Abstract
Background
Sarcopenia is a significant geriatric syndrome with both health care expenditure and personal burden. Most recently, the European Working Group in Sarcopenia in Older Adults has established a consensus definition and assessment criteria for sarcopenia that includes a below-normal muscle mass and muscle function (either or both of below-normal muscle strength and physical performance). Using these criteria, work is needed to identify the prevalence and risk factors among the old, and those most susceptible to sarcopenia, the very old. This manuscript describes the recruitment and data collection methodology, and direct burden to participants, among a very old cohort residing in a residential aged care (RAC) setting.
Methods
Eleven RAC facilities participated in the study. Potential participants were identified by the facility service manager and then randomised into the study. All participants gave self or substitute decision maker consent. Participants undertook a single one on one assessment that included measures of sarcopenia, functional capacity, cognitive and nutritional health, falls, activity, facility and hospital history, physical activity and assessment burden. A sub-study of physical activity and sedentary behaviours measured by activPAL3™ inclinometer was also conducted.
Results
Of 709 residents, 328 were ineligible to participate. Two hundred and seventy-three residents were randomised to the study and 102 gave informed or substitute decision maker consent. Participants were 84.5 ± 8.2 years of age and had been in care for 1,204.2 ± 1,220.1 days. The groups need for care was high (Aged Care Funding Instrument score of 2.6 ± 1.7) and they had a below-normal functional (Short Physical Performance Battery summery score of 3.5 ± 2.4). The larger percentage of participants had no depression and normal cognitive capacity. A total of 33 residents participated in the activPAL study. Each assessment took an average of 27.0 ± 7.0 min, with a low assessment burden reported by participants.
Conclusions
The successful assessment of sarcopenia and physical activity in a RAC setting is labour intensive to establish, but feasible to conduct. Low recruitment numbers and the restrictive exclusion criteria, may have limited the accuracy of this work. However, this work is a primary step in establishing the level of sarcopenia and its risk factors for those in end-of-life care.
Keywords: Sarcopenia, Residential aged care, Prevalence, Risk factors, Methodology
Introduction
Over the next two decades, the direct cost of residential aged care (RAC) service expenditure in Australia will increase substantially compared with other primary health services due to population ageing and an increased prevalence of complex health conditions such as dementia ($8 billion), cardiovascular disease ($1.9 billion) and musculoskeletal disorders ($1.7 billion) [1]. When coupled with increasing need for intensive RAC services and the growth of the ≥85 years population to 3.1 million by 2054 [2], the economic impact will be substantial. A primary cause in institutionalisation is the age-associated loss in muscle mass and muscle function, termed sarcopenia [3, 4].
Sarcopenia is a significant geriatric syndrome, a major cause of disability and increasing healthcare utilisation that cost the US health care system > $US18.5 billion in 2000 [4, 5]. Recently, due to a lack of term clarity, a number of international organisations have been working to establish a consensus definition. Among these, the European Working Group on Sarcopenia in Older People (EWGSOP) have developed a definition and diagnostic criteria that required the presence of both low muscle mass and low muscle function (muscle strength or physical performance) [6]. While earlier interpretations had evaluated sarcopenia as an age-associated decline in muscle mass alone, a recognised precursor to disease and disability, it is the loss in muscle function which has the most debilitating consequences for later life disability [7]. In particular, for those in end-of-life care (residential aged care or a nursing home) declining functional muscle strength and physical performance equate directly to increased dependency and care needs [8]. Previous research had estimated the prevalence of sarcopenia to be 6–50 % among the very old [5, 9, 10]. Using the revised EWGSOP definition and assessment criteria, a prevalence of 33 % among very old adults has been shown [11, 12]. However, prevalence may be under-estimated due to the exclusion of the disabled, bedridden, non-ambulatory or those with advanced disease [3]. With this, the population assumed to be most vulnerable, those in end-of-life care are significantly understudied in relation to prevalence.
