Novel interaction between DAP12 and KIR2DS significantly alters surface expression and may impact subsequent cellular functions.
Keywords: innate immunity, receptor maturation, receptor internalization
Abstract
KIR aid in the regulation of NK cell activity. In this study, the effect of the interaction between the KIR2DS and their adapter, DAP12, was investigated beyond the previously defined signaling function. Flow cytometry analysis showed enhanced KIR2DS surface expression on NKL cells when cotransfected with DAP12. Conversely, KIR2DS4 surface expression on primary cells was decreased when the cells were treated with DAP12-specific siRNA. Treatment of the KIR2DS and DAP12-transfected cells with CHX or BFA repressed KIR2DS surface expression, revealing a role for DAP12 in trafficking newly synthesized KIR to the cell surface. Immunoprecipitation of DAP12 revealed an interaction of DAP12 with an immature isoform of KIR2DS, indicating that the interaction likely initiates within the ER. An internalization assay demonstrated a significant impact of DAP12 on KIR2DS surface stability. Confocal microscopy showed that internalized KIR2DS molecules are recruited to lysosomal compartments independent of DAP12 expression. Our results suggest that in vivo conditions that adversely affect DAP12 expression will indirectly reduce surface expression and stability of KIR2DS. These effects could significantly impact ligand recognition and strength of signaling through KIR2DS molecules.
Introduction
The polymorphic KIR family is comprised of inhibitory and stimulatory receptors that impact NK cell function. The receptors express two or three extracellular Ig-like domains. The two- and three-domain-inhibitory KIR are known to recognize specific HLA-C and HLA-B allelic products, respectively [1, 2]. Upon ligand binding, the ITIMs, located on the long cytoplasmic tail of the inhibitory KIR, recruit and activate the phosphatases, SHP-1 and SHP-2, that inhibit propagation of activating signals within the cell [3, 4]. This recognition of HLA molecules prevents autoimmune responses by NK cells, as predicted by the missing self-hypothesis, and plays a role in determining the activation potential of an individual NK cell [5, 6].
The activating receptors, KIR2DS1 and KIR2DS4, are also capable of binding HLA ligands. KIR2DS1 is known to recognize the same HLA-C molecules as its inhibitory counterpart KIR2DL1 but with a lower affinity [7–9]. The full-length allelic product, KIR2DS4*001, recognizes HLA-C*04 and HLA-A*11:02, as well as an unidentified non-HLA ligand [10–12]. Ligands for the other stimulatory KIR, KIR2DS2, KIR2DS3, KIR2DS5, and KIR3DS1, remain unknown.
Transmission of intracellular signals from stimulatory KIR differs from the inhibitory KIR. The shorter cytoplasmic tail of the stimulatory KIR lacks classical ITAMs. The absence of ITAMs causes these receptors to rely on their adapter molecule, DAP12, for efficient transmission of signaling. The DAP12 gene encodes a disulfide-bonded homodimer containing two ITAMs within its cytoplasmic region. KIR and DAP12 interact noncovalently through a lysine located in the transmembrane region of the stimulatory KIR and an aspartic acid residue of DAP12. Upon ligand binding of the receptor, DAP12 recruits ZAP-70 and Syk protein tyrosine kinases to initiate activation cascades within the cell [13]. Signal transduction by KIR2DS2 in the absence of DAP12 has been observed in T cells upon costimulation of the TCR, suggesting that stimulatory KIR may also interact with an alternate adapter molecule [14, 15].
Adapter molecules also function beyond their signaling capabilities. Another adapter molecule, DAP10, plays an essential role in regulating proper expression of its associated receptor, NKG2D. The data suggest that DAP10 prevents degradation of NKG2D and directs its transport to the cell surface [16, 17]. Similar roles have been suggested for DAP12, as ex vivo culture of NK cells with the combination of IL-15 and IL-21 reduces expression of DAP12 with a correlated decrease in surface expression of the associated activating receptor, NKp44 [18]. KIR3DS1 surface expression has also been correlated with DAP12 expression in a transfected model system [19]. In this study, we sought to determine the impact of DAP12 on KIR2DS surface expression and to elucidate mechanisms underlying the outcome. Our data demonstrate a significant role of DAP12 in driving KIR2DS maturation and transport to the cell surface. We also describe a significant role for DAP12 in stabilizing these receptors at the cell surface. Understanding these mechanisms may help clarify KIR2DS function and signaling capabilities under conditions where DAP12 expression is altered significantly.
