Abstract
Osteopenia/osteoporosis, sarcopenia, and obesity are commonly observed in the process of aging, and recent evidence suggests a potential interconnection of these syndromes with common pathophysiology. The term osteosarcopenic obesity has been coined to describe the concurrent appearance of obesity in individuals with low bone and muscle mass. Although our understanding of osteosarcopenic obesity’s etiology, prevalence, and consequences is extremely limited, it is reasonable to infer its negative impact in a population that is aging in an obesogenic environment. It is likely that these individuals will present with poorer clinical outcomes caused by the cascade of metabolic abnormalities associated with these changes in body composition. Clinical outcomes include but are not limited to increased risk of fractures, impaired functional status (including activities of daily living), physical disability, insulin resistance, increased risk of infections, increased length of hospital stay, and reduced survival. These health outcomes are likely to be worse when compared to individuals with obesity, sarcopenia, or osteopenia/osteoporosis alone. Interventions that utilize resistance training exercise in conjunction with increased protein intake appear to be promising in their ability to counteract osteosarcopenic obesity.
Keywords: Osteopenia/osteoporosis, Sarcopenia, Obesity, Body composition
Introduction
Body composition refers to the amount and distribution of fat and fat-free tissues of the body; it extends beyond body weight and body mass index (BMI) because the units of body weight are evaluated for the relative proportions and distribution of fat and fat-free tissues [1]. Body composition analysis becomes particularly important in situations or clinical conditions where body weight and BMI do not accurately depict nutritional status and when abnormalities in body composition emerge [1]. Examples include but are not limited to elderly individuals who may present with normal body weight and BMI but have significant depletion in both muscle strength and mass (dynapenia/sarcopenia). These individuals may also present with deteriorated bone, undetected by assessment of body weight alone [2, 3]. Likewise, marked increases in visceral adipose tissue may occur regardless of changes in total body weight. In clinical situations such as cancer, muscle wasting with or without changes in adipose tissue also occurs regardless of BMI. In fact, obese individuals may present with depleted muscle mass and strength, similar to emaciated or cachectic patients [1].
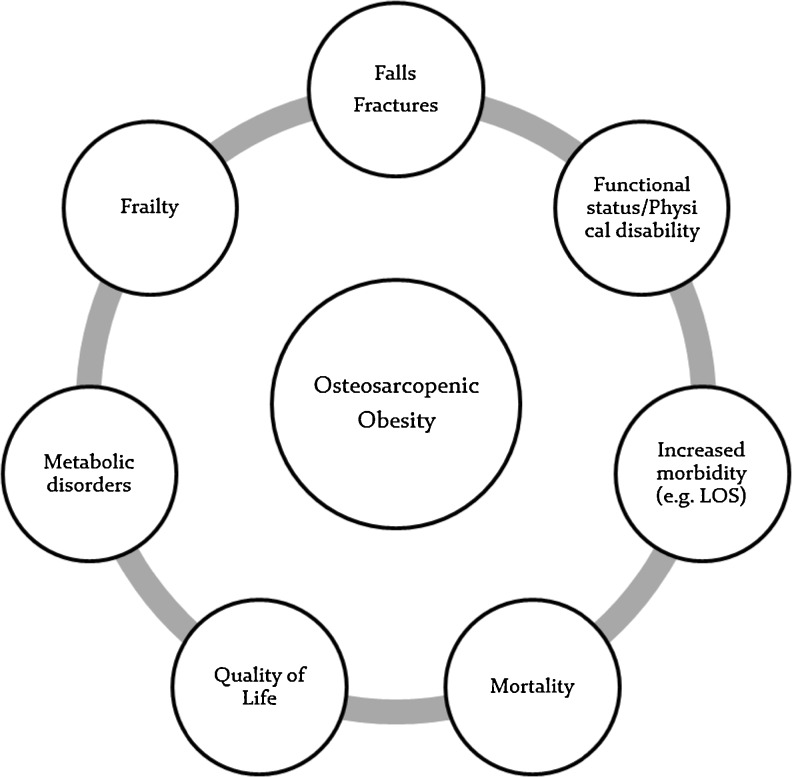
Whether abnormalities in body composition are related to losses of bone and muscle or increases in adipose tissue, these unfavorable changes are likely to impact health. We use the term “osteosarcopenic obesity” to describe the concurrent appearance of obesity in individuals with low bone and muscle mass. We hypothesize that this phenotype may be associated with poorer functional and metabolic outcomes than each of these conditions alone, ultimately affecting quality of life, morbidity risk, and survival (Fig. 1). In this paper, we discuss potential mechanisms, populations at risk, health outcomes, and countermeasures to osteosarcopenic obesity.
Fig. 1.
Clinical implications of changes in body composition. Legend: Abnormal body composition is defined as abnormalities in the amount and/or distribution of tissues in the body. Examples include low muscle mass (sarcopenia), low bone mass/density (osteoporosis), high levels of adipose tissue (obesity), abnormal patterns of adipose tissue distribution (visceral adiposity), and a combination of these abnormalities. Metabolic disorders include but not limited to insulin resistance, decrease production of anabolic hormones, and inflammation. LOS, length of hospital stay
Definition and assessment of abnormal body composition phenotypes: osteopenia/osteoporosis, sarcopenia, and obesity
Osteopenia/osteoporosis
Osteopenia/osteoporosis is a skeletal disorder characterized by compromised bone strength leading to increased risk of fractures. Osteoporosis affects approximately 12 million adults over the age of 50 years in the USA [4]. In 2005, more than 2 million incident fractures were reported in the USA alone, with a total cost of $17 billion. Even if rates stay the same, the aging of the world population is expected to increase the number of fractures and costs in the USA by 48 % to greater than 3 million fractures associated with a cost of $25.3 billion [5].