Several factors lead to the onset and progression of sarcopenia including muscular disuse and age-related alterations in sex hormones, protein synthesis, proteolysis, neuromuscular integrity, endocrine function, nutritional balance and an increase in muscle fat content [13]. Whereas a number of these mechanisms are well-understood, the effects of lifestyle choices as risk factors for sarcopenia are less clear [14]. To date, data are mixed in relation to the influence of body weight, level of disease, gender, health and physical activity background. Further, the importance of other factors known to influence healthy ageing (e.g., education, socioeconomic background or smoking history) as risk factors for sarcopenia is currently poorly understood. It is established that reduced muscle mass, muscle strength and physical performance increase the risk of disability, institutionalisation and mortality [8]. However, a broader understanding of these as risk factors in relation to sarcopenia is needed [13]. In addition, physical inactivity and muscle disuse are suggested to influence the development of disability later in life [15], but more work is needed to define the relationship with sarcopenia.
This paper describes the methodologies employed to collect sarcopenia prevalence and risk factor data from old and very old Australians residing in high- and low-care RAC. In addition to the recruitment and assessment strategies, we describe participation burden. Given the renewed interest in the area of sarcopenia due to its detrimental effect on disability and associated economic burden, the updated diagnostic criteria and the difficulty recruiting frail older people, this paper reports a detailed protocol on recruitment strategy and collecting information on risk factors and prevalence of sarcopenia within residential care settings, and may serve as valuable guide for others looking to collect data in a similar setting. The aims of the present study were to establish the prevalence and risk factors to sarcopenia among a group of older adults with compromised wellbeing residing in RAC facilities. As a risk factor to sarcopenia, the use of an activPAL3™ motion inclinometer was trialled, and study feasibility was assessed through participant burden.
Methods
Study design and recruitment
A cross-sectional study with random sampling was undertaken to measure the prevalence of sarcopenia and its association with functional and clinical risk factors in older adults residing in RAC facilities. Eleven purposefully selected RAC facilities within one care organisation and a 100-km radius of Bond University, Gold Coast, in South East Queensland, Australia, were identified and invited to participate. At pre-organised times, facilities were visited by members of the research team and a discussion undertaken with the facilities Service Manager or Director of Nursing as to the eligibility of all residents to participate in the study. Inclusion criteria were (i) age over 60 years, (ii) residing in a RAC facility and (iii) could provide informed consent, or if unable, proxy-informed consent obtained from the substitute decision maker. Residents were excluded if, (i) they had a pacemaker due to reported contraindications to bioelectrical impedance analysis, (ii) they were end-stage palliative or terminal (iii), they had difficult behaviours that would limit data collection or (iv) they had medical or other issues that would limit data collection. Medical issues raised as a reason for exclusion were total uncommunicable deafness and significantly advanced dementia or a comatose status.
Eligible participant were randomly selected within three levels of care (low- and high-care or residing in a secure dementia ward). Randomisation was undertaken using a random number generator (http://stattrek.com/statistics/random-number-generator.aspx). Reasons for ineligibility or refusal to participate were recorded. The study was approved by the human ethics committees of UnitingCare Queensland, Bond University and the University of Queensland.
Following randomisation, at a pre-organised time, facilities were re-visited and participant information sheet and consent forms delivered to the service manager or director of nursing. In addition, facilities were supplied with a list of residents randomised to participate and an instruction document outlining information sheet dissemination and consent. In brief, facilities were asked to deliver the information sheet to the participant with a request for the participant to read and sign the consent form if interested. For those cognitively challenged participants, the facility service manager or director of nursing was asked to contact the substitute decision maker, inform them of the study and inform them of the need for consent for participation. All participants were required to give informed consent directly or by the substitute decision maker, or by the service manager or director of nursing following discussion with the substitute decision maker. Facilities were then followed up 1 week later and a date set for participant assessments to take place. All participants were de-identified and assigned a study registration number.
