Abstract
Muscle wasting is a common complication afflicting maintenance hemodialysis (HD) patients, and it is associated with decreased muscle function, exercise performance, physical function, and quality of life. Meanwhile, numerous epidemiologic studies have consistently shown that greater muscle mass (ascertained by body anthropometry surrogates, body composition tests such as dual x-ray absorptiometry, and/or serum creatinine in patients with little to no residual kidney function) is associated with increased survival in this population. The pathophysiology of muscle wasting in HD patients is complex and may be caused by poor dietary intake, catabolic effects of dialysis therapy, hormonal alterations (e.g., decreased levels or resistance to anabolic hormones, increased levels of catabolic hormones), inflammation, metabolic acidosis, and concurrent comorbidities. Muscle disuse resulting from low physical activity is an important yet under-appreciated risk factor for muscle wasting. Intra-dialytic resistance exercise training has been suggested as a potential strategy to correct and/or prevent this complication in HD patients, but prior studies examining this exercise modality as an anabolic intervention have shown mixed results. In a recently published 12-week randomized controlled trial of a novel intra-dialytic progressive resistance exercise training (PRET) program vs. control therapy conducted in HD and non-HD patients, PRET resulted in increased muscle volume and strength in both groups. At this time, further study is needed to determine if anabolic improvements imparted by resistance exercise translates into improved physical function and quality of life, decreased hospitalization and mortality risk, and greater cost-effectiveness in HD patients.
Main text
Approximately 20 to 50 % of maintenance hemodialysis (HD) patients suffer from protein-energy wasting (PEW), a condition of decreased body protein and fat mass that potently predicts morbidity and mortality in this population [1–3]. Muscle wasting is a key component of PEW, and it adversely affects multiple patient-centered outcomes including muscle function, exercise performance, physical function, and quality of life (QOL) [4, 5].
Numerous epidemiologic studies have also consistently shown that reduced muscle mass is associated with decreased survival in end-stage renal disease patients, including those receiving HD, peritoneal dialysis, or kidney transplantation [6–14]. While various body anthropometry surrogates (e.g., mid-arm muscle circumference [9]) and sophisticated equations have been utilized to estimate muscle mass in these studies [10], serum creatinine has been found to be a reliable marker of muscle mass in dialysis patients with little to no residual kidney function [13]. Indeed, a number of studies have shown that lower serum creatinine levels in HD patients are associated with increased death risk [6, 7, 11]. Recent data also suggest that decreased muscle strength may be an even more potent mortality predictor than muscle mass [15].
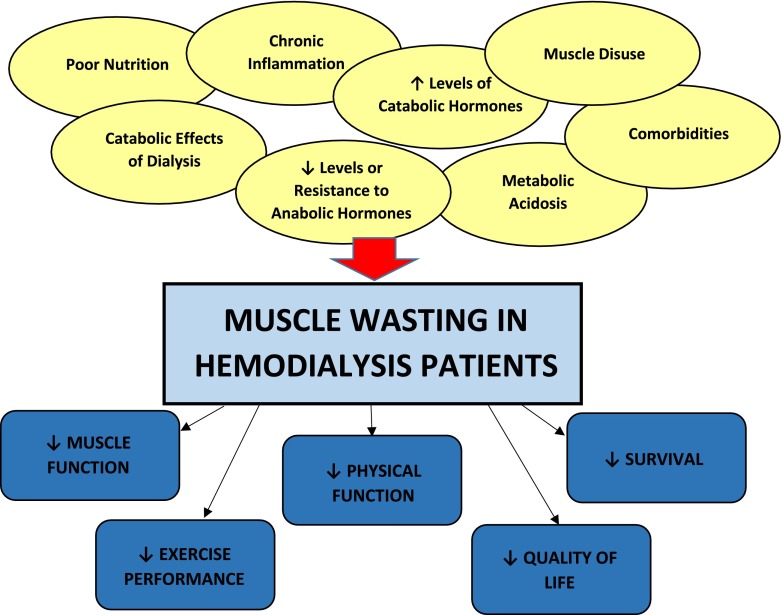
Given its high prevalence and dire sequelae, considerable study has been made to understand the underlying etiologies of muscle wasting in HD patients, in order to identify strategies that correct and/or prevent this complication (Fig. 1). The pathophysiology of muscle wasting and weakness appears to be complex and multifactorial and may be attributed to one or more of (1) insufficient nutritional intake; (2) catabolic effects of dialysis therapy; (3) hormonal aberrations including low levels or increased resistance to anabolic hormones (e.g., testosterone, growth hormone, insulin-like growth factor-1 [IGF-1]), increased levels of catabolic hormones (e.g., cortisol), and possible thyroid hormone deficiency; (4) chronic inflammation; (5) metabolic acidosis; and (6) concurrent comorbidities (e.g., diabetic gastroparesis) [1, 4, 16, 17].
Fig. 1.