Although osteoporosis is a complex entity, we can measure areal bone mineral density (BMD), which is used as a proxy to define bone strength and to diagnose osteopenia/osteoporosis [6, 7]. Dual-energy X-ray absorptiometry (DXA) is the most common diagnostic imaging technique for assessing BMD. DXA uses low-radiation X-rays of two different photon energy levels that pass through the body and are identified by a photon detector that measures the amount of energy absorbed by soft tissue and bone at each pixel [8]. This method measures both bone and soft tissue [8]. Soft tissue is further subdivided into fat and lean [also called lean body mass (LBM), Table 1] based on the empiric attenuation of both pure fat and bone-free soft tissue [9]. Therefore, bone mineral content and BMD, as well as fat and fat-free soft tissues at the whole-body and regional levels can be assessed by DXA [8, 10]. Uniform standards for diagnosing osteopenia and osteoporosis by DXA do exist. According to the World Health Organization (WHO), the operational definition of osteopenia is a BMD that lies from 1.1 to 2.4 standard deviations below (a T-score of 1.1–2.4) and osteoporosis is a BMD that lies 2.5 standard deviations or more below the average value for young healthy women (a T-score of < −2.5). Increased BMD augments the strength of bone. On the contrary, significant losses in BMD predispose an individual for increased risk of fracture.
Table 1.
Body composition terminology
| ASM (appendicular skeletal muscle mass) | Lean body mass from limbs, a surrogate measure of skeletal muscle mass. It can be expressed adjusted for height in squared meters (kg/m2) and named ASM index. |
| FFM (fat-free mass) | Sum of LBM plus bone mineral content |
| FM (fat mass) | Amount of fat, also known as body fat |
| LBM (lean body mass) | Also called lean soft tissue, it is the sum of the lean compartments of the body (excluding bone mineral content) (total body water, total body protein, carbohydrate, nonfat lipid, and soft tissue minerals) |
| Obesity | Body mass index ≥ 30 kg/m2 |
| Osteosarcopenic Obesity | Concurrent osteoporosis, low muscle mass, and obesity |
| Sarcopenia | Low skeletal muscle mass or muscle wasting |
| Sarcopenic obesity | Concurrent obesity and low muscle mass |
Sarcopenia
Muscle and bone mass, as well as their strength/quality, are both lost during aging starting in the late 20s and accelerating in the 50s [11–13]. In the presence of chronic disease, drug therapy, environmental factors, poor nutrition, and physical inactivity, this loss can be accelerated.
One important advantage of using DXA is its ability to estimate appendicular skeletal muscle (ASM) mass which is of particular interest for the assessment of sarcopenia. ASM corresponds to the amount of LBM in the arms and legs, which is mainly muscle (except for a small amount of connective tissue and skin) [14].
Sarcopenia is a term originally used to describe age-related decreases in muscle mass [15]. The term has evolved to indicate a point where skeletal muscle mass and/or strength has declined past a threshold in which health is affected. The European Consensus [16] has recently defined sarcopenia as a progressive, generalized loss of muscle mass and strength which will increase the risk for physical disability, diminished physical performance, and poor quality of life. Although losses of both muscle mass and strength can occur simultaneously, this relationship is not linear [16–18].
Although there are several working definitions of sarcopenia, one of the most commonly used criteria is a level of skeletal muscle mass (ASM index, Table 1) lower than 2 SD below the expected mean for healthy young adults, as established by DXA. This sex-specific definition corresponds to ≤7.26 kg/m2 for men and ≤5.45 kg/m2 for women [19]. Importantly, individuals below this cut point have a significantly increased risk of adverse functional status, such as higher risk of disability, falls, and fractures [20].
It is important to highlight that establishing the threshold to define sarcopenia has been a challenge for the past several years. A variety of cut points in the literature have been reported, which were developed using different body composition techniques such as DXA, bioelectrical impedance, skinfold thickness measurements, and computerized tomography (CT images) [21]. Although most cut points are based on measurements of muscle mass, muscle quality has also been used as a criterion to identify sarcopenia. Measurements of muscle strength (handgrip, hip, or knee strength) are popular for this purpose because of their cost-effectiveness and availability in clinical settings [22]. In summary, definitions of sarcopenia are somewhat arbitrary and are limited to the availability of large clinical and epidemiological studies (population-representative cut points) using gold-standard body composition assessment tools [23]. The reader is referred to an extensive review on the topic for an in depth discussion [24].
Obesity
Obesity is a condition of excess weight, specifically adipose tissue, in the body. The most commonly accepted definition of obesity is the WHO categories of BMI [weight (kg)/height (m2)] of 30 kg/m2 or higher. According to this definition, there are three grades of obesity: grade I (BMI 30.0–34.9 kg/m2), grade II (BMI 35.0–39.9 kg/m2), and grade III (BMI ≥ 40 kg/m2) [25]. Nonetheless, BMI is an imperfect and controversial criterion [26] since it does not differentiate between lean and fat tissues. Using percent body fat is another way to classify individuals as obese. Normal percent body fat for an adult man is considered to be approximately 12–20 % of total body weight and 20–30 % for an adult woman [27]. Alternatively, the WHO has suggested that obesity is identified at levels of higher than 25 % body weight for men and 35 % for women [28], with somewhat higher levels established for elderly individuals (>28 % for men and >40 % for women) [29]. Percent body fat values for obese individuals separated by sex, age, and ethnicity have also been proposed by Gallagher et al. [30]. Interestingly, the distribution of body fat is also associated with increased health risks, with android adiposity being more problematic compared to the gynoid adiposity. These are most easily identified with waist circumference measures (>88 cm for men and >102 cm for women) [27, 31] or a waist to hip ratio calculation (>0.90 for men and >0.80 for women) [32].