MATERIALS AND METHODS
Cell lines and culture
The NKL cell line was a gift of Dr. Francisco Borrego (National Institute of Allergy and Infectious Diseases, Rockville, MD, USA) and was maintained in RPMI 1640 containing 10% FBS, 1 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 100 U/ml IL-2 (BD Biosciences, Franklin Lakes, NJ, USA). PBMCs were obtained from SeraCare Life Sciences (Milford, MA, USA) and genotyped to identify KIR2DS4*001-positive donors, as described previously [20]. PBMCs were cultured in RPMI 1640 containing 10% FBS, 1 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 2% spent culture media from the myeloma cell line, J558L, transfected with the human IL-2 gene (a kind gift of Dr. Louis Weiner, Georgetown Medical Center, Washington, DC, USA). The KHYG-1 cell line was obtained from the Japanese Collection of Research Bioresources cell bank (Osaka, Japan) and was maintained in the same culture media as the primary PBMCs. HEK293 T cells were a gift of Dr. Todd Waldman (Georgetown Medical Center) and were maintained in DMEM with 10% FBS, 1 mM L-glutamine, 10 mM HEPES, and 1 mM sodium pyruvate. Jurkat cells were obtained from the tissue culture-shared resources at the Lombardi Comprehensive Cancer Center (Georgetown Medical Center) and were maintained in RPMI 1640 containing 10% FBS, 1 mM L-glutamine, 10 mM HEPES, and 1 mM sodium pyruvate.
DNA constructs
The cDNA encoding KIR2DS1*002 and KIR2DS4*001 was cloned into the expression vector pEF-DEST51 (Invitrogen Life Technologies, Carlsbad, CA, USA), as described previously [21, 22]. KIR2DS1*002 cDNA was also cloned into the pLenti4/V5-Dest gateway vector (Invitrogen Life Technologies) using the same primer sets described previously [22]. The cDNA encoding KIR2DS2*001 was obtained from Origene Technologies (Rockville, MD, USA), and the DAP12 cDNA was obtained from Invitrogen Life Technologies. These cDNAs were amplified using the following primers: 2DS2-forward (CACCATGTCGCTCATGGTC) and 2DS2-reverse (TCCTGCGTATGACACCTCCTG) for KIR2DS2, with a glycine in place of the stop codon, and DAP12-forward (CACCATGGGGGGACTTGAAC) and DAP12-reverse (TCATTTGTAATACGGCCTC). These amplicons were cloned into the expression vector, pEF-DEST51, through Gateway Technology, using the entry vector pCR8/GW/TOPO, following the manufacturer's protocol (Invitrogen Life Technologies). Following the manufacturer's protocol for QuikChange II (Stratagene, La Jolla, CA, USA), site-directed mutagenesis was performed to insert nucleotides encoding the HA-tag YPYDVPDYA, immediately following the leader sequence of DAP12. The mutant DAP12 construct was created by site-directed mutagenesis using the following primers: D50A-forward (CAGGGATCGTGATGGGAGCCCTGGTGCTGACAGTGCTC) and D50A-reverse (GAGCACTGTCAGCACCAGGGCTCCCATCACGATCCCTG). The LAMP1-GFP and Rab5-GFP expression vectors (pCMV6-AC-GFP) were obtained from Origene Technologies. All constructs were prepared as per the manufacturer's instructions using the HiSpeed Plasmid Maxi Kit (Qiagen, Valencia, CA, USA).