Data collection
At a pre organised time, the study research assistant (RA), an experienced allied health professional, met with the facility’s service manager and resident nurse to establish the level of care required for each participant. Following this discussion, a carer accompanied the RA to visit and make the initial contact with the participants. For low-care participants, the RA was then left to conduct the data collection without assistance. For high-care and dementia participants, the carer remained. Where necessary and with the aim of a reduced falls risk, carers assisted the RA with any physical mobilisation of high care and dementia participants. In addition, for dementia participants, the carer was specifically from the participants ward. Their presence served to increase familiarity for the participants and thereby reduce anxiety, and to help the RA identify assessment components, the participant was unable to complete. Participants were assessed individually in a one-on-one format, and data were collected from all participants at one facility before moving to the next facility. To reduce any potential burden to participants undertaking assessment, participants were encouraged to rest as needed and were given verbal support and encouragement.
Measures
Although facility staff had previously familiarised participants with the project, the RA reviewed these documents before commencing the assessment. Participants who did not provide consent were asked a short series of questions about why they did not want to participate, and then thanked for their time. All measures are validated for use among old and very old adults. Data for each participant was entered on site and then forwarded to the project manager at Bond University at the completion of each day. Prevalence was determined using the project’s primary outcome of sarcopenic status and the EWGSOP definition and assessment criteria [6]. Secondary measures were collected to determine risk factors for sarcopenia in this population. In case a participant was unable to undertake a physical measure, self-report or answer a question, this variable was left blank, except for the 2.4-m walk which was scored as 100 s to ensure a walking speed of <0.8 m/s and that they were scored 0 for this component of the Short Physical Performance Battery (SPPB) summary score.
Primary outcome: sarcopenic status
According to the EWGSOP, the diagnosis of sarcopenia requires the presence of both low muscle mass and low muscle function (muscle strength or physical performance). In accordance with EWGSOP and appropriate for use among very old low functioning adults, (a) muscle mass was measured using Bioelectrical Impedance Analysis (BIA), (b) muscle strength by hand grip strength and (c) performance by the SPPB 2.4-m walk [11]. The cut-off point to define low muscle mass are ≥2 standard deviations below the norm of a young healthy population, to define low muscle strength are <30 and <20 kg for men and women, respectively, and to define low physical performance is a gait speed of <0.8 m/s [6].
BIA (Maltron BF-906, Maltron International Ltd., Rayleigh, UK) is a non-invasive, non-intrusive diagnostic system that estimates the volume of fat and lean body mass, BMI and resistance (ohms). Participants lay supine during testing, with electrodes placed at the top of the right wrist and distal end of the central metacarpal, and over the right foot talus and distal end of the central metatarsal.
Muscle mass is calculated from the BIA equation of Janssen et al.[5], namely, skeletal muscle mass (kg) = [height (cm)2/BIA resistance (ohms) × 0.401) + (gender × 3.825) + (age (years) × −0.071)] + 5.102. BIA was deemed as the most appropriated measure for this group, given their limited mobility and function, and the difficulties in transport participant to an offsite venue and the invasive nature of dual-energy x-ray absorptiometry (DXA) for this cohort.
Handgrip muscle strength was measured by isometric Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL). Three trials were conducted for dominant hand and the best result used in analysis. Participants were seated, instructed to keep their elbow at 90 ° and to squeeze the dynamometer maximally [16].
Physical performance was measured by the SPPB 2.4-m walk test [17, 18]. This measure was selected in preference to the 4 or 6 m, due to the convenience of assessing participants in the confines of their own room, most of which had restricted space [19]. In addition, the two other SPPB measures where collected. The additional two tests for the SPPB are (1) the hierarchical test of standing balance and (2) the five-time repeated chair stands. Measures were collected in triplicate and the best result was used in analysis.