Osteosarcopenic obesity: a new face of an old problem
Although osteopenia/osteoporosis, sarcopenia, and obesity have been recognized and assessed for decades, the concurrent appearance of these problems has just begun to be discussed. From a historical perspective, the combination of sarcopenia and obesity termed sarcopenic obesity, was the first term to be introduced [33] and is extensively studied [21].
Sarcopenic obesity is an emerging health problem characterized by the simultaneous manifestation of excess body fat and low muscle mass/strength, and it has been described by Roubenoff as the confluence of two epidemics: the aging and the obesity epidemics [34]. Since there are no standard definitions for combined sarcopenia and obesity, a variety of indices has been used, and we refer the reader to an extensive review [21]. Regardless of indices used to define this condition, the majority of studies have found sarcopenic obesity to predict worse clinical outcomes when compared to sarcopenia or obesity in isolation [21, 34, 35].
The term “sarco-osteopenia” or “ sarco-osteoporosis” was first introduced by Binkley and Buehring [36]. The authors proposed that patients with both low bone and muscle mass/performance would be diagnosed with this condition. Therefore, sarco-osteopenia or sarco-osteoporosis is an interconnected syndrome which should be combined into a single term. Individuals presenting with sarco-osteopenia or sarco-osteoporosis would be at higher risk for falls and fractures and, hence, increased morbidity, reduced quality of life, and increased mortality [36].
Although the term has only recently been proposed, the association of muscle and bone mass has been extensively studied [37–41]. Specifically, the dominant role of muscle on BMD of various skeletal sites in younger and older women was reported earlier [3]. Furthermore, when there is a lack of weight training, muscle mass begins to decline during the third decade of life, and bone loss follows due to the lack of strain [42]. Even the various modes of habitual and low-impact physical activity (gardening, stair climbing, heavy housework) had a positive influence on bone in postmenopausal women [2].
As the prevalence of elderly individuals increases in the USA and throughout the world, so does the prevalence of sarcopenia and osteopenia/osteoporosis. The addition of obesity to these existing conditions exacerbates the metabolic abnormalities likely leading to reduced physical function and quality of life. Osteosarcopenic obesity represents a change in paradigm that has gone unrecognized until recently, due to the lack of accurate technology to assess human body composition, as sophisticated tools are needed to accurately assess fat, lean, and bone tissue compartments. Furthermore, as suggested by Stenholm et al. [43], although in healthy young and older individuals bone and muscle change concurrently with changes in body weight, this process may be impaired in some individuals when the excess of body weight occurs without concurrent increases in bone and muscle mass [43].
The association among bone, muscle, and fat mass was explored by Sowers et al. [37] in adult women. The authors categorized fat and lean tissue mass into tertiles, reporting a linear increase in mean femoral neck BMD for each tertile of muscle mass. Conversely, there was a nonlinear increase in BMD for each tertile of fat mass. BMD was similarly and equally greater in the high-muscle/low-fat and high-muscle/high-fat body composition types, suggesting that greater weight alone was not associated with increased BMD. Hence, if muscle does not grow in parallel with increased body weight, BMD is not optimized [37]. The authors concluded that low muscle mass was a risk factor for low BMD in young adult women, while higher fat mass was only protective when muscle mass was adequate.
Finally, as bone and muscle loss can appear concurrently with obesity, it is reasonable to propose a new term encompassing these three conditions. The acknowledgment of osteosarcopenic obesity as an emerging public health problem increases not only scientific but also public awareness for the identification, prognostic significance, public health costs, and ultimately the development of behavioral, nutritional, and possibly pharmacological interventions to prevent or reverse this condition.
Challenges of operationalization and applicability
Unfortunately, the advantage of proposing this new abnormal body composition phenotype introduces challenges to future research endeavors. Primarily, it leads to additional debate on the diagnosis of osteosarcopenic obesity, which in turn can impact risk prediction and treatment strategies. Although the definition of osteopenia/osteoporosis has been quite widely accepted, substantial debate still exists regarding the definition of sarcopenia and even obesity [21]. Likewise, the expected prevalence of osteosarcopenic obesity in the general population is uncertain. The combination of three diagnostic criteria (for abnormal bone, muscle, and fat) may limit the number of individuals presenting with this condition, which will impact the identification of this phenotype in non-epidemiological studies in healthy populations. On the contrary, we hypothesize that the prevalence of osteosarcopenic obesity will be pronounced in those presenting with clinical conditions (e.g., cancer, diabetes, etc.).
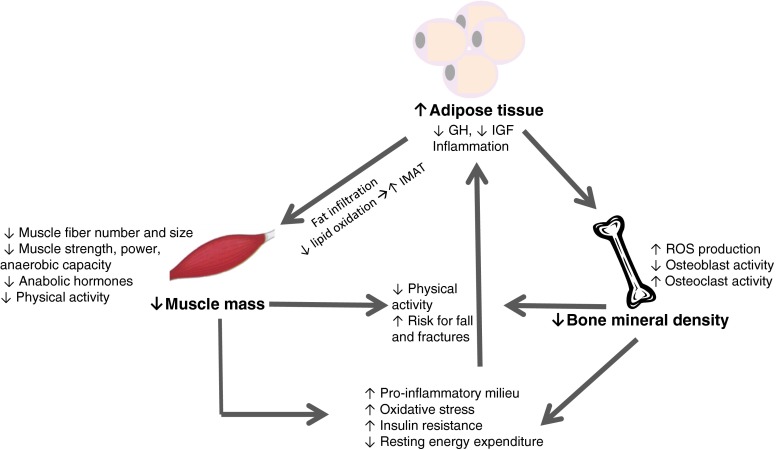
Additional opportunities exist to identify the onset, cause, and effect of this condition. Although osteosarcopenic obesity leads to health complications (Fig. 1), it is unclear if the health implications are a cause or consequence of osteosarcopenic obesity (Fig. 2). In fact, further studies are needed to investigate the occurrence of potential reverse causality. Importantly, although future studies are needed, we anticipate that this combined phenotype will represent an advantage for risk prediction and preventive/treatment strategies in primary and secondary/tertiary care.
