Abstract
Genetics in health care is shifting, and responsibilities of genetic and nongenetic specialists are changing, requiring new guidance on how to adapt health care to advances in genetic services. This paper explores facilitators and barriers in the process of implementation of innovations in genetic health care. Furthermore, lessons learnt for optimizing development of new genetic services are summarized. Barriers and facilitators in transition processes were identified using mixed methods, including an online open-ended questionnaire among professionals and an international expert meeting. A multi-case study approach was used to explore recent experiences with innovations in genetic services in different phases of implementation. Barriers encountered in transitions in genetic service provision include the following: lack of genetic knowledge and skills among nongenetic health care providers, resistance to new divisions of responsibilities, and a need for more close collaboration and communication between geneticists and nongeneticists. Facilitating factors include the following: statutory registration of genetic specialists, availability of essential staff and equipment, and existence of registries and guidelines. Other challenges are experienced in the establishment of the appropriate legal and financial structures. A set of points to consider for genetic innovation processes is proposed, addressing, e.g., transition management and cooperation and communication strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-014-0189-x) contains supplementary material, which is available to authorized users.
Keywords: Genetic services, Translational medical research, Diffusion of innovation, Interdisciplinary communication, Genetic education
Introduction
Developments in genetic service provision
In 2010 the Council of Europe stated that “The development of genetics in health care services has a major impact on the organization of health care, leading to shifting from curative to preventive services, from in-patient to out-patient treatment, from specialized genetic services to genetics as an integral part of general health services.” (Committee of Ministers and Europe 2010). A variety of organizations have also recognized that “mainstream medicine” is increasingly encompassing genetic aspects of diseases and that specialist clinical and laboratory elements are being integrated (Burton 2011). Both nongeneticists are taking over genetic services previously conducted exclusively by genetic specialists, and genetic specialist services are becoming more visible and better recognized by nongeneticists. This development is still ongoing and as a result, responsibilities of clinical geneticists and nongenetic specialists are changing. Because of these developments, genetic service provision is currently undergoing many changes, with varying success and efficiency.
Among the most promising opportunities for integration of genetics in mainstream medicine is genetic testing for monogenic forms of common diseases. Identification of patients with subtypes of, e.g., cancer, sudden cardiac death or diabetes caused by a single-gene mutation, might effectively reduce morbidity and mortality by offering more appropriate treatment. Moreover, family members of affected individuals can benefit from genetic testing by monitoring, prevention, and/or timely treatment in case of genetic predisposition to the disease (van El and Cornel 2011).
Although different initiatives have implemented genetic services aimed at monogenic forms of common diseases, they have not all been equally effective, and currently, accessibility is still far from optimal in many places (Malecki 2010; Pujol et al. 2013; Sharaf et al. 2013; Shields et al. 2010; Teekakirikul et al. 2013). Challenges include multidisciplinary cooperation, merging of different cultures and practices (from within and outside of clinical genetics), and the development of appropriate genetic competencies in all actors involved. It is expected that similar challenges are also involved in the implementation of other novel genetic services, for example, the expansion or introduction of genetic screening programs (e.g., preconception carrier testing and noninvasive prenatal testing (ACOG Committee on Genetics and SMFM Publications Committee 2012; Grody et al. 2013) and clinical application of next generation sequencing (Ormond et al. 2010)).
While different scholars have identified several barriers and facilitators in transitions in health systems in general, not much is known about the factors specifically influencing the process of genetic service innovation. Although, e.g., seven steps to be undertaken for strategic implementation of innovations in health care have been applied to clinical genetic services recently by Hamilton et al., little practical further guidance exists for the planning and execution of such transition processes related to genetic health services (Bennett et al. 2010; Berwick 2003; Hamilton et al. 2013; Manolio et al. 2013; Rogowski et al. 2009).
In this paper, we describe general and case-specific facilitators and barriers in different phases of the process of implementation of innovations in genetic services and summarize what can be learnt for optimization of development of new (or adapted) genetic services. For this purpose, we addressed the following questions: (1) what factors are expected to be of influence in the development and implementation of genetic health services, (2) what barriers and facilitators for the implementation of genetic health services are encountered in recent examples of testing for monogenic forms of common diseases, and (3) what can be learnt for future planning and execution of transition processes related to new (or adapted) genetic health services?
Towards effective and efficient implementation of genetic services: a transition perspective
Since technology in genetic health-care services is developing at a high speed, exerting a major impact on the provision of health-care services, new guidance is urgently needed in how to adapt to changes in genetic service provision in many countries.