KIR expression
For analysis of KIR surface expression on transfected NKL cells, NKL cells (107 cells) were cotransfected with 5 μg of a KIR2DS-encoding construct and 5 μg empty vector (pEF-DEST51) or 5 μg of the DAP12-encoding construct using a Nucleofector II (Lonza, Cologne AG, Cologne, Germany) with the protocol O-017. KIR surface expression was determined by flow cytometry using PE-conjugated antibodies specific for CD158a/h (KIR2DS1), CD158b/j (KIR2DS2), and CD158i (KIR2DS4; Beckman Coulter, Fullerton, CA, USA). Unless noted, cells were collected 16 h post-transfection and stained externally with the appropriate antibody for 30 min at 4°C. Total KIR expression was also determined by flow cytometry using a FITC-conjugated V5 specific for the internal, C-terminal V5 tag linked to each KIR molecule (Invitrogen Life Technologies), as described previously [21]. Variation in total KIR expression across independent expreiments was controlled for by representing KIR surface expression as a ratio to total expression. The ratios were normalized to the ratios obtained for KIR2DS cotransfected with DAP12. Each experiment was performed with three independent biological samples resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. The data shown are representative of these experiments. The differences in surface expression were examined from each experiment using a one-way ANOVA, followed by Bonferroni's multiple comparison analysis using GraphPad Prism version 4.
Analysis of KIR trafficking
NKL cells were cotransfected simultaneously with KIR2DS, along with empty vector or DAP12, as described above. Beginning 10 h post-transfection, cells were treated for 6 h with 100 μg/ml CHX (Sigma-Aldrich, St. Louis, MO, USA) or 1× BFA (eBioscience, San Diego, CA, USA). CHX and BFA were replenished every 2 h for the duration of the experiment. After the treatment, KIR2DS surface expression was determined by flow cytometry, as described above. Each experiment was performed with three independent biological samples resulting from three independent transfections/condition for each time-point. Each experiment was repeated and analyzed in two independent experiments. The results from each experiment were statistically analyzed using a one-way ANOVA, followed by Bonferroni's multiple comparison analysis.
Gene silencing and rqRT-PCR
Primary PBMCs from two individuals positive for KIR2DS4*001 were separately expanded in culture for 7 days as described above. PBMCs (3×105) or flow cytometry-sorted, KIR2DS4-positive cells (3×105) from the expanded PBMCs were cultured in serum-free Accell siRNA delivery media (siRNA technologies; Dharmacon, Lafayette, CO, USA) with 1 μM nonsilencing siRNA or 1 μM DAP12-specific siRNA (Dharmacon) for 72 h. KIR2DS4 surface expression was determined by flow cytometry as described above. Total RNA was isolated following the manufacturer's protocol with a RNeasy Mini kit (Qiagen). cDNA was generated from ∼200 ng total RNA using TaqMan reverse transcription reagents from Applied Biosystems (Foster City, CA, USA), as per the manufacturer's instruction. DAP12 and β-actin mRNA levels were determined by relative quantitative RT-PCR using a StepOnePlus RT-PCR instrument using DAP12 and β-actin-specific, predesigned probes and primer sets and TaqMan Gene Expression Master Mix, as per the manufacturer's protocol (Applied Biosystems). β-Actin mRNA levels served as the internal control. The relative quantities of DAP12 mRNA, obtained after targeted siRNA treatment, were normalized to the values obtained following scrambled siRNA treatment. The data presented were obtained from five independent experiments performed in duplicate from two individuals for the whole PBMC population. Results from KIR2DS4 positively sorted cells were also obtained from five independent experiments performed in duplicate. Differences in DAP12 mRNA expression and KIR2DS4 surface expression following siRNA treatment were statistically analyzed using Mann-Whitney U-tests.