Fig. 2.
Hypothesized interrelationships between bone, muscle, and adipose tissue in the osteosarcopenic syndrome. IMAT, intramuscular adipose tissue; GH, growth hormone; IGF, insulin-like growth factor I; ROS, reactive oxygen species. Adapted from Zamboni et al. [35], Ezzat-Zadeh et al. [44], and Roubenoff [33]
Osteosarcopenic obesity: hypothesized mechanisms
Although the etiology of osteosarcopenic obesity can only be hypothesized at this time, Fig. 2 illustrates potential mechanisms leading to progressive losses of bone and muscle mass and an increase in adipose tissue. Regardless of the initiating metabolic abnormalities, an increase in total and/or abdominal adipose tissue causes an increase in pro-inflammatory cytokines, as well as some hormonal disturbances leading to losses of both muscle and bone tissues through a variety of mechanisms which ultimately affects clinical outcomes such as increase in risk for falls and fractures and potentially a variety of other problems (discussed below). The decreases in muscle and bone are associated with decreases in physical activity; once losses hit a threshold, physical activity becomes even more limited, leading to a vicious cycle of progressive loss of muscle and bone and gain in fat, of unveiled complexity (Fig. 2).
While obesity is clearly a multifactorial condition, the primary causes appear to be related to an excess energy consumption, low levels of physical activity, and high genetic susceptibility [45]. An increase in body fat from a lack of physical activity is primarily due to the low level of energy expenditure compared to a higher level of energy intake. Interestingly, this resultant increase in adiposity is highly correlated with excess inflammation that leads to impairments in skeletal muscle function and size [46, 47]. Furthermore, while further research is needed to clarify potential mechanisms, a loss of skeletal muscle mass and function is also associated with a sedentary lifestyle [48]. Therefore, lack of activity further exacerbates the loss of muscle mass and function resulting from obesity-derived inflammation. Adding to this problem is that loss of muscle mass also accounts for a reduced level of physical activity [48]. It is clear that a sedentary lifestyle contributes to sarcopenic obesity [48, 49] and, unfortunately, leads to both disability and mobility issues greater than those with only one of these conditions [50]. The reader is referred to a comprehensive review on the topic [51].
Osteosarcopenic obesity: potential clinical implications
The financial burden of each representative component of osteosarcopenic obesity is substantial. For instance, in 2008, direct costs of obesity were estimated to total almost 14 billion US dollars [52], with even more capital lost in indirect costs such as absenteeism, disability, and premature mortality [53]. Direct medical expenditure related to sarcopenia was estimated in 2000 to be around 18.5 billion dollars per year [54]. Those with osteoporosis with a concurrent hip fracture may contribute more than 6 billion dollars on health-care costs per year in order to treat the fracture and underlying osteoporosis; those with osteoporosis without a fracture may contribute to 3.79 billion dollars of health-care costs [55], though both of these estimates are conservative compared to an alternative 13.7–20.3 billion-dollar estimate [5]. Cleary, these three conditions substantially augment the direct cost of the US health-care expenditure with extensive presumed indirect costs. A disease state such as osteosarcopenic obesity would therefore prove to be a considerable economic encumbrance.
Obesity alone and its related consequences is perhaps one of the most comprehensively studied and debated modern epidemics. The prevalence of obesity across a multitude of countries has accelerated in the last decade [56]. In the USA, 32.2 % of men and 35.5 % of women are classified as obese according the WHO cut points [57]. Though the mechanisms of obesity are yet to be elucidated, excess adiposity has been shown to be related to heart disease, type II diabetes, osteoarthritis, sleep apnea, reproductive abnormalities, certain cancers, high blood pressure, dyslipidemia, stroke, and liver/gallbladder disease [58]. In a clinical setting, obese patients may be more susceptible to infections, notably nosocomial, periodontal, postsurgical, and respiratory infections [59]. Moreover, those who are obese may experience greater mobility-related functional impediments [60] as well as walking limitations [61], which is particularly important in osteosarcopenic obesity.
Sarcopenia has its own assortment of detrimental clinical outcomes. Disability assessed by questions concerning activities of daily living can aggravate the development of sarcopenia, especially in those with severe sarcopenia (defined as a ASM index < 8.50 kg/m2 in men and <5.75 kg/m2 in women); due to its bidirectional nature, sarcopenia may lead to disability, and disability may further initiate sarcopenia [18, 62], thus creating an infinite cycle. Elderly individuals who lose fat-free mass (FFM) are over two times more likely to report disability compared to those who do not lose FFM [63]. Additionally, sarcopenia is also associated with increased mortality in the oldest (80+ years of age) frail elderly [64]. Smaller muscle mass and greater fat infiltration in muscle has been shown to have a negative relationship to lower extremity performance repeatedly measured by walking and standing/sitting assessments [65].