Risk factors and sequelae of muscle wasting in hemodialysis patients
Muscle disuse due to reduced physical activity (defined as “any bodily movement produced by contraction of skeletal muscle that increases energy expenditure above a basal level” [18]) is another important yet relatively under-appreciated risk factor for muscle wasting in HD patients [19]. Epidemiologic data show that physical activity levels in HD patients are exceedingly low. Among 1,547 ambulatory dialysis patients in the Comprehensive Dialysis Study, physical activity scores ascertained by the Human Activity Profile were below the fifth percentile of healthy individuals based on age and sex norms [20]. Similarly, among 134 patients in the Dialysis Outcomes and Practice Patterns Study, 64 % of patients had sedentary or low physical activity levels [21]. While the 2005 Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines on Cardiovascular Disease in Dialysis Patients recommend that nephrology and dialysis staff routinely counsel dialysis patients on increasing their physical activity levels [22], survey data has shown that less than one-third of nephrologists routinely recommend exercise (a form of physical activity that is “planned, structured, repetitive, and purposive in the sense that the improvement or maintenance of one or more components of physical fitness is the objective” [23, 24]) [25]. This may in part relate to providers’ uncertainties regarding the benefits of exercise upon short- and long-term outcomes in HD patients as well as its optimal modality, prescription, and associated risks in this population.
A number of studies have examined various modalities of exercise training in dialysis patients over the past three decades [26]. While most studies have focused on aerobic exercise, which primarily improves cardio-respiratory endurance and fitness, there have been fewer studies of resistance exercise, which promotes muscle growth, mass, and strength; has been shown to be an effective anabolic intervention in elderly patients and other chronic disease populations [27]; and is theoretically a more optimal exercise modality in enhancing physical function [4, 26, 28]. Furthermore, there has been particular interest in studying resistance exercise during or around the time of dialysis treatment, which is thought to enhance compliance and to counter-act muscle wasting at a time when catabolism is at its peak [1]. In a sentinel study by Johansen et al., among 79 HD patients randomized to a 2 × 2 factorial trial of moderate-intensity intra-dialytic resistance exercise training and anabolic steroid administration (nandrolone decanoate) for 12 weeks, exercise resulted in increased quadriceps muscle cross-sectional area measured by MRI, increased strength, and improved self-reported physical function [29]. However, resistance exercise did not increase lean body mass detected by dual-energy x-ray absorptiometry nor improve physical performance (e.g., walk test, stair climb, chair rise). Shortly thereafter, an elegant study conducted by Kopple et al. showed that, among 80 patients randomized to intra-dialytic strength/resistance training, endurance training, strength/resistance + endurance training, vs. no exercise training, those assigned to resistance or endurance training experienced increases in muscle mRNA, muscle IGF-1 protein, and lean body mass ascertained by anthropometric measurements [30]. However, other randomized controlled trials of moderate- to high-intensity resistance training alone, or in combination with intra-dialytic nutritional supplementation, have not shown improvements in muscle cross-sectional area nor lean body mass and have been inconsistent with respect to effects upon muscle strength [31–33]. Hence, the utility of intra-dialytic resistance exercise as an anabolic intervention in HD patients has remained unclear.
A recent study by Kirkman et al. published in the Journal of Cachexia, Sarcopenia, and Muscle entitled “Anabolic Exercise in Haemodialysis Patients: A Randomized Controlled Pilot Study” has sought to address this knowledge gap by examining the impact of a novel intra-dialytic progressive resistance exercise training (PRET) program on muscle volume, strength, and physical function in HD patients as well as in non-HD healthy patients during university visits [19]. In this single-blind controlled study, 23 HD and 9 non-HD patients were randomized to PRET, which consisted of thrice-weekly high-intensity leg press exercises (three sets of eight to ten repetitions at 80 % of their predicted one-repetition maximum, which is the maximum weight that can be lifted one time with proper technique), vs. control (SHAM) therapy, which consisted of low-intensity lower body stretching activities using ultra-light resistance bands. An important innovation of this study was to incorporate an incremental increase in the weekly training load/volume in the PRET arm of the trial. After a 12-week interventional period, PRET resulted in a significant increase in (1) the primary outcome of thigh muscle volume ascertained by MRI and (2) the secondary outcome of knee extensor strength measured by isometric dynamometer in both HD and non-HD patients. Furthermore, patients randomized to SHAM therapy, particularly those in the HD group, experienced clinically significant amounts of muscle volume loss.
However, PRET did not enhance HD patients’ (3) performance in physical function tests (e.g., sit-to-stand, get-up-and-go, walk test) or (4) self-reported QOL ascertained by the Short Form-36, although improvements in non-HD patients were observed. This stands in contrast to prior studies in the elderly, those with other catabolic states, and dialysis patients in whom similar interventions have resulted in improved physical function [27, 34]. This study’s discrepant findings may have been due to (1) selection of particular physical function assessment tests that have a high degree of variability, are subject to a ceiling effect, or have limited ability to capture improvements in activities of daily living as compared to other physical function instruments and metrics (i.e., self-reported physical function) [29]; (2) type 2 error due to the small sample size of the pilot study; or (3) true absence of effect on physical function and QOL.