Immunoprecipitation and Western blot
NKL cells were cotransfected with a KIR2DS construct and empty vector or DAP12, as described above. Sixteen hours post-transfection, cells were lysed for 30 min at 4°C using 0.5% Nonidet P-40 in PBS. Lysates were immunoprecipitated using a DAP12-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoprecipitants or whole cell lysates from NKL cells or PBMCs were electrophoresed on 4–15% polyacrylamide Tris-HCl ready gels, transferred to nitrocellulose membranes, and the membranes were blocked using 5.0% nonfat dry milk, as described previously [22] (Bio-Rad, Hercules, CA, USA). The membranes were probed with a 1:5000 dilution of a V5 (Invitrogen Life Technologies)-, HA (Sigma-Aldrich)-, or β-actin (Abcam, Cambridge, MA, USA)-specific primary antibody or a 1:500 dilution of a DAP12-specific antibody (Santa Cruz Biotechnology). Following primary antibody incubation and washing with 0.1% Tween 20 in PBS, the membranes were incubated with a secondary HRP-conjugated antibody specific for mouse (Jackson ImmunoResearch, West Grove, PA, USA) or rabbit (Santa Cruz Biotechnology) IgG and detected as described previously [22]. Densitometry values were obtained using AlphaImager software (AlphaInnotech, San Leandro, CA, USA). The density values for DAP12 expression in primary cells were obtained from five independent experiments performed in duplicate and were normalized to density values for the loading control, β-actin. The normalized data were statistically analyzed using a Mann-Whitney U-test.
Internalization analysis
The internalization of KIR2DS molecules on NKL cells cotransfected with KIR2DS plus empty vector or DAP12 was analyzed as described previously [23]. Briefly, transfectants were probed 16 h post-transfection with the corresponding PE-conjugated, KIR-specific antibody and cultured at 37°C for 0, 10, 30, and 60 min. Samples were collected at indicated time-points and were untreated or treated with 200 μl PBS containing 100 mM glycine and 100 mM NaCl (pH 2.5) for 2 min to strip away externally bound antibodies. Cells were washed, and the amount of receptor internalization was analyzed by flow cytometry. The percent of KIR2DS internalized was calculated using the following equation: 100 × (MFIexp−MFIstripped)/(MFItotal−MFIstripped), where MFIexp represents internalized KIR staining at the time-point taken. MFItotal and MFIstripped are the values representing KIR expression in samples not treated with the acidic solution or samples stained and then treated immediately with the acid at the “zero” time-point representing background values, respectively. Each experiment was performed with three independent biological samples, resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. The results from each experiment were statistically analyzed using a two-way repeated-measures ANOVA, followed by Bonferroni post-tests.
Viral transduction and stable KIR2DS1 expression
Viral particles for stable expression of KIR2DS1 in the KHYG-1 cell line were produced using the ViraPower Lentiviral Expression System (Invitrogen Life Technologies), following the manufacturer's protocol. The KHYG-1 cells (1.5×106) were incubated overnight with a 1:1 ratio of culture media containing viral particles to complete growth media for the cell line. After the overnight incubation, the media were replaced with complete growth media for 24 h. Following this recovery period, the cells were grown in selection media containing 100 μg/ml Zeocin and 20% FBS, and KIR2DS1-positive cells were selected via flow cytometry using a PE-conjugated antibody specific for KIR2DS1 (Beckman Coulter).
Confocal microscopy
HEK293T cells (5×104) were plated on a 35-mm glass Fluorodish (World Precision Instruments, Sarasota, FL, USA). The cells were transfected with 1 μg LAMP1-GFP or Rab5-GFP, 1 μg KIR2DS1, and 1 μg DAP12 or empty vector (pEF-DEST51) constructs with FuGENE 6 (Roche, Indianapolis, IN, USA). Sixteen hours post-transfection, the cells were probed with an APC-conjugated antibody specific for KIR2DS1 (Miltenyi Biotech, Bergisch Gladbach, Germany) for 2 h at 4°C or 37°C. After the antibody incubation, cells were fixed using a 4.0% PFA solution (Pierce Scientific, Rockford, IL, USA). For confocal analysis of KIR2DS1 localization in the KYHG-1/KIR2DS1 stable cell line, the cells were probed with an APC-conjugate antibody specific for KIR2DS1 for 2 h at 37°C. The cells were fixed with 4.0% PFA, followed by permeabilization with 0.1% Triton X-100 (Sigma-Aldrich) solution. The cells were probed with a LAMP1-specific antibody (Abcam), followed by a FITC-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch). The samples were viewed with an Olympus FluoView FV300 laser-scanning confocal system.