Theoretical framework
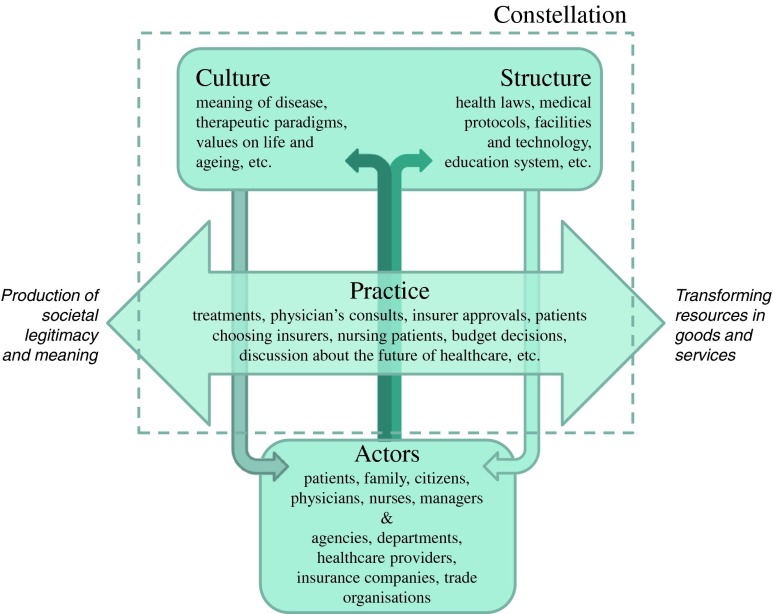
To unravel and structure transition processes related to new (or adapted) genetic health services, we have adopted elements of models and concepts used in the field of Health System Innovation and Transition. We will use a model that is based on the “constellation perspective,” referring to a set of interrelated practices and relevant structuring elements that together are both defining and fulfilling a function in a larger societal system in a specific way (van Raak 2010). In medicine, the dominant constellation is often determined by a group of individuals or actors (professionals and patients) that are used to working in a certain structure, culture, and practice (see Fig. 1).
Fig. 1.
Operationalization of the constellation concept into structure, culture, practice, and associated actors (adapted from van Raak (2010))
Dynamics within or outside this constellation may cause instability and thereby provide an opportunity for change. When the change process leads to fundamental changes in structure, culture, and practice, a transition of the constellation has occurred. However, such a fundamental change process, called a “transition,” is difficult to realize because constellations are inherently resistant to change (Essink 2012). In general, the driving force in a transition is a sense of urgency for change in a group of individuals or key actors within a constellation. Key actors initiate and push for changes in technological options (e.g., development of new genetic tests or treatment options) are involved in or respond to the development of facilities and services (e.g., offering genetic tests from general clinic laboratories instead of clinical genetic laboratories), demand changes from a user perspective (e.g. more interest in personalized medicine), and/or are influential on political and cultural acceptability.
Often, changes start at a small scale and need deepening, broadening, and scaling up before a full transition is accomplished and a new constellation (with adapted culture, structure, and/or practice) is formed. In this paper, the concept deepening illustrates the small-scale development of new ways of doing and identifying best practices (focusing on problems and solutions in a protected (experimental) space: a niche), while broadening refers to the adaption of niche practices to other contexts and alignment of visions of the different actors (partnering with other niche experiments and linking it to the existing constellation). Scaling up involves embedding the results of niche experiments in the existing culture, structure, and practice, requiring fundamental changes within the constellation by structurally embedding the new best practices in policy and legislation (changing dominant ways of thinking and doing) (van den Bosch 2010).
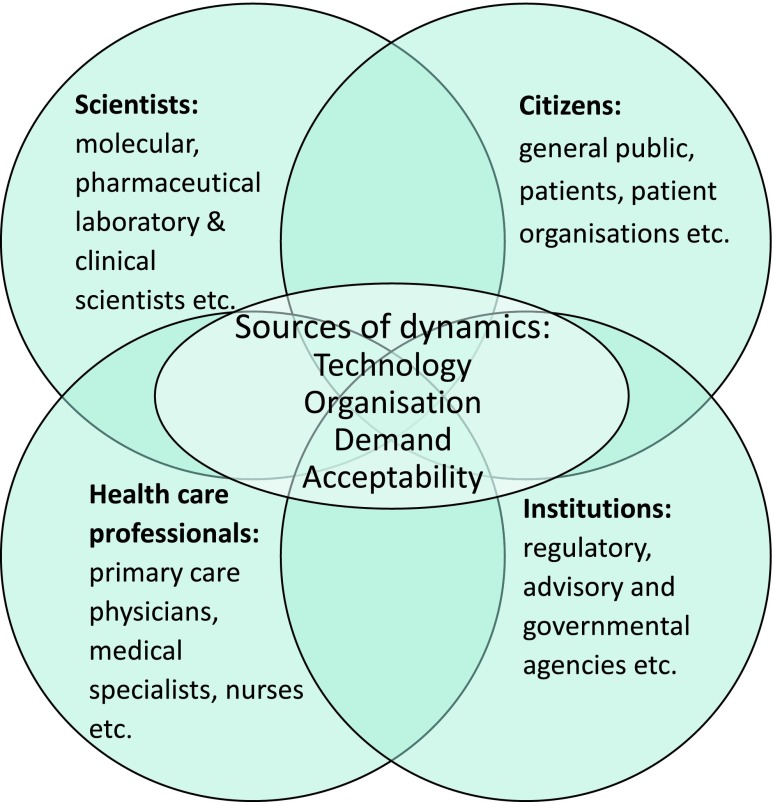
The key actors initiating and pushing transitions (also called “change agents”) are often involved in attuning different actors involved in transition processes in health-care systems, which can be grouped following a framework previously described by Achterbergh et al. (2007) (see Fig. 2).