Secondary outcomes
Demographics and health status
The RA collected height (cm) and weight (kg) by standardised methodologies. Demographics and clinical data including gender, level of education, occupation or spouse’s occupation if not the primary income earner, fall history in the last 6 months, history and present frequency of smoking and exercise. In addition, women where asked about age of menopause. From facility records, number and type of diseases and medications were recorded, and from the organisational database, date of birth and entry into the facility, marital status, language spoken, hospitalisation history and duration of stay in the last 12 months, falls history, bone mineral density diagnosis (normal, osteopenic or osteoporotic) and the Australian Aged Care Funding Instrument (ACFI) rating at entry and at present were recorded.
Mental health
The extent of cognitive impairment and depression were assessed by the Mini-Mental State Examination questionnaire (MMSE) [20] and the Geriatric Depression Scale (GDS-15), respectively. The GDS consists of a series of 15 questions in a simple yes/no format [21]. From MMSE summary scores, participants were classified as having normal cognition (25–30), mild (21–24), moderate (14–20) or severe (<13) cognitive impairment [22]. From GDS summary scores, participants were classified as without depression (normal (0–4)), or having mild depression (5–8), moderate depression (9–11) or severe depression (12–15) [21].
Nutritional status
The Mini-Nutritional Assessment Instrument (MNA) assesses nutritional status. The MNA consists of four main components (anthropometric and a global, dietary, and subjective assessment) and is a Dieticians Association of Australia-recommended screening tool in all levels of care [23, 24].
Falls history and fear of falling
A fall was defined as an event resulting in a person coming to rest unintentionally on the ground or lower level, not as a result of a major intrinsic event (such as a stroke) or an overwhelming hazard [25]. The Activity-Specific Balance Confidence questionnaire was used to assess fear of falling [26]. In addition, participants were asked directly to recall the number of falls they had experienced in the last 6 months as a reference point for comparison to facility-recorded falls [27].
Facility audit of services and activities
All participating facilities underwent a 35-question audit to provide information on the services and activities accessible and available to participants. Examples of the services, activities and facilities audited included ‘doctor or resident nurse’, ‘group exercise classes’ and ‘swimming pool.’ These were scored as ‘available’ or ‘not available’.
Participant burden
To evaluate the impact of data collection, participants were asked a short battery of eight questions and sessions were timed. Questions assessed length and fatigue during assessment, and clarity of instructions and terminology, and were scored on a 5-point Likert Scale (strongly agree, agree, neither, disagree or strongly disagree). Participants were also asked whether they would be willing to be involved in follow-up work and if they required a rest during assessment. The RA noted if participants required more than one session to collect individual data.
ActivPAL3™ sub-study
The activPAL3™ (Pal Technologies Ltd., Glasgow, UK) is a small (dimensions = 53 × 35 × 7 mm; 15 g), unobtrusive device/inclinometer worn on the thigh 24 h per day for the duration of the measurement protocol and classifies time as sitting/lying, standing or ambulation [28]. All participants in the primary study were invited to participate in the activPAL3™ sub-study, excluding those with dementia or non-ambulatory. All participants consent to the sub-study independent of the primary study. The device was fitted by the RA to the participant’s anterior mid-line of the right thigh using a hypoallergenic bandage, and worn continuously for 7 days. If removed, participants and facility staff were instructed on how to replace the device appropriately. Sleep times and any time where the activPAL3™ was removed were recorded in a sleep diary. A batch of 10 monitors were charged, initialised and waterproofed each week then sent to the RA for distribution to participants. Each monitor has a recording life of 14 days after the initialised start date. Following the protocol period, the device was returned to the research team by centre staff using a reply paid express post bag. Raw data from the activPAL3™ monitor were used to generate overall activity averages, as well as per day and per hour averages. These data are presented elsewhere [29].