KIR2DS degradation
KHYG-1 cells stably expressing KIR2DS1 were treated with 100 μM chloroquine (Sigma-Aldrich) for 2 h. Following treatment, the cells were fixed and permeabilized as described for confocal analysis and then probed with a FITC-conjugated, V5-specific antibody and analyzed by flow cytometry. Degradation of the KIR2DS molecules in NKL cells was analyzed following cotransfection with the empty vector or DAP12. Ten hours post-transfection, the NKL cells were left untreated and stored at 4°C or treated with 100 μg/ml CHX at 37°C for 6 h. After the treatment, the cells were fixed, permeabilized, probed for total KIR expression using a V5-specific antibody, and analyzed by flow cytometry as described above. The percent degraded was calculated using the equation, 100 × (MFI10 h−MFI16 h)/MFI10 h. Each experiment was performed with three independent biological samples, resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. The data were statistically analyzed using Student's unpaired t-tests.
Online Supplemental material
Shown is Western blot analysis of DAP12 protein expression in NKL cells following transfection with empty vector, WT DAP12, or mutated DAP12 (D50A). Transfections and Western blot analysis were performed as described above. RT-PCR for DAP12 and β-actin mRNA expression was performed on cDNA obtained from the Jurkat and NKL cell lines. The cDNA from Jurkat and NKL cells was amplified using the following primers: DAP12-forward (GTAAGTGGTCTCCGTCCTGTGC) and DAP12-reverse (GTAATACGGCCTCTGTGTGTTG) and β-actin-forward (GTGGCCATCTCTTGCTCGAAGTC) and β-actin-reverse (GTTTGAGACCTTCAACACCCC). The results for DAP12 protein and mRNA expression were reproduced in three independent experiments.
RESULTS
DAP12 impacts KIR2DS surface expression
To analyze the potential impact of DAP12 on KIR2DS expression, KIR surface expression was determined on NKL cells in the presence or absence of exogenous DAP12. The NKL cells, which do not express endogenous KIR, were cotransfected with a singular KIR2DS construct and empty vector or DAP12. The KIR2DS molecules analyzed for surface expression were KIR2DS1, KIR2DS2, and KIR2DS4. KIR2DS3 was excluded from the study, as we have demonstrated only minimal surface expression of this receptor [22]. As a result of the lack of a specific antibody, KIR2DS5 was also excluded based on observations that an N-terminal tag impacted trafficking patterns of the receptor.
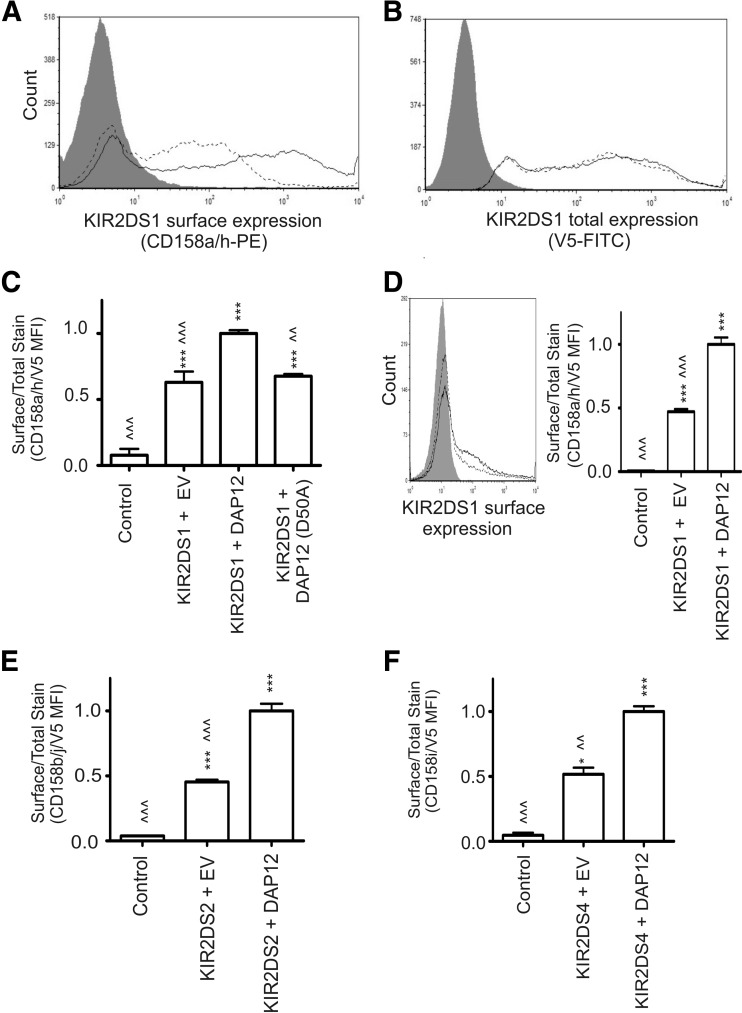