On its own, osteopenia/osteoporosis is a known risk factor for fractures. Fractures in particular have numerous adverse clinical implications, including an increased risk of mortality. In fact, those who experience a hip fracture have a mortality rate that is three times higher than the general population, in part due to complications faced after the fracture [66]. Men have a lower life expectancy after hip fracture than women [67], though only 25–30 % of hip fractures occur in men [68]. Those who are victims of a fracture face adverse consequences such as compromised ability to perform activities of daily living [69], increased risk of subsequent fractures, and negatively altered quality of life [70]. The fracture can negatively affect an elderly individual’s ability to walk independently and complete daily activities; more alarmingly, these patients may have an increased risk of premature entrance into nursing facilities [71].
In addition to each component’s individual implications, combined disorders can influence the development of even more adverse outcomes, and one disorder may cause another. For example, in addition to the separate side effects of sarcopenia and obesity, sarcopenic obese patients have a higher incidence of impaired function [72], diminished quality of life [43], knee osteoarthritis [73], falls, disability [29], and chemotherapy toxicity [1, 74] and shorter survival in cancer patients [75] in comparison to individuals with normal body composition; this is most likely due to the dual affliction of both low muscle mass and excess adiposity. Though obesity may have a protective role on osteoporosis [76], some research has suggested that a high body weight may actually perpetuate the development of osteoporosis [77]. A large examination of over 60,000 women reported that high body weight is not protective against fracture incidence, but may in fact be associated with ankle and upper leg fractures [78]. Furthermore, those with the most muscle wasting and lowest grip strength have much higher odds of having osteoporosis, fractures, or falls than those who have more muscle mass and strength [79]. Clearly, these conditions are interrelated, and the occurrence of one may aid in the development of another and thus lead to compounded clinical implications.
Importantly, osteosarcopenic obesity is not a syndrome of elderly individuals. There are a variety of disease states and conditions where the disease itself or its treatment is associated with losses of skeletal muscle and bone alongside gains in adipose tissue. For instance, low BMD in adulthood can be caused by type 1 diabetes mellitus (DM), though the association between type II DM and osteoporosis is less clear [80]. Diabetes treated with insulin therapy can lead to weight gain [81], and insulin resistance can lead to accelerated muscle loss mainly due to metabolic and hormonal factors [82]. Therefore, those with diabetes are likely to present with osteosarcopenic obesity. Sarcopenia has been found to occur independently of BMI in patients diagnosed with respiratory and gastrointestinal cancers and can precipitate the loss of functional status [75]. Patients undergoing cancer therapies (especially victims of breast cancer) may be at risk for bone loss, as some treatments may suppress estrogen production (which has a protective effect on bone) as well as directly adversely affect bone metabolism [83]. Yet another clinical manifestation, chronic obstructive pulmonary disease (COPD), is associated with muscle wasting and weakness which can make even daily tasks difficult, leading to decreased activity that further intensifies sarcopenia development [84]. COPD patients display numerous risk factors for osteoporosis including smoking, physical inactivity, vitamin D deficiency, low body weight, and hypogonadism. Of particular concern is glucocorticoid use which may directly adversely affect BMD, leading to secondary osteoporosis [85]. In HIV patients, antiretroviral interventions are associated with weight gain [86] along with bone loss through multiple mechanisms; the disease itself is associated with a loss in lean mass [87], suggesting that these patients commonly suffer from osteosarcopenic obesity.
Clearly, the direct impact of osteosarcopenic obesity is a potential threat to clinical and public health. Considering the effects of low muscle and bone tissues combined with the influence of obesity, it is likely that these individuals will present with poorer clinical outcomes caused by the cascade of metabolic abnormalities associated with these changes in body composition. Regardless of the specific diagnosis cut points for muscle, bone, and adipose tissue that have yet to be determined, there is no doubt that future research is needed to explore the clinical outcomes and appropriate interventions associated with the concurrent appearance of all three conditions.
Countermeasures and future directions
Future research is needed to identify the countermeasures to osteosarcopenic obesity. As this is a complex condition, it is very likely that a multifactorial approach would be the best strategy. An option for clinical practice may be non-pharmacological approaches that may play a vital therapeutic role in ameliorating potential negative consequences of osteosarcopenic obesity.
Substantial evidence exists to suggest that long-term resistance training (RT) alone can promote favorable changes in body composition, muscular strength and endurance, and lipid metabolism thereby having a positive impact on obesity [88]. Other research has demonstrated increases in FFM with resistance training [89].
Resistance exercise can also be used as an effective intervention to improve BMD; however, the precise physiological actions that modulate bone remodeling are not clearly understood. Although beyond the scope of this review, some potential mechanism of action involve mechanical mechanotransduction [90, 91], production of nitric oxide (NO) as a result of mechanical loading [92–94], and prostaglandin release [92, 95].
Additionally, resistance exercise improves muscle strength and quality through a variety of different mechanisms that include satellite cell recruitment [96] and stimulation of the mammalian target of rapamycin (mTOR) pathway (which regulates skeletal muscle growth) [96]. Certain hormones (e.g., growth hormone, insulin-like growth factor I, and testosterone) are also implicated in promoting anabolism [97–99]. Finally, resistance exercise has been shown to increase lipolysis and fat oxidation [100] in overweight and obese individuals, and the combination of resistance and aerobic exercise has been shown to favorably alter body adiposity [101, 102]. Recently, the combination of aerobic and resistance training was also shown to improve weight loss and physical function among elderly obese individuals (potentially susceptible to osteosarcopenic obesity) [103].