Fig. 2.
Network of actors that need to be attuned in transition processes that could be initiated by dynamics in technology, organisation, demand, and/or acceptability in health-care systems (adapted from Achterbergh et al. (2007))
Apart from creating a collective sense of urgency to change practice among the relevant actors within an existing constellation, key actors in change processes also need new competencies (knowledge, attitude, and skills) to efficiently achieve transition. In addition, establishing coalitions, transparent organizational structures, and a clear division of responsibilities are crucial to efficiently and effectively form or change the structure of a constellation and accomplish deepening of the new practice. Establishment of robust coalitions among strategically chosen parties is crucial, for example, because an appropriate legal and financing framework should eventually be organized in order to scale up new services (van den Bosch 2010).
From the description of the key processes and actors in each phase of a transition related to genetic health care provision, we aimed to identify relevant points to consider for future (genetic) innovation processes.
Materials and methods
A multi-case study approach
Barriers and facilitators for future processes of innovations in genetic services were identified using mixed methods. Data collection methods involved an online open-ended questionnaire (see supplementary information) among 20 professionals with different backgrounds from 13 different countries,1 to gain insight into what is perceived as good practice in genetic service provision. A link to the online questionnaire was posted on the Eurogentest website (ww.eurogentest.org) and promoted in the monthly Eurogentest newsletter. The data were qualitatively analyzed. They were open-coded in an iterative process using a computer software program (Atlas.ti), to identify returning topics. These topics were then grouped in themes. Results of the questionnaire were summarized and used as part of a background document for a subsequent expert meeting in November 2011. This expert meeting (with 25 professionals from 11 countries2) titled “Building excellence in clinical genetic services” was organized to validate the results of the questionnaire and to deepen the subject by using a multi-case study approach (program and background document: see supplementary information). In both the questionnaire and the expert meeting, clinical genetic specialists were overrepresented, due to their broader (and in general longer) experience with the topics discussed and the recruitment methods. Three recent examples of transitions within genetic services for monogenic forms of common diseases (for more details on respondents, participants, and procedures: see Online Resource 1). The examples used were chosen as examples of recent developments in genetic service provision and reflect genetic services in different phases of development and implementation in health care: (i) monogenic diabetes moving from research to practice in designated centers, (ii) cardiogenetics upcoming in some countries, and (iii) oncogenetics that is well developed in many countries. Results of the discussions of the expert meeting were member-checked by the key actors within the examples used. Document analysis of published experiences and theories from different sectors in health care was conducted to frame and verify our results.
This article reflects the expert contributions to both the questionnaire and the meeting, supported by additional international literature.
Results
Expected influencing factors in transitions in genetic health care
Barriers and facilitators for transitions in genetic health care expressed by participants of the questionnaire and/or the expert meeting have been summarized for changes in practice, culture, and structure. Main needs have been summarized in Table 1, together with illustrative quotes from the questionnaire.
Table 1.
Needs expressed by respondents to the questionnaire (n = 20) for changes in practice, culture and structure
| Theme: | Need expressed: | Illustrative quote: |
|---|---|---|
| Needs for changing practice | More focus on pre-test and post-test counseling | “[What currently is missing is] good preparation for people having diagnostic tests but who are unprepared for the result (often tests offered by non-genetic specialists). More time [is needed] for psychological care after testing.” (#10 Clinical Geneticist) |
| New division of responsibilities | “[A weak link in current genetic services] may be the understanding (by the geneticists) that times are changing and there should be a new way of dividing the work: an increasing role for other specialists and maybe only diagnostics/counselling of the rare diseases left as the special goal for only the clinical geneticists.” (#20 Clinical geneticist)” | |
| Close collaboration and communication between geneticists and non-geneticists | “I think there needs to be development of [professional] teams around the different human disorders, and a clinical geneticists should be represented in such teams.” (#12 Clinical Geneticist) | |
| Needs for changing culture | Attention for genetics in mainstream medicine | “The main problem is that other health care specialists (with few exceptions) do not recognize the influence of genetics in their field. Maybe publishing more genetics related articles in [more general] medicine journals would [lead to] more attention.” (#8 Clinical Geneticist) “[What is missing is] greater awareness of genetic risk in wider health system (outside genetics, especially in primary care).” (#10 Clinical Geneticist) |
| Needs for changing structure | Statutory registration of staff trained in genetics | “Statutory registration of genetic counsellors and technical staff [is missing and] would provide quality assurance of the professional standards of these members of the workforce” (#5 Clinical Geneticist) |
| Education for non-geneticists | [In order to make developments within genetic services meaningful there needs to be] better education for non-geneticists in genetic testing in their discipline.” (#7 Clinical Geneticist) | |
| Availability of essential staff and equipment | “Staff and finance [are] needed. [There currently is] poor secretarial support, not enough facility for follow up of families needing screening etc. [and there is a] need for better genetic registers” (#7 Clinical Geneticist) | |
| Existence of guidelines/protocols | “[An example of good practice is the availability of ] agreed protocols for offering predictive/presymptomatic testing.” (#10 Clinical Geneticist) “What could be improved are the protocols outside clinical genetics.” (#3 Researcher in Health Sciences) |
|
| Legal structures | “Perhaps some policy statement [is needed] saying that if you offer genetic tests you must also be able to offer genetic counselling.” (#12 Clinical Geneticist) | |
| Financial structures | “[There currently is a] lack of reimbursement of counselling and support activities by non-medical professionals” (#2 Researcher in Psychology) “[What is missing is] reimbursement for non-diagnostic tests (carrier, predictive/pre-symptomatic).” (#14 Clinical Geneticist) “[Good aspects of current genetic services include] […] reimbursement for genetic testing in patients under obligatory health care [the basic health care package], if testing meets established criteria of "medical indication".” (#6 Clinical Geneticist) |
Changing practice: different ways of doing
When genetic services are implemented in mainstream medicine, this will involve changes in the existing practice. Genetic aspects of disorders will require more attention, not only enabling more effective diagnostics in some cases but also providing opportunities for predictive testing in family-members. This shift towards more predictive medicine will require practices that involve counselling and monitoring besides the traditional focus on diagnostics and treatment in mainstream medicine. Furthermore, a more family-oriented practice is required to adequately detect family members at increased risk of a genetic disorder, making the practice of taking family history of a patient essential.
