, and Cd36 gene expression and upregulated AMPK–sirtuin 1–PGC-1α pathway, altogether suggesting that WR induced revascularization and modified mitochondrial biogenesis as well as glucose and fatty acid metabolism. Body weight gain was greater in females than males, possibly due to the greater WR activity displayed in female groups. Finally, we identified robust strain differences, R1 mice exhibiting superior WR activity and enhanced balance, coordination, and body strength than P8 mice, despite that these differences were less apparent in female mice.
Consistent with other studies, we observed striking strain differences in the patterns of WR–P8 mice travelling shorter distances in the wheels in comparison to R1 mice, despite that both strains decreased WR activity with age (Samorajski et al. 1987; Pang et al. 2004). Additionally, we also found clear gender differences, female mice running longer distances than males (De Bono et al. 2006) and, as a result, possibly showing a greater increase in body weight gain and superior behavioral benefits from the WR intervention [(Pietropaolo et al. 2008) for a review on gender-specific effects of WR]. Based on the correlation analysis, we can propose the increase in the distances travelled on the wheels as a possible causation for the increase in muscle mass and therefore body weight in female mice. In future experiments, the inclusion of muscle mass weight might help elucidate this hypothesis.
In the present study, and in accordance with another study showing that WR can lessen the impact of age on motor behavior in mice (Marlatt et al. 2012), we observed a dramatic improvement in the equilibrium performance of WR mice, suggesting that physical exercise programs could be valuable tools to prevent balance deterioration in aged populations. Additionally, muscle strength has been shown to have a mediating role between physical activity and disability (Crane et al. 2013). Results from the hanging test revealed that WR activity extended the latency to fall, these effects expressed more acutely in the P8 male mice. Collectively, considering the fact that the great majority of injuries among the elderly occur as a result of a fall impact (Myers et al. 1991), these findings emphasize the relevance of long-term exercise as a preventive practice to improve balance and gait and may, as a result, increase coping with environmental hazard.
The increased locomotor activity in female mice is another positive effect of the WR intervention observed in our study. As previously reported, P8 mice show a moderate to severe degree of activity loss (Takeda 2009). Here, we only accounted activity loss in female P8 versus female R1 but not in male mice, WR increasing the level of activity in the home cage test. We therefore propose the model of voluntary WR as an active intervention that is able to prevent motor dysfunction in the senescent female mice. Additional work is needed to further characterize the differences that exist between males and females and how sex may influence WR-related changes in muscle function and locomotor activity observed herein. Previous reports indicate that gender may be an important factor in mediating some of the WR-induced differential effects observed in other markers such microglia (Kohman et al. 2013) or BDNF gene expression (Zajac et al. 2010).
Partial benefits of the reported increments in balance and muscle strength from a long-term WR intervention can result from the skeletal muscle adaptations reported in our study and by others (Valdez et al. 2010). Improvements in the latter are of major relevance, as P8 mice present features of sarcopenia and muscle aging from a young age and significantly faster than other mouse models (Derave et al. 2005). Gene expression data suggest that the increase in locomotor activity in WR P8 female mice is a consequence of WR-induced improvements of revascularization, mitochondrial biogenesis, and glucose and fatty acid metabolism in skeletal muscle. Indeed, in addition to the angiogenic and regenerative potential of Vegfa in skeletal muscle (Item et al. 2013), adequate PGC-1α levels in muscle are required for normal glucose homeostasis, mitochondrial gene expression, and insulin signaling (Jager et al. 2007). Intriguingly, none of the genes analyzed were modified in R1 mice, and, with the exception of Glut4, they presented slight but significantly higher expression levels in P8 compared with R1 CON mice. This observation may reflect compensatory mechanisms in the senescent muscles that were intensified by the WR intervention. On the other hand, Glut4 expression, which was lower in P8 than in R1 CON mice, reached levels undistinguishable from those observed in controls after the WR intervention. Results on gene expression were partially confirmed by protein analysis. Although we did not find differences in sirtuin 1 expression in the skeletal muscle that correlate with the gene expression increase detected in fP8WR mice, we did find a significant increase in PGC-1α and in the sirtuin 1 activator AMPK in these mice. Interestingly, the activation of the sirtuin 1–AMPK–PGC-1α pathway is involved in mitochondrial biogenesis (Chen et al. 2008; Liu et al. 2012) and has been previously proposed as a possible target of molecular changes induced by exercise (Liu and Fielding 2011; Canto and Auwerx 2009). Indeed, evidence suggests that moderate levels of regular physical activity increase a larger number of mitochondrial biogenesis-related gene expression in aged subjects, though to a lesser extent than in younger individuals (Bori et al. 2012). As we only examined a single age (9 months), future studies addressing different time points may help elucidate further interactions of WR on the trajectory of aging (Valdez et al. 2010).