Several important contributions made by Kirkman et al.’s study should be noted. First, their incorporation of a graded increase in weekly training load/volume in the study arm may be necessary to promote an adequate anabolic response in HD patients, and the absence of this intervention in prior studies of resistance training may in part explain the inconsistencies across these collective data [19, 29–33]. Second, while earlier studies of intra-dialytic resistance have employed combination anabolic interventions (i.e., anabolic hormone administration [29], intra-dialytic nutritional supplementation [33]) given concerns that exercise both stimulates muscle protein synthesis and breakdown, the findings of the Kirkman et al. study suggest that PRET alone can augment muscle volume and strength. Third, while there may be theoretical concerns related to the risk of exercise in HD patients (e.g., particularly musculoskeletal injury, cardiac ischemia, and sudden cardiac death as well as exercise-related hypo- and hypertension, electrolyte abnormalities, and hypoglycemia [26, 28]), the low frequency and rather mild nature of adverse events observed in HD patients assigned to the PRET arm provides further reassurance that resistance exercise is safe in this population. However, it is of our opinion that HD patients with underlying history or risk factors for cardiopulmonary disease and related comorbidities should undergo exercise testing prior to the initiation of a resistance exercise training program.
Taken together, these data corroborate that resistance exercise training administered at incrementally higher levels is an effective and safe anabolic intervention in HD patients. At this time, further study is needed to determine if these short-term augmentations in muscle volume and strength translate into improved physical function, QOL, hospitalization and mortality risk, and cost-effectiveness.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Conflict of interest
Connie M. Rhee and Kamyar Kalantar-Zadeh declare that they have no conflict of interests related to the present submission.
Funding source
The authors are supported by the research grants from the NIH/NIDDK including K24-DK091419 (KKZ), R01-DK078106 (KKZ), and philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang.
Contributor Information
Connie M. Rhee, Phone: (714) 456-5142, Email: crhee1@uci.edu
Kamyar Kalantar-Zadeh, Phone: (714) 456-5142, Email: kkz@uci.edu.
References
- 1.Dong J, Ikizler TA. New insights into the role of anabolic interventions in dialysis patients with protein energy wasting. Curr Opin Nephrol Hypertens. 2009;18:469–75. doi: 10.1097/MNH.0b013e328331489d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4:89–94. doi: 10.1007/s13539-013-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikizler TA. Exercise as an anabolic intervention in patients with end-stage renal disease. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found. 2011;21:52–6. doi: 10.1053/j.jrn.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–7. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175:793–803. doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant Off J AmSoc Transplant Ame SocTransplant Surg. 2011;11:725–36. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–68. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noori N, Kovesdy CP, Bross R, Lee M, Oreopoulos A, Benner D, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis Off J Natl Kidney Found. 2011;57:130–9. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88:479–86. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J, Mehrotra R, Rhee CM, Molnar MZ, Lukowsky LR, Patel SS, et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc. 2013;28:2146–55. doi: 10.1093/ndt/gft213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013;4:19–29. doi: 10.1007/s13539-012-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:1463–73. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clinical Journal of the American Society of Nephrology: CJASN. 2014. Epub 2014/07/31. [DOI] [PMC free article] [PubMed]
- 16.Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2013;8:593–601. doi: 10.2215/CJN.06920712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee CM, Brent GA, Kovesdy CP, Soldin OP, Nguyen D, Budoff MJ, et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2014. Epub 2014/02/28. [DOI] [PMC free article] [PubMed]
- 18.Painter P, Marcus RL. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013;8:861–72. doi: 10.2215/CJN.06590712. [DOI] [PubMed] [Google Scholar]
- 19.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle. 2014. Epub 2014/04/09. [DOI] [PMC free article] [PubMed]
- 20.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–70. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avesani CM, Trolonge S, Deleaval P, Baria F, Mafra D, Faxen-Irving G, et al. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc. 2012;27:2430–4. doi: 10.1093/ndt/gfr692. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2005; 45(4 Suppl 3):S1–153. [PubMed]
- 23.Physical Activity Guidelines Advisory Committee: Physical Activity Guidelines Advisory Committee Report, 2008, Washington, DC, U.S. Department of Health and Human Services, 2008.
- 24.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis Off J Natl Kidney Found. 2003;41:171–8. doi: 10.1053/ajkd.2003.50001. [DOI] [PubMed] [Google Scholar]
- 26.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18:1845–54. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 27.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726–34. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 28.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis Off J Natl Kidney Found. 2012;59:126–34. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–14. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 30.Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W, et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007;18:2975–86. doi: 10.1681/ASN.2006070794. [DOI] [PubMed] [Google Scholar]
- 31.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis Off J Natl Kidney Found. 2007;50:574–84. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 33.Dong J, Sundell MB, Pupim LB, Wu P, Shintani A, Ikizler TA. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found. 2011;21:149–59. doi: 10.1053/j.jrn.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Headley S, Germain M, Mailloux P, Mulhern J, Ashworth B, Burris J, et al. Resistance training improves strength and functional measures in patients with end-stage renal disease. Am J Kidney Dis Off J Natl Kidney Found. 2002;40:355–64. doi: 10.1053/ajkd.2002.34520. [DOI] [PubMed] [Google Scholar]