Needs expressed to improve practices within current genetic services include close collaboration and communication between geneticists and nongenetic health-care workers and new division of responsibilities.
Changing culture: different ways of thinking
The new practices within the constellation will require changes in the existing culture, for example, raising awareness of the importance of genetic services (often in a nongenetic professional community), changing from a single-patient perspective to a family approach, setting other priorities (e.g., from a focus on diagnostics and treatment to presymptomatic counselling and monitoring), and/or converging the experience of disease itself with the experience of being at risk for disease (Aronowitz 2009).
Previously, a change in mind set (or culture) has often been established by one or more so-called focusing events (organizing meetings and/or media attention) to accomplish agenda setting (Achterbergh et al. 2007). Key actors therefore clearly need to be active in agenda setting to strategically involve all players necessary for effective implementation of new services (Loorbach 2007; van Raak 2010). To accomplish efficient broadening of new genetic services, a sense of urgency needs to be present in all actors involved in the changing system. This means that not only the scientists, health-care professionals, regulatory agencies, but also the (potential) users of the genetic services need to be convinced of the benefits of implementation.
Multiple respondents to the questionnaire mentioned the lack of genetic knowledge and skills among nongenetic health-care providers to be the weakest link in current genetic services, creating a barrier for efficient implementation.
Changing structure: different ways of organizing
Genetic education and training for nongenetic specialists is deemed important, but is also mentioned by the respondents to the questionnaire to be lacking. Other important changes acknowledged include the statutory registration of genetic specialists (including counsellors and technical staff), availability of sufficient secretarial support and other general facilities, existence of genetic registers, and existence of guidelines for genetic services (including pre and posttest counselling and follow-up).
For the successful introduction of genetic services in mainstream medicine, the establishments of legal and financial structures are considered barriers and missing in many current service as well as prerequisites for adequate service delivery (or facilitators).
The organization of the required legal and financial frameworks for new practice will require commercial as well as project management skills to mobilize the necessary actors and to get the new constellation established. For accommodating the structure of a constellation to new practice, key actors will have to use entrepreneurial and policy skills such as negotiation, as well as insights into the relevant institutional networks, among others to adjust rules and to create room and resources for experimentation, including the establishment of coalitions.
Examples of recent innovations in genetic services for monogenic subtypes of common disorders: barriers and facilitators encountered
Since the development of PCR techniques and an increase in linkage studies in the 80s, genetic testing has been increasingly integrated into health care. In the early 90s (diagnostic and presymptomatic), genetic testing for breast cancer became possible due to the cloning of major genes involved in pathogenesis of breast and colon cancer enabling establishment of a clinical oncogenetic service, now available in most countries (Offit 2011). Genes involved in sudden cardiac death were identified later and clinical cardiogenetic services began to be implemented by early 2000; however, systematic clinical testing and accompanying services are still lacking or not fully effective in many places. Key genes causing monogenic subtypes of diabetes were identified in the late 1990s but only few specialist centers currently aim at detection and follow-up of maturity-onset diabetes of the young (MODY) patients (Thanabalasingham et al. 2012).
Three examples of service development in Europe are described in more details below, together with an analysis of the main (general and more context-specific) barriers and facilitators within the concerning constellations that led to change (Table 2).
Table 2.