Nutrition also plays an important role in the development of obesity and sarcopenia. Protein intake is important for both preventing loss of muscle mass and promoting increased muscular strength and endurance [104]. In fact, evidence is mounting to suggest that manipulation of the protein/carbohydrate ratio in the diet may improve muscle mass, fat mass, and BMD in overweight and obese individuals [101, 105]. In a study of 90 free-living overweight and obese adults, it was demonstrated that a whey protein supplement providing 56 g per day of protein (with no other dietary alterations) resulted in a significantly lower body weight (−1.8 kg; p < 0.006) and fat mass (−2.3 kg; p < 0.005) over 23 weeks compared to an isocaloric carbohydrate supplement [106]. This higher protein intake is thought to best manage body composition via its influence on metabolism, satiety, and muscle mass [48, 107, 108].
Resistance training and protein supplementation combined together are possible therapies to attenuate many of the previously mentioned side effects that are associated with osteosarcopenic obesity due to lack of physical activity. A high-protein dietary intake combined with exercise training has been shown to effectively reduce both body weight and body fat in obese men and women [101] while also helping to maintain or even improve muscle mass in overweight or obese men and women [101, 105] and in sarcopenic individuals [109]. Data indicate that exercise training (six times per week) combined with a higher protein intake (40 % of total energy intake) over a period of 12 weeks improves body weight (−6.2 %), percent total body fat (−15.8 %), percent abdominal body fat (−26.4 %), and BMI (−6.0 %) in healthy but overweight and obese men and women [101]. Likewise, it was reported [105] that moderate protein intake (25 % of total daily energy intake) and exercise training (6 days per week) resulted in significant decreases in body weight (90.8 ± 4.9 to 85.3 ± 4.7 kg), percent total body fat (36.8 ± 3.0 to 33.2 ± 3.2 %), percent abdominal fat (39.4 ± 2.8 to 36.6 ± 3.0 %), and BMI (32.1 ± 1.0 to 30.1 ± 1.1 kg/m2) in overweight/obese men and women both with exercise (6 days/week) and without exercise. Interestingly, high-protein intake (40 %) in the non-exercising group had significant improvements over 12 weeks in body composition marked by decreases in body weight (94.5 ± 6.5 to 89.3 ± 5.9 kg), percent body fat (40.3 ± 2.4 to 38.3 ± 2.6 %), and waist circumference (102.2 ± 5.8 to 94.3 ± 4.8 cm). These improvements in body composition were made without the addition of an exercise program.
Taken together, increased physical activity and protein intake (likely above the current recommended reference intakes) appear to be useful for reducing adiposity and maintaining muscle and bone mass in an effort to stave of osteosarcopenic obesity. This holds true for the oldest of the old and in a variety of clinical populations, although more clinical trials are needed.
As part of the multifactorial concept of osteosarcopenic obesity, future studies on the relationship between both psychological and physical fatigue in the development and exacerbation of this syndrome must be explored. Fatigue is a behavioral marker for reduced ability to adapt to stressors, leading to reduced physical function and poorer quality of life. Reductions in fatigue by using holistic measures may be proven beneficial as a potential countermeasure for osteosarcopenic obesity [110].
Finally, given the novelty of the identification of osteosarcopenic obesity, the development of animal models will be helpful to identify the pathophysiology of this syndrome. Ultimately, experimental animal models may provide insight in how to apply these mechanisms to the human condition.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
Michael Ormsbee, Carla Prado, Jasminka Ilich, Sarah Purcell S, Mario Siervo, Abbey Folsom, and Lynn Panton declare that they have no conflict of interest.
References
- 1.Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE. Assessment of nutritional status in cancer—the relationship between body composition and pharmacokinetics. Anti Cancer Agents Med Chem. 2013;13:1197–203. [DOI] [PubMed]
- 2.Ilich JZ, Brownbill RA. Habitual and low-impact activities are associated with better bone outcomes and lower body fat in older women. Calcif Tissue Int. 2008;83:260–71. [DOI] [PubMed]
- 3.Ilich-Ernst J, Brownbill RA, Ludemann MA, Fu R. Critical factors for bone health in women across the age span: how important is muscle mass? Medscape Womens Health. 2002;7:2. [PubMed]
- 4.Force USPST. Screening for osteoporosis: recommendation statement. Am Fam Physician. 2011;83:1197–200. [PubMed]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. [DOI] [PubMed]
- 6.Rosen CJ. Primer on the metabolic bone diseases and disorders of mineral metabolism. New York: Wiley; 2013.
- 7.Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab. 2004;89:3651–5. [DOI] [PubMed]
- 8.Lohman TG, Chen Z. Dual-energy x-ray absorptiometry. In: Heymsfield SB, Lohman T, Wang Z, Going S, editors. Human body composition. 2nd ed. Champaign: Human Kinetics; 2005. p. 523.