In addition to measuring older adult’s activity patterns, the feasibility of using activPAL3™ monitors in a RAC population was investigated. To facilitate this, participants could express any concerns associated to wearing the monitor by writing in a designated section of the sleep diary. Further, the researchers undertaking this sub-study conducted a telephone interview with each RAC centre asking them for any comments and/or difficulties experienced due to the monitors. As a final measure, a research member visited one RAC centre to personally conduct interviews with the primary contact person for the study. Questions of concern included, but are not limited to, ‘did participants report any concerns regarding wearing the monitor?’, ‘did the process impede on staff work?’ and ‘what are your suggestions for future studies?’
Sample size and analysis plan
Based on previous non-consensual sarcopenic prevalence estimates of 30 % in RAC [13, 30, 31], and assuming a level of confidence Z = 95 %, and precision of 5 %, it was estimated that 210 adults would be required for this study [32]. With allowance for a dropout for 30 %, 273 adults were randomised into the study. The primary aim of this study is to measure the prevalence of sarcopenia in RAC.
Cases of sarcopenia were diagnosed using the EWGSOP criteria [6]. Specifically, participants were classified pre-sarcopenic if found to have below-normal muscle mass only, sarcopenic if they had below-normal muscle mass and function (either muscle strength or physical performance), and severe sarcopenic if they had below-normal muscle mass, strength and performance [6]. Prevalence was based on a proportion of cases of sarcopenia per total study population.
Subgroup analyses were conducted on the prevalence of sarcopenia according to gender, age and care settings. Comparisons were made on demographic, functional and clinical variables between participants with and without sarcopenia. Data were analysed using parametric and nonparametric statistics based on data distribution. Risk factors were determined by statistical analysis of the association of the secondary measures on the primary measure of sarcopenic status by regression analysis. Statistical significance was based on two-tailed tests with p < 0.05 considered as significant.
Results: baseline characteristics
Participants
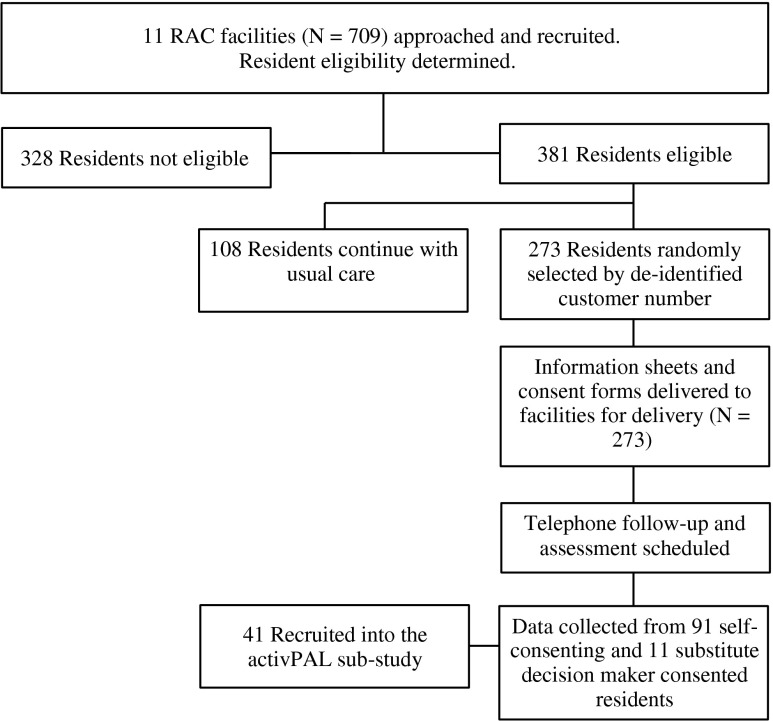
Of the 709 residents residing in the 11 RAC facilities, 328 were considered by facility service managers or directors of nursing ineligible to participate. The reasons for ineligibility were: 3 % had a pacemaker, 8 % were end-stage palliative or terminal, 31 % had dangerous behaviours and 58 % had medical or other problems that would have made them difficult to include in data collection, including the inability to get substitute decision maker consent. Three hundred and eighty-one residents were considered eligible and capable of participating. Of these, 273 were randomly selected to participate in the study. Within this sample, level of care was represented as per the total eligible sample (N = 381). This was: 29 % low care, 58 % high care and 13 % in a secure dementia ward. Of the 273 residents invited to participate, 91 provided consent and 11 were consented by proxy (N = 102; 37 %). Therefore, of the total available sample, 53 % were eligible for participation and 14 % consented to participate. Participant recruitment and assessment occurred over October to December 2012. The flow of recruitment to assessment is represented in Fig. 1.