Analysis of changes within the constellations towards existing services for monogenic disorders
| Diagnostic Genetic Testing for Maturity Onset Diabetes of the Young (MODY) in the UK | Cardiogenetic Services, South Sweden | Hereditary Cancer Program in Catalonia, Spain | |
|---|---|---|---|
| Constellation | Diabetes care in the UK | Cardiology in South Sweden | Oncology in Catalonia |
| Niche experiment | Deepening/Broadening | Broadening/Scaling up | Scaling up |
| Function | From general diabetes care for all diabetic patients to specialized services for patients with monogenic diabetes. | From occasional cascade screening with sudden cardiac death of cardiomyopathy to a multidisciplinary network with (regional) guidelines | From incidental detection and counselling of people at increased risk of hereditary cancer to systematic service provision |
| Structure | - From unstructured/fragmented care to structured services for MODY - From individual initiatives to a central referral center for monogenic patients - From patches of knowledge about MODY to “spreading the word” through genetic diabetes nurses - From no funding, to research funded testing, to testing provided by the local health care provider |
- From individual initiatives to a structured network with guidelines - From single specialist to multidisciplinary approach |
- From individual initiatives to centralized care - From unstructured to structured services - From single specialist to multidisciplinary approaches |
| Culture | - From (type 1/2) diabetes as a clear diagnosis to genetic forms of diabetes as a separate subgroup - From initial focus on healthy blood-sugar levels to focus on the right (genetic) diagnosis and appropriate treatment |
- From individual case to family care - From treatment to presymptomatic counselling and follow-up |
- From individual patient to family care - From focus on treatment to pre-symptomatic counselling and follow-up |
| Practice | - From no specific attention for MODY patients to accustomed services | - From little specific attention to family members of cardiac patients to structured service provision | - From no specific attention to family members of patients with hereditary cancer to a tailored service |
| Main change agent(s) | A consultant diabetologist, molecular geneticist and a diabetes specialist nurse who joined forces and started training genetic diabetes nurses to educate diabetes teams about monogenic diabetes. | Cardiologist who activated a group of enthusiasts to form a network | Oncologist, geneticist and a nurse who joined forces and started a Cancer Genetic Counselling Unit |
| Main persistency in current phase | Finding stable long-term funding for ongoing training for genetic diabetes nurses | Regional initiatives (until 2011), first national meetings in 2012 | Regional Services are trying to build a National Web, and services available are mainly focused on oncology (difficult to convince other disciplines) |
Diagnostic genetic testing for maturity-onset diabetes of the young, Exeter, UK
The introduction of a service for diagnostic genetic testing for MODY in the UK has started with the initiative of a consultant diabetologist, a molecular geneticist, and a diabetes specialist nurse. Through convincing their network and by recognizing the general consensus that genetics is an important part of mainstream medicine (Burton 2011), they initiated a UK referral center for monogenic diabetes in Exeter in 2000. In this center, diabetes specialist nurses have been trained as genetic diabetes nurses (GDNs) in the UK since 2002, to raise awareness of monogenic diabetes, as approximately 80 % of these patients are initially misdiagnosed as having type 1 or type 2 diabetes leading to inappropriate treatment Shields et al. 2010. The center is currently still focused on conducting research in monogenic diabetes and also in promoting the translation of these research findings into improvements in clinical care for patients, e.g., treatment outcomes for MODY patients. Furthermore, a website with information on monogenic diabetes is available for professionals and patients and an online MODY probability calculator has been developed (Njolstad and Molven 2012). Through the center’s activities, knowledge about MODY among diabetologists in the UK has increased and more patients with monogenic diabetes are now diagnosed and treated accordingly. The majority of individuals are referred for genetic testing via the GDNs or diabetologists within secondary-care diabetes teams, with a small number from clinical geneticists or general practitioners. Some services delivered by the center are currently still research-funded, and because of a lack of experience of monogenic diabetes, it is still perceived difficult to convince some clinicians of the need for genetic testing.
The main facilitating factor in this example was the sense of urgency that was recognized by different key actors at the start of the development of this service. The change agents had extensive networks in the (scientific as well as health care) professional and patient community and funding agencies were aware of the need to accommodate mainstream medicine for genetics. Furthermore, the structure of care for diabetes patients required minimal changes because diabetes specialist nurses were already actively involved in diagnosis and follow-up of patients. Consequently, only a relatively small group of nurses needed training as GDN in order to reach a large group of diabetes professionals and raise awareness of monogenic diabetes among them. One of the main barriers to further broaden the current services is the need for long-term follow-up and evaluation of outcomes in order to provide robust evidence for the clinical utility of the service provision. Furthermore, organizing structural financing from regular health-care budgets instead of research funding will be essential in order to eventually scale up the GDN project to become a fully integrated part of diabetes care in the UK.
Cardiogenetic services, South Sweden
The example of a cardiogenetic service in the Southern Swedish health-care region started from an incident: in 2005, a case of sudden cardiac arrest of a young football player required genetic counselling due to inheritable aspects of his previously undiagnosed heart condition. This incident initiated collaboration between cardiologists and clinical geneticists, who subsequently (acting as change agents) created a multidisciplinary network consisting of adult and pediatric cardiologists and clinical geneticists and involving a pathologist and a forensic specialist. This network is since then having regular meetings (five to six meetings a year). Furthermore, they organize education for cardiologists in the 15 referring hospitals and developed and adopted regional guidelines and standardized notes of admission. Since the initiation, awareness for possible genetic causes and cascade testing of family members of cases of sudden cardiac arrest has increased in the Southern region of Sweden: in 2010, 68 families with mutations have been referred by their cardiologist to the cardiogenetic clinic in Lund. Here, specialized clinical geneticists and a cardiologist offer counselling according to the guidelines. Costs for the services are provided by the regional health-care system. As the approach to service delivery seems successful in the region, further health-care regions have recently implemented similar services one way or another, and a national cardiogenetics expert group consisting of cardiologists and clinical geneticists was formed in 2013. The aim of this professional group is to develop national guidelines for genetic testing and provide training and education for colleagues.