- 9.Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB. Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a gold standard". Am J Clin Nutr. 1993;58:589–91. [DOI] [PubMed]
- 10.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–58. [DOI] [PubMed]
- 11.Marcus R, Kosek J, Pfefferbaum A, Horning S. Age-related loss of trabecular bone in premenopausal women: a biopsy study. Calcif Tissue Int. 1983;35:406–9. [DOI] [PubMed]
- 12.Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton 3rd LJ. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–35. [DOI] [PMC free article] [PubMed]
- 13.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–24. [DOI] [PubMed]
- 14.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–8. [DOI] [PubMed]
- 15.Rosenbe0rg I. The epidemiologic and methodologic problems in determining nutritional status of older persons. (Summary comments). Am J Clin Nutr. 1989;50:1231–33. [PubMed]
- 16.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. [DOI] [PMC free article] [PubMed]
- 17.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. [DOI] [PubMed]
- 18.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21. [DOI] [PubMed]
- 19.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. [DOI] [PubMed]
- 20.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. [DOI] [PubMed]
- 21.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. [DOI] [PubMed]
- 22.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. [DOI] [PubMed]
- 23.Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–50. [DOI] [PMC free article] [PubMed]
- 24.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. [DOI] [PMC free article] [PubMed]
- 25.World Health Organization. Obesity: preventing and managing the global epidemic. WHO obesity technical report series 894. Geneva: World Health Organization 2000. [PubMed]
- 26.Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238–52. [DOI] [PubMed]
- 27.Pi-Sunyer FX. Obesity: criteria and classification. Proc Nutr Soc. 2000;59:505–9. doi: 10.1017/S0029665100000732. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–52. [PubMed]
- 29.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. [DOI] [PubMed]
- 30.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–39. [DOI] [PubMed]
- 31.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. [DOI] [PMC free article] [PubMed]
- 32.Center for Disease Control and Prevention. National obesity trends. In: Services USDoHaH, ed. Atlanta: Center for Disease Control and Prevention; 2011.
- 33.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. [DOI] [PubMed]
- 34.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. [DOI] [PubMed]
- 35.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–95. [DOI] [PubMed]
- 36.Binkley N, Buehring B. Beyond FRAX: it's time to consider "sarco-osteopenia". J Clin Densitom. 2009;12:413–6. [DOI] [PubMed]
- 37.Sowers MF, Kshirsagar A, Crutchfield MM, Updike S. Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol. 1992;136:257–65. [DOI] [PubMed]
- 38.Sowers M, Kshirsagar A, Crutchfield M, Updike S. Body composition, age and femoral bone mass of young adult women. Ann Epidemiol. 1991;1:245–54. [DOI] [PubMed]
- 39.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–51. [DOI] [PubMed]
- 40.Proctor DN, Melton LJ, Khosla S, Crowson CS, O'Connor MK, Riggs BL. Relative influence of physical activity, muscle mass and strength on bone density. Osteoporos Int. 2000;11:944–52. [DOI] [PubMed]
- 41.Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–46. [DOI] [PubMed]
- 42.Frost HM. Mechanical usage, bone mass, bone fragility: a brief overview. In: Kleerekoper M, Krane SM, editors. Clinical disorders of bone and mineral metabolism: proceedings of the Laurence and Dorothy Fallis International Symposium. New York: Mary Ann Liebert; 1989. pp. 15–40. [Google Scholar]
- 43.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. [DOI] [PMC free article] [PubMed]
- 44.Ezzat-Zadeh Z, Dodge BG, Elam M, Feresin R, Browne J, Kim JS et al., eds. The underlying mechanisms by which estrogen regulates energy metabolism and body composition. FASEB J. 2012;26:56.
- 45.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. [DOI] [PubMed]
- 46.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 2011;203:259–69. [DOI] [PMC free article] [PubMed]
- 47.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2. [DOI] [PMC free article] [PubMed]
- 48.Li Z, Heber D. Sarcopenic obesity in the elderly and strategies for weight management. Nutr Rev. 2012;70:57–64. [DOI] [PubMed]
- 49.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas. 2012;71:109–14. [DOI] [PubMed]
- 50.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–28. [DOI] [PMC free article] [PubMed]
- 51.Ilich, J. Z., Kelly, O. J., Inglis, J. E., Panton, L. B., Duque, G., & Ormsbee, M. J. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15C;51–60. [DOI] [PubMed]
- 52.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev. 2011;12:50–61. [DOI] [PMC free article] [PubMed]
- 53.Trogdon JG, Finkelstein EA, Hylands T, Dellea PS, Kamal-Bahl SJ. Indirect costs of obesity: a review of the current literature. Obes Rev. 2008;9:489–500. [DOI] [PubMed]
- 54.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5. [DOI] [PubMed]
- 55.Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16:359–71. [DOI] [PubMed]
- 56.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metrics. 2012;10:22. [DOI] [PMC free article] [PubMed]
- 57.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. [DOI] [PubMed]
- 58.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the Evidence report. National Institutes of Health1998 Sep. Report No.: 1071-7323 (Print) 1071-7323 (Linking).
- 59.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. [DOI] [PMC free article] [PubMed]
- 60.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–9. [DOI] [PubMed]
- 61.Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res. 2007;19:277–83. [DOI] [PubMed]
- 62.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. [DOI] [PubMed]
- 63.Fantin F, Di Francesco V, Fontana G, Zivelonghi A, Bissoli L, Zoico E, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci. 2007;62:1375–81. [DOI] [PubMed]
- 64.Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–9. [DOI] [PubMed]
- 65.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. [DOI] [PubMed]
- 66.Panula J, Pihlajamaki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011;12:105. [DOI] [PMC free article] [PubMed]
- 67.Frost SA, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Excess mortality attributable to hip-fracture: a relative survival analysis. Bone. 2013;56:23–9. [DOI] [PubMed]
- 68.Orwig DL, Chan J, Magaziner J. Hip fracture and its consequences: differences between men and women. Orthop Clin N Am. 2006;37:611–22. [DOI] [PubMed]
- 69.Osnes EK, Lofthus CM, Meyer HE, Falch JA, Nordsletten L, Cappelen I, et al. Consequences of hip fracture on activities of daily life and residential needs. Osteoporos Int. 2004;15:567–74. [DOI] [PubMed]
- 70.Boonen S, Autier P, Barette M, Vanderschueren D, Lips P, Haentjens P. Functional outcome and quality of life following hip fracture in elderly women: a prospective controlled study. Osteoporos Int. 2004;15:87–94. [DOI] [PubMed]
- 71.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S–7S. discussion 7S-9S. [DOI] [PubMed]
- 72.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am J Clin Nutr. 2009;89:1895–900 [DOI] [PubMed]
- 73.Lee S, Kim TN, Kim SH. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. 2012;64:3947–54. [DOI] [PubMed]
- 74.Prado CM. Body composition in chemotherapy: the promising role of CT scans. Curr Opin Clin Nutr Metab Care. 2013;16:525–33. [DOI] [PubMed]
- 75.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. [DOI] [PubMed]
- 76.Oros S, Ianas O, Vladoiu S, Giurcaneanu M, Ionescu L, Neacsu E, et al. Does obesity protect postmenopausal women against osteoporosis? Acta Endocrinol-Buch. 2012;8:67–76.