In sum, a decline in muscle function is widely believed to underlie the decrease in quality of life, in addition to the increase in susceptibility to disease associated with aging. As the elderly population grows, identifying the mechanisms involved and possible preventive treatments would be highly beneficial for ensuring healthy aging and reducing health care costs (Blankevoort et al. 2010). Collectively, this study demonstrates for the first time that long-term voluntary WR leads to metabolic muscle adaptations, in addition to ameliorating the locomotor-activity detriments in the WR senescent female mice. Our findings also demonstrate gender-dependent differences, male mice improving their balance and muscle strength in response to exercise, despite their lower WR activity in comparison with females. These findings, as well as those of previous studies, emphasize the importance of accounting for genotype and gender–environment interaction in aging paradigms. WR may become an effective intervention to counteract or ameliorate the age-associated decrease in motor function.
Electronic supplementary material
(DOC 43.0 kb)
Acknowledgments
This study was supported by funding from the Dirección General de Investigación on grants PSI 2011-29807-C02/PSIC and PSI 2008-06417-C03-03. J.F.L. was supported by a predoctoral fellowship from the Generalitat de Catalunya (FI-DGR 2011).
References
- Álvarez-López MJ, Castro-Freire M, Cosin-Tomas M, Sanchez-Roige S, Lalanza JF, Del Valle J, Parrizas M, Camins A, Pallas M, Escorihuela RM, Kaliman P. Long-term exercise modulates hippocampal gene expression in senescent female mice. J Alzheimers Dis. 2013;33(4):1177–1190. doi: 10.3233/JAD-121264. [DOI] [PubMed] [Google Scholar]
- Bayod S, Del Valle J, Lalanza JF, Sanchez-Roige S, de Luxan-Delgado B, Coto-Montes A, Canudas AM, Camins A, Escorihuela RM, Pallas M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol. 2012;47(12):925–935. doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Blankevoort CG, van Heuvelen MJ, Boersma F, Luning H, de Jong J, Scherder EJ. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord. 2010;30(5):392–402. doi: 10.1159/000321357. [DOI] [PubMed] [Google Scholar]
- Bori Z, Zhao Z, Koltai E, Fatouros IG, Jamurtas AZ, Douroudos II, Terzis G, Chatzinikolaou A, Sovatzidis A, Draganidis D, Boldogh I, Radak Z. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47(6):417–424. doi: 10.1016/j.exger.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104(5):1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GH, Wang C, Yangcheng HY, Liu RY, Zhou JN. Age-related changes in anxiety are task-specific in the senescence-accelerated prone mouse 8. Physiol Behav. 2007;91(5):644–651. doi: 10.1016/j.physbeh.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22(13):1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67(1):41–47. doi: 10.1093/gerona/glr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Macneil LG, Tarnopolsky MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci. 2013;68(6):631–638. doi: 10.1093/gerona/gls237. [DOI] [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- del Valle J, Duran-Vilaregut J, Manich G, Pallas M, Camins A, Vilaplana J, Pelegri C. Cerebral amyloid angiopathy, blood-brain barrier disruption and amyloid accumulation in SAMP8 mice. Neurodegener Dis. 2011;8(6):421–429. doi: 10.1159/000324757. [DOI] [PubMed] [Google Scholar]
- Derave W, Eijnde BO, Ramaekers M, Hespel P. Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp Gerontol. 2005;40(7):562–572. doi: 10.1016/j.exger.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Duan W. Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci. 2013;5:36. doi: 10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes T, Nakamuta JS, Magalhaes FC, Roque FR, Lavini-Ramos C, Schettert IT, Coelho V, Krieger JE, Oliveira EM. Exercise training restores the endothelial progenitor cells number and function in hypertension: implications for angiogenesis. J Hypertens. 2012;30(11):2133–2143. doi: 10.1097/HJH.0b013e3283588d46. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez L, Comes G, Bolea I, Valente T, Ruiz J, Murtra P, Ramirez B, Angles N, Reguant J, Morello JR, Boada M, Hidalgo J, Escorihuela RM, Unzeta M. LMN diet, rich in polyphenols and polyunsaturated fatty acids, improves mouse cognitive decline associated with aging and Alzheimer’s disease. Behav Brain Res. 2012;228(2):261–271. doi: 10.1016/j.bbr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44(1):53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22(1):1–20. doi: 10.1016/S0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/S0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- Item F, Nocito A, Thony S, Bachler T, Boutellier U, Wenger RH, Toigo M. Combined whole-body vibration, resistance exercise, and sustained vascular occlusion increases PGC-1α and VEGF mRNA abundances. Eur J Appl Physiol. 2013;113(4):1081–1090. doi: 10.1007/s00421-012-2524-4. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SM, Vykoukal J, Lechler P, Zeitler K, Gehmert S, Schreml S, Alt E, Bogdahn U, Prantl L. Noninvasive in vivo assessment of muscle impairment in the mdx mouse model–a comparison of two common wire hanging methods with two different results. J Neurosci Methods. 2012;203(2):292–297. doi: 10.1016/j.jneumeth.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27(1):101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD + -dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287(31):25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD. Linking sirtuins, IGF-I signaling, and starvation. Exp Gerontol. 2009;44(1–2):70–74. doi: 10.1016/j.exger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Carrey N, Langevin LM, Jaworska N, Crawford S. Disorder-specific volumetric brain difference in adolescent major depressive disorder and bipolar depression. Brain Imaging Behav. 2014;8(1):119–127. doi: 10.1007/s11682-013-9264-x. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Gollisch KS, Holton L, Kim YB, Brandauer J, Fujii NL, Hirshman MF, Goodyear LJ. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013;280(3):916–926. doi: 10.1111/febs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6 J mice. Dev Neurobiol. 2012;72(6):943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JD, Miller JP, Pang KC. Age-related changes in the spontaneous motor rhythms of the senescence-accelerated mouse (SAMP8) Exp Aging Res. 2004;30(1):113–127. doi: 10.1080/03610730490251513. [DOI] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49(1):131–143. [PubMed] [Google Scholar]
- Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 Mouse: a model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des. 2012;18(8):1123–1130. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- Myers AH, Baker SP, Van Natta ML, Abbey H, Robinson EG. Risk factors associated with falls and injuries among elderly institutionalized persons. Am J Epidemiol. 1991;133(11):1179–1190. doi: 10.1093/oxfordjournals.aje.a115830. [DOI] [PubMed] [Google Scholar]
- Pang KC, Miller JP, McAuley JD. Circadian rhythms in SAMP8: a longitudinal study of the effects of age and experience. Neurobiol Aging. 2004;25(1):111–123. doi: 10.1016/S0197-4580(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192(1):42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samorajski T, Rolsten C, Przykorska A, Davis CM. Voluntary wheel running exercise and monoamine levels in brain, heart and adrenal glands of aging mice. Exp Gerontol. 1987;22(6):421–431. doi: 10.1016/0531-5565(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153(1):13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282(3):E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34(4):639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17(2):183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM) Exp Gerontol. 1997;32(1–2):117–127. doi: 10.1016/S0531-5565(96)00068-X. [DOI] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SM, Wojcicki TR, McAuley E. Physical activity and quality of life in community dwelling older adults. Health Qual Life Outcomes. 2009;7:10. doi: 10.1186/1477-7525-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20(5):621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 43.0 kb)