In this example, the initiating facilitating factor was the sense of urgency for changes in current practice, which was raised in a relatively small group of professionals through an incident of sudden cardiac death. This case quickly received broader attention, not only in the (public) media but also in the professional society by effective agenda setting by the initial professionals involved. Broadening of the service was achieved effectively by organizing training for cardiologist in multiple referring hospitals in the region and the establishment of regional coalitions for development and efficient implementation of new guidelines. Possibly due to the evident clinical utility of presymptomatic detection of patients with hereditary cardiac disorders (by effectively preventing early sudden cardiac arrest), financial structures were organized efficiently, opening doors for scaling up to national organization of cardiogenetic services.
Hereditary cancer program in Catalonia, Spain
The oncogenetic services in Catalonia, offering genetic testing and counselling of people at increased risk for hereditary cancer, were formally initiated within the Catalan Institute of Oncology by a group of clinicians and geneticists at the Department of Prevention and Cancer Control in 1998. Initially, a Cancer Genetic Counselling Unit was started in 1999 by a geneticist, an oncologist, and a nurse, which has since then grown from one daily clinic to three full-time operating locations (since 2008). The oncogenetic services are currently organized as a Hereditary Cancer Program with three Cancer Genetic Counselling Units and one central Molecular Diagnostic Unit.
Most referrals come from medical specialists, and since 1999, more than 1,000 carriers of highly penetrant cancer-predisposing genes have been identified and are under surveillance by the multidisciplinary team (consisting of two oncogeneticists, four oncogenetic nurses, a psycho-oncologist, and a geneticist as coordinator). Furthermore, more than 900 healthy relatives have been withdrawn from intensive surveillance because they had not inherited the pathogenic mutation identified in their families. The service is paid for by the Catalan health system. Nowadays, similar services are available in other regions in Spain, and in Catalonia as well, without clear national guidance. Some of the regional governments, such as the Catalan Regional Government, however have implemented Clinical Guidelines for Cancer Genetic Counselling. Little comparable services are offered for other conditions than cancer because it seems hard to convince other disciplines of the importance of cascade screening. In addition, private companies are increasingly offering testing, which could keep patients and/or family members from utilizing the services offered in regular health care.
In this example, the service was developed as a response to the growing international awareness of the clinical utility of oncogenetic services to diminish the population impact of cancer, using genetic information to individualize preventive and treatment strategies (Cabrera et al. 2010). This sense of urgency that was felt by all relevant actors may be seen as one of the main facilitators in the early phases of deepening the development of services. Furthermore, the main change agents effectively established regional coalitions to accommodate the existing structures for the new practices by systematically organizing a multidisciplinary program. In this specific example, the lack of scaling up to national oncogenetic services is probably mainly due to the regional organisation of health care in Spain. Opportunities still exist to broaden the services for other (monogenic) disorders, making strategic involvement of other disciplines and creating a sense of urgency among them relevant.
Lessons to be learnt for optimization of development of new (or changed) genetic services: asking the right questions
From the analysis of the survey and the cases presented above, we aimed to define lessons for different phases of transition. By defining the main topics and questions to be addressed for deepening, broadening, and scaling up new developments, we aim to give guidance for new genetic services. Given a new technological development or an opportunity for better service provision is available, the themes addressed in Table 3 should be acknowledged.
Table 3.
Points to consider for transitions in genetic service provision
| Main topics to address | Questions to address |
|---|---|
| Deepening implementation | |
| Clinical need | Is the service needed to optimize clinical practice? |
| Analytical validity and clinical validity | What is the evidence for analytical and clinical validity? |
| Clinical utility | What is the evidence for clinical utility? |
| Perceived need | |
| Stakeholder’s priority and awareness (patients, payers, doctors, etc.) | Do stakeholders perceive the need for introduction of the service? Why (not)? |
| Public priority and awareness | |
| Surveillance possibilities (economic, technical) | How will the implementation of the service be monitored? |
| Broadening implementation | |
| Learning from others | What can be learnt from similar services existing elsewhere? |
| Cooperation and communication strategies | How to ensure and maintain cooperation and communication between different stakeholders? |
| Scaling up implementation | |
| Educating stakeholders (payer, doctors, nurses) | How should the actors involved in the execution of the service be educated? |
| Public education | How to educate the public about the existence, characteristics, and implications of the service? |
| Information material for the target group | What should be the content of the information material? |
| Where should it be available? (internet, leaflets, tv, etc.) | |
| Dissemination strategies | What is the target population and how should the service be disseminated to them? |
| Availability of genetic counselling | What are the needs for information and pre and posttest counselling and how to ensure the availability for the potential users of the service? |
| Acceptability (legal, political, cultural) | What are legal, moral, and financial prerequisites that need to be met? |
| Health economic evaluation | Are the benefits outweighing the costs? |
| Continuous evaluation and quality control | How will the (quality of the) service be evaluated? |
| What are the outcome measures (user satisfaction, clinical outcome, etc.) | |
| Who will be responsible for the evaluation? | |
| Monitoring results | How will the results of the service be monitored? |
| Technology development | How to ensure that new developments/notions will be integrated in the service? |
Although deepening, broadening, and scaling up is generally occurring in chronological order, it should be recognized that strategies for all themes should be anticipated early in the process.