- 77.Jankowska EA, Rogucka E, Medras M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia. 2001;33:384–9. [DOI] [PubMed]
- 78.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–50. [DOI] [PMC free article] [PubMed]
- 79.Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–80. [DOI] [PubMed]
- 80.Inzerillo AM, Epstein S. Osteoporosis and diabetes mellitus. Rev Endocr Metab Disord. 2004;5:261–8. [DOI] [PubMed]
- 81.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812. [DOI] [PubMed]
- 82.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–69. [DOI] [PubMed]
- 83.Hadji P, Gnant M, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treat Rev. 2012;38:798–806. [DOI] [PubMed]
- 84.Wust RC, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chronic Obstructive Pulm Dis. 2007;2:289–300. [PMC free article] [PubMed]
- 85.Biskobing DM. COPD and osteoporosis. Chest. 2002;121:609–20. [DOI] [PubMed]
- 86.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retrovir. 2013;29:435–40. [DOI] [PMC free article] [PubMed]
- 87.Dudgeon WD, Phillips KD, Carson JA, Brewer RB, Durstine JL, Hand GA. Counteracting muscle wasting in HIV-infected individuals. HIV Med. 2006;7:299–310. [DOI] [PubMed]
- 88.Prabhakaran B, Dowling EA, Branch JD, Swain DP, Leutholtz BC. Effect of 14 weeks of resistance training on lipid profile and body fat percentage in premenopausal women. Br J Sports Med. 1999;33:190–5. [DOI] [PMC free article] [PubMed]
- 89.Treuth MS, Ryan AS, Pratley RE, Rubin MA, Miller JP, Nicklas BJ, et al. Effects of strength training on total and regional body composition in older men. J Appl Physiol. 1994;77:614–20. [DOI] [PubMed]
- 90.Burger EH, Klein-Nulend J. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–12. [PubMed]
- 91.Zernicke R, MacKay C, Lorincz C. Mechanisms of bone remodeling during weight-bearing exercise. Appl Physiol Nutr Metab. 2006;31:655–60. [DOI] [PubMed]
- 92.Fan X, Roy E, Zhu L, Murphy TC, Ackert-Bicknell C, Hart CM, et al. Nitric oxide regulates receptor activator of nuclear factor-kappaB ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology. 2004;145:751–9. [DOI] [PubMed]
- 93.Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, et al. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U S A. 1994;91:3569–73. [DOI] [PMC free article] [PubMed]
- 94.MacIntyre I, Zaidi M, Alam AS, Datta HK, Moonga BS, Lidbury PS, et al. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A. 1991;88:2936–40. [DOI] [PMC free article] [PubMed]
- 95.Turner CH, Robling AG. Exercise as an anabolic stimulus for bone. Curr Pharm Des. 2004;10:2629–41. [DOI] [PubMed]
- 96.Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97:643–63. [DOI] [PubMed]
- 97.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–22. [DOI] [PubMed]
- 98.Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–95. [DOI] [PubMed]
- 99.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–61. [DOI] [PubMed]
- 100.Ormsbee MJ, Thyfault JP, Johnson EA, Kraus RM, Choi MD, Hickner RC. Fat metabolism and acute resistance exercise in trained men. J Appl Physiol. 2007;102:1767–72. [DOI] [PubMed]
- 101.Arciero PJ, Gentile CL, Martin-Pressman R, Ormsbee MJ, Everett M, Zwicky L, et al. Increased dietary protein and combined high intensity aerobic and resistance exercise improves body fat distribution and cardiovascular risk factors. Int J Sport Nutr Exerc Metab. 2006;16:373–92. [DOI] [PubMed]
- 102.Ormsbee MJ, Choi MD, Medlin JK, Geyer GH, Trantham LH, Dubis GS, et al. Regulation of fat metabolism during resistance exercise in sedentary lean and obese men. J Appl Physiol. 2009;106:1529–37. [DOI] [PubMed]
- 103.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. [DOI] [PMC free article] [PubMed]
- 104.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7. [DOI] [PubMed]
- 105.Arciero PJ, Gentile CL, Pressman R, Everett M, Ormsbee MJ, Martin J, et al. Moderate protein intake improves total and regional body composition and insulin sensitivity in overweight adults. Metab Clin Exp. 2008;57:757–65. [DOI] [PubMed]
- 106.Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr. 2011;141:1489–94. [DOI] [PMC free article] [PubMed]
- 107.Phillips SM. Role of protein absorption and nutrient timing on muscle mass accretion. 110th Abbott Nutrition Research Conference; Ross Park Conference Centre, Columbus, Ohio2009.
- 108.Churchward-Venne TA, Murphy CH, Longland TM, Phillips SM. Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans. Amino Acids. 2013;45:231–40. [DOI] [PubMed]
- 109.Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. [DOI] [PubMed]
- 110.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46. [DOI] [PMC free article] [PubMed]