Fig. 1.
Project CONSORT diagram of recruitment and assessment
Reasons for non-consent were (i) ‘didn’t want to participate’ (79 %), ‘My GP doesn’t want me to participate’ (7 %), they were not available during the facility testing period (2 %), a change in their situation occurred (generally health related) (7 %) or they died after being identified as potentially eligible prior to consent (3 %). Five people claimed they were too sick on the day of their assessment and four did not provide a reason.
Participants ranged in age from 60.7 to 101.3 years with the average age of 84.5 ± 8.2 years and were in the RAC facility for 1,204.2 ± 1,220.1 days (65–7165 days). Level of disability and need for care was represented by a group ACFI score of 2.6 ± 1.7 (1 being the highest needs and 7 the lowest), with low functional status supported by a SPPB score of 3.5 ± 2.4 (1–11). Participants had an average of 1.8 ± 2.0 chronic diseases (0–6) and took 11.8 ± 4.9 medications (2–31). The BMI for participants was 27.4 ± 5.7 kg/m2 (13.4–44.5 kg/m2). Six participants were unable to complete the MMSE and GDS. Of the remaining group, 33 had normal cognitive capacity, 19 were mildly, 30 moderately and 14 severely cognitively impaired. Nine adults had severe depression, 13 were moderately and 22 mildly depressed and the remaining 52 had no depression.
Burden
Time to complete the data collection ranged from 11.0 to 59.0 min dependant of the participant capacity to complete measures, with an average of 27.0 ± 7.0 min across the group. When asked about the burden of assessment, 84 % of participants either agreed or strongly agreed that the assessment was an appropriate length and 83 % either agreed or strongly agreed that the instructions given with each measure were clear. In relation to the number of questionnaires, terminology employed and assessment fatigue, >70 % of participant felt the assessment protocol was appropriate for the target cohort. Of those who participated, only one individual required a break during testing and 87 % said they would be happy to be followed up for future work in this area.
ActivPAL sub-study
For the activPAL3™ sub-study, a total of 41 residents agreed to participate from an eligible 86. Most common reasons for not consenting were ‘didn’t want to participate’ (73 %) and ‘believe it will be too difficult’ (19 %). Two people were unavailable during the testing period, one person saw no benefit to participating, and six residents said ‘My GP doesn’t want me to participate’. Six monitors were lost during the course of the study, and two participants were subsequently found to be ineligible. The final participant sample consisted of 33 residents. Of those, 2 did not return their diaries, 3 did not complete the diary, 14 (42 %) partially completed the diary, and 14 (42 %) completed the diary in full. Five (15 %) provided at least one comment regarding wearing the monitor. All comments were positive and participants did not report any adverse outcomes.
Informal interviews with key contact personnel at each centre reinforced that residents were not burdened by wearing the monitor. There were no reported cases of skin irritation or complaints from residents. Furthermore, staff members indicated the monitor wear did not significantly impede on their daily duties. Discussions during the face-to-face interview indicated there was a need to include RAC staff members in the activPAL3™ process from the very beginning. Furthermore, staff members suggested that more information regarding the monitors and the overall goal of the sub-study would have been beneficial in order to better explain the study to residents and answer questions they may have had, and to avoid confusion and minimise the risk of losing monitors.