Discussion
Although the three case examples described in this study take place in different legal, governmental, and financial health-care structures and embodied different phases of implementation, similar challenges and facilitators could be identified for deepening, broadening, and scaling up of new innovation in genetic service provision. Main barriers in transition processes of genetic services include a lack of essential skills and genetic knowledge among nongenetic health-care providers, a resistance to new divisions of responsibilities among important actors, and a need for closer collaboration and communication between geneticists and nongeneticists. Facilitating factors include statutory registration of genetic specialists, availability of essential staff and equipment, and existence of registries and guidelines for specific genetic services. Other relevant challenges are experienced in the establishment of the appropriate legal and financial structures.
Some of the needs for changes in culture, structure, and practice expressed by our respondents to the questionnaire were also expressed as concerns by Godard et al. (2003). For example, professional education, developing a multidisciplinary approach, and division of tasks are clearly important challenges that have been and still are encountered in the process of implementation of genetic innovations in mainstream medicine. Furthermore, Battista et al. (2012) expressed the need for reconfiguration of professional roles and responsibilities and a lack of preparedness for enhanced sharing of expertise between professionals in first-, second-, and third-line medical care for optimal integration of genetic services into the health-care system. Moreover, a more recent study by Hamilton et al. (2013) identified overlapping characteristics of genetic services that hinder or facilitate adoption within health-care organizations.
In our study, it also became clear that often, diffusion and dissemination of new practices (when broadening and scaling up) provide specific challenges. Referring to the steps described by Hamilton et al. (2013) (reflecting the processes needed for broadening, deepening, and scaling up), it seems that making early adopters visible, creating “slack for change” (by creating a sense of urgency among the relevant stakeholder) and enabling reinvention are not always receiving the required attention. This may be due to the fact that change agents are more focused on trying to obtain proof of principle (deepening) than on having a long-term vision. This has also been observed by Essink (2012) in his study on innovations in service provision in long-term care. The efficient implementation of genetic services for MODY in the UK described here however shows that when key actors are specifically focused on scaling up early in the process, this could aid in overcoming these challenges. Specifically, for relatively rare genetic disorders, this is very relevant, since in many cases, it may not be cost-effective to only provide local services. It is therefore relevant to acknowledge that “diffusion of innovation” as previously referred to (Hamilton et al. 2013; Lomas 1993) might not be the optimal term when discussing efficient implementation of genetic services in mainstream medicine, since this requires a more active process. “Transition management” might be a term that better describes this process (Loorbach 2007; van Raak 2010).
Participants that collaborated in the online questionnaire and the workshop often had a genetic service background. In Table 1, the (most clearly formulated) opinions are from genetic experts. Other professionals than clinical geneticists in general mentioned similar topics, but their quotes appeared to be less clear. Patient organizations clearly are also very relevant stakeholders. They were invited for the workshop, but sent a medical specialist to represent them. Priorities for mainstreaming genetics from a nongenetic expert’s stance need further investigation.
Although most of the challenges and facilitators presented in this study have been described elsewhere for different contexts, the use of practical examples with focus on the different phases of transitions could give more insight in the prerequisites for efficient implementation of genetic services in mainstream medicine. Furthermore, the points to consider (Table 3), defined after analysis of the results of the questionnaire and the three case examples, depict a structured approach to genetic service development and could be useful for all countries that are further developing genetic services. It is conceivable that these conclusions are applicable to other fields of health care as well, but this should be further explored. Actors who we think could benefit most from this structured approach include commissioners from different agencies (including health insurance and maybe even direct-to-consumer companies), health economists, health-care departments, and governmental agencies.
Electronic supplementary material
(DOC 600 kb)
Acknowledgment
We wish to express our great gratitude to all participants in this study. The study was undertaken as part of the work of Unit 2, Work Package 8 of the EuroGentest2 Coordination Action 2011 project (funded by the European Commission Contract no: HEALTH-F4-2010-261469). T.R. also received funding from the Netherlands Genomics Initiative in the program of CSG Centre for Society and the Life Sciences and Centre for Medical Systems Biology. M.S. is funded by the NIHR Exeter Clinical Research Facility, University of Exeter.
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
This study complies with the current laws of the country in which they were performed.
Footnotes
Responses to questionnaire received from: Belgium, China, Finland, Germany, Israel, Lithuania, Norway, Spain, Sweden, Switzerland, The Netherlands, Turkey, and the UK. Background respondents: clinical genetics (n = 13), primary care, psychology, health sciences, molecular genetics, cytogenetics, sociology, anthropology, and health economics.