Following transfection of the NKL cells, all KIR2DS molecules analyzed were expressed at the cell surface as detected by flow cytometry. As a representative example, surface expression of KIR2DS1 is shown (Fig. 1A). As exemplified by KIR2DS1, surface expression of all the KIR2DS molecules was detected, independent of exogenous DAP12, but the level of surface expression was greatly enhanced in the presence of exogenous DAP12. The observed difference in the level of surface expression between transfection conditions was not a result of variation in protein production, as total KIR2DS1 expression was comparable, independent of exogenous DAP12 (Fig. 1B). To analyze data from independent experiments, the data are expressed as a ratio of KIR surface expression to total KIR expression, as described in Materials and Methods (Fig. 1C). The ratio of surface-expressed KIR2DS1 to total KIR2DS1 expression was increased twofold in the presence of exogenous DAP12 compared with KIR2DS1 cotransfected with empty vector. A similar twofold increase in KIR2DS1 surface expression was observed on Jurkat cells following cotransfection with exogenous DAP12 compared with cotransfection with empty vector (Fig. 1D).
Figure 1. KIR2DS surface expression is increased by exogenous DAP12 expression.
(A) Surface expression of KIR2DS1 on NKL cells following cotransfection of KIR2DS1 with DAP12 (solid line) or empty vector (EV; dashed line). NKL cells transfected with KIR2DS4 (gray shade) that does not interact with the KIR2DS1-specific antibody, was used as a negative control. (B) Total KIR2DS1 expression in NKL cells transfected with empty vector (gray) or NKL cells cotransfected with KIR2DS1 and empty vector (dashed line) or with DAP12 (solid line). Cells were permeablized and probed with a V5-specific antibody for the internal, C-terminal V5 tag linked to each KIR molecule. (C) KIR2DS1 surface expression presented as a ratio of surface-expressed KIR2DS1 (A) to total KIR2DS1 expression (B) following cotransfection with empty vector, WT DAP12, or mutant DAP12 (D50A). All ratios obtained were normalized to results for KIR2DS with exogenous DAP12. (D) KIR2DS1 surface expression on Jurkat cells following cotransfection with DAP12 (solid line), empty vector (dashed line), or transfection with KIR2DS4 (gray shade). The results are presented as a ratio of surface-expressed KIR2DS1 to total KIR2DS1 expression, as described in Materials and Methods. (E and F) Surface-expression data presented in relative ratios for KIR2DS2 and KIR2DS4. KIR2DS1 was used as a negative control for external staining for KIR2DS2 and KIR2DS4, whereas KIR2DS4 was used as the negative control for the KIR2DS1. Each experiment was performed with three independent biological samples, resulting from three independent transfections/condition. The data (mean±sem) represent the results observed in two independent experiments. All experiments were performed in triplicate, and the data (mean±sem) represent the results observed in two independent experiments. Statistical analysis was performed using a one-way ANOVA, followed by Bonferroni's