Discussion
Demonstrated by a topical presence at the 2013 International Association of Geriatrics and Gerontology Congress and Global Aging Research Network symposium, sarcopenia is re-emerging as a growing area of research interest (see Journal of Nutrition, Health and Aging, Issue 6, Supplementary, June 2013). While sarcopenia has always generated some research, the revised European Working Group in Sarcopenia in Older People diagnostic criteria has significantly influenced the clinical acknowledgment of the consequences of sarcopenia, and the association to disease and disability [33]. Using this definition and assessment criteria, more work is needed at the community and in-care level to establish population prevalence and the associated risk factors. This paper describes in detail the feasibility and processes undertaken in studying these variables in an Australian population of very old RAC adults. It is suggested this work could service as a guide for others interested in undertaking similar research among other international populations.
The feasibility of our approach is indicated by the low participant burden, efficient collection time and agreement by participants that the methodologies were appropriate for the cohort. The group in question are typical of RAC in Australia in relation to age, length of stay and ACFI score [34], with a low functional status supported by a SPPB summary score indicative of negative health outcomes [18]. We involved the RAC facility in the research, using the facility service manager or director of nursing to identify potential participants, and coordinating through the facility the delivery of project information, obtaining of consent and assistance in data collection. However, while the study had a liberal inclusion criteria that aimed to capture adults independent of disease or disability [3], ethical and patient difficulty limitations excluded almost half of the total available population. This exclusion may limit the accurate reflection of the population in question. This is a concern for all end-of-life research, but unavoidable given the invasive nature of data collection, and the need for consent and the level of communication in collecting secondary variables questionnaires included to identify risk factors [35]. Of further concern is that 67 % of those randomised to the study declined, commonly affirming a lack of interest in participation. This could be related to a number of factors including no participation incentives such as the benefits gained from a trial intervention (i.e. exercise intervention), a fear of something new, the need for greater project understanding by the staff responsible for disseminating information, as was voiced in the sub-study, and/or cognitive wellbeing, where depression is a known de-motivator to participation [36–38]. While this low participation rate may reduce the generalisability of results across RAC population, they are by no means unusual [36]. Another methodological limitation was that data were collected within one organisation and one region of Australia. Organisational differences in delivery of care and the effect of remote and rural living could influence levels of sarcopenia [39]. The present work had budget restriction that influenced recruitment, but to allow for these differences a broader scope analysis is planned.
The sub-study analysis of the activPAL monitor was also found to be feasible in this population. In order to improve the protocol, facility staff suggested that the sleep diary be simplified, more information provided to centre staff, and greater communication within centre be encouraged, to minimise monitors loss and adequately address residents’ questions.
In conclusion, certain limitations restrict blanket data collection in a RAC setting. However, the assessment of sarcopenia and sarcopenic risk factors such as physical inactivity is feasible among those without ethical considerations such as palliative status, behavioural or communications problem. To facilitate projects in this setting, key staff members need to be involved in the planning and execution of this process from the beginning in order to ensure their full understanding and leadership of the protocol within centres.
Acknowledgments
This project was supported by a Bond University Seeding Grant. The research team would like to thank the staff of Blue Care who supported the project and assisted with resident recruitment and assessment. The authors certify that they have complied with the ethical guidelines for authorship and publishing set down by the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
Timothy R. Henwood, Justin W. Keogh, Natasha Reid, Will Jordon and Hugh E. Senior declare no conflict of interest in undertaking this research or delivering this manuscript.