Expert meeting 25 participants from 11 countries: Czech Republic, Hungary, Finland, France, Iceland, Norway, Portugal, Spain, Sweden, The Netherlands, and the UK. Background participants: clinical genetics (n = 10), cardiology, genetic counselling (n = 10), health sciences/research (n = 5), oncology, neurology, primary care, psychology, and sociology.
References
- Achterbergh R, Lakeman P, Stemerding D, Moors EH, Cornel MC. Implementation of preconceptional carrier screening for cystic fibrosis and haemoglobinopathies: a sociotechnical analysis. Health Policy. 2007;83:277–286. doi: 10.1016/j.healthpol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Genetics and SMFM Publications Committee Committee opinion no. 545: noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;Obstet Gynecol 120:1532–1534. doi: 10.1097/01.AOG.0000423819.85283.f4. [DOI] [PubMed] [Google Scholar]
- Aronowitz RA. The converged experience of risk and disease. Milbank Q. 2009;87:417–442. doi: 10.1111/j.1468-0009.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista RN, Blancquaert I, Laberge AM, van SN HK, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15:34–45. doi: 10.1159/000328846. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Burke SE, Burton H, Farndon PA. A toolkit for incorporating genetics into mainstream medical services: learning from service development pilots in England. BMC Health Serv Res. 2010;10:125. doi: 10.1186/1472-6963-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- Burton H. Genetics and mainstream medicine. Cambridge, UK: Report PHG Foundation; 2011. [Google Scholar]
- Cabrera E, Blanco I, Yague C, Zabalegui A. The impact of genetic counseling on knowledge and emotional responses in Spanish population with family history of breast cancer. Patient Educ Couns. 2010;78:382–388. doi: 10.1016/j.pec.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Committee of Ministers, Council of Europe (2010) Recommendation CM/Rec (2010) 11 of the Committee of Ministers to member states on the impact of genetics on the organisation of health care services and training of health professionals. Available at: https://wcd.coe.int Accessed December 11, 2013.
- Essink DR. Sustainable health systems: the role of change agents in health system innovation. Amsterdam: Dissertation VU University; 2012. [Google Scholar]
- Godard B, Kaariainen H, Kristoffersson U, Tranebjaerg L, Coviello D, Ayme S. Provision of genetic services in Europe: current practices and issues. Eur J Hum Genet. 2003;11(Suppl 2):S13–S48. doi: 10.1038/sj.ejhg.5201111. [DOI] [PubMed] [Google Scholar]
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482–483. doi: 10.1038/gim.2013.47. [DOI] [PubMed] [Google Scholar]
- Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2013 doi: 10.1038/gim.2013.101. [DOI] [PubMed] [Google Scholar]
- Lomas J. Diffusion, dissemination, and implementation: who should do what? Ann N Y Acad Sci. 1993;703:226–235. doi: 10.1111/j.1749-6632.1993.tb26351.x. [DOI] [PubMed] [Google Scholar]
- Loorbach D. Transition management: new mode of governance for sustainable development. Dissertation: Erasmus University Rotterdam; 2007. [Google Scholar]
- Malecki MT. The search for undiagnosed MODY patients: what is the next step? Diabetologia. 2010;53:2465–2467. doi: 10.1007/s00125-010-1908-4. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njolstad PR, Molven A. To test, or not to test: time for a MODY calculator? Diabetologia. 2012;55:1231–1234. doi: 10.1007/s00125-012-2514-4. [DOI] [PubMed] [Google Scholar]
- Offit K. Personalized medicine: new genomics, old lessons. Hum Genet. 2011;130:3–14. doi: 10.1007/s00439-011-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond KE, Wheeler MT, Hudgins L, et al. Challenges in the clinical application of whole-genome sequencing. Lancet. 2010;375:1749–1751. doi: 10.1016/S0140-6736(10)60599-5. [DOI] [PubMed] [Google Scholar]
- Pujol P, Lyonnet DS, Frebourg T, et al. Lack of referral for genetic counseling and testing in BRCA1/2 and Lynch syndromes: a nationwide study based on 240,134 consultations and 134,652 genetic tests. Breast Cancer Res Treat. 2013;141:135–144. doi: 10.1007/s10549-013-2669-9. [DOI] [PubMed] [Google Scholar]
- Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–495. doi: 10.1038/nrg2606. [DOI] [PubMed] [Google Scholar]
- Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093–1100. doi: 10.1016/j.cgh.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504–2508. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- Teekakirikul P, Kelly MA, Rehm HL, Lakdawala NK, Funke BH. Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. J Mol Diagn. 2013;15:158–170. doi: 10.1016/j.jmoldx.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Thanabalasingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206–1212. doi: 10.2337/dc11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch S (2010) Transition experiments: exploring societal changes towards sustainability. Dissertation, Erasmus University Rotterdam.
- van El CG, Cornel MC. Genetic testing and common disorders in a public health framework. Eur J Hum Genet. 2011;19:377–381. doi: 10.1038/ejhg.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raak R. The transition (management) perspective on long-term change in healthcare. In: Broerse JEW, Bunders JFG, editors. Transitions in health systems: dealing with persistent problems. Amsterdam: VU University Press; 2010. pp. 49–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 600 kb)