References
- 1.Goss J. Projection of Australian health care expenditure by disease, 2003 to 2033. Canberra: Australian Institute of Health and Welfare; 2008. [Google Scholar]
- 2.3222.0—population projections, Australia, 2006 to 2101. Canberra: Australian Bureau of Statistics; 2008. [Google Scholar]
- 3.Chumlea WMC, Cesari M, Evans WJ, Ferrucci L, Fielding RA, Pahor M, et al. Sarcopenia: designing phase IIB trials international working group on sarcopenia. J Nutr Health Aging. 2011;15:450–5. doi: 10.1007/s12603-011-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. JAMDA. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, Salive M, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 12.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, Perez-Zepeda MU. Prevalence of sarcopenia in Mexico City. Eur Geriatr Med. 2012;3:157–60. [Google Scholar]
- 13.Janssen I. The epidemiology of sarcopenia. Clin Geriatr Med. 2011;27:355–63. doi: 10.1016/j.cger.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Taaffe DR. Sarcopenia—exercise as a treatment strategy. Aust Fam Physician. 2006;35:130–4. [PubMed] [Google Scholar]
- 15.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–34. [PubMed] [Google Scholar]
- 16.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Kurlowicz L. The Geriatric Depression Scale (GDS) Geriatr Nurs. 1999;20:212–3. [PubMed] [Google Scholar]
- 22.Woodford HJ, George J. Cognitive assessment in the elderly: a review of clinical methods. QJM. 2007;100:469–84. doi: 10.1093/qjmed/hcm051. [DOI] [PubMed] [Google Scholar]
- 23.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature—what does it tell us? J Nutr Health Aging. 2006;10:466–85. [PubMed] [Google Scholar]
- 24.Saka B, Kaya O, Ozturk GB, Erten N, Karan MA. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr. 2010;29:745–8. doi: 10.1016/j.clnu.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–22. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 26.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 27.Hewitt J, Refshauge K, Goodall S, Henwood T, Clemson L. Does progressive resistance and balance exercise reduce falls in residential aged care? Randomized Controlled Trial Protocol for the SUNBEAM Program. Clin Interv Aging. Accepted 2013: In Press. [DOI] [PMC free article] [PubMed]
- 28.Grant PM, Granat MH, Thow MK, Maclaren WM. Analyzing free-living physical activity of older adults in different environments using body-worn activity monitors. J Aging Phys Act. 2010;18:171–84. doi: 10.1123/japa.18.2.171. [DOI] [PubMed] [Google Scholar]
- 29.Reid N, Eakin E, Henwood T, Keogh JW, Senior HE, Gardiner PA, et al. Objectively measured activity patterns among adults in residential aged care. Int J Environ Res Public Health. 2013;10:6783–98. doi: 10.3390/ijerph10126783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillette-Guyonnet S, Vellas B. Body composition and age-related diseases. Mech Ageing Dev. 2003;124:247–8. doi: 10.1016/s0047-6374(02)00191-4. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 32.Daniel WW. Biostatistics: a foundation for analysis in the health sciences. Hoboken: Wiley; 1999. [Google Scholar]
- 33.Zhong K, Lei SF, Yang F, Chen XD, Tan LJ, Zhu XZ, et al. The differences of sarcopenia-related phenotypes: effects of gender and population. Eur Rev Aging Phys Act. 2012;9:63–9. [Google Scholar]
- 34.Residential aged care in Australia 2008–09: a statistical overview. Canberra: Australian Institute of Health and Welfare; 2010. [Google Scholar]
- 35.O’Reilly M, Courtney M, Edwards H. How is quality being monitored in Australian residential aged care facilities? A narrative review. Int J Qual Health Care. 2007;19:177–82. doi: 10.1093/intqhc/mzm002. [DOI] [PubMed] [Google Scholar]
- 36.Nyman SR, Victor CR. Older people’s recruitment, sustained participation, and adherence to falls prevention interventions in institutional settings: a supplement to the Cochrane systematic review. Age Ageing. 2011;40:430–6. doi: 10.1093/ageing/afr016. [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. JAMDA. 2012;13:418–28. doi: 10.1016/j.jamda.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Hsu YC, Wright CL. The association between participation in social activity and depressive symptoms in institutionalized elders in Taiwan. Geriatr Nurs. 2014;35:31–6. doi: 10.1016/j.gerinurse.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly M, Courtney M, Edwards H, Hassall S. Clinical outcomes in residential care: setting benchmarks for quality. AJA. 2011;30:63–9. doi: 10.1111/j.1741-6612.2010.00447.x. [DOI] [PubMed] [Google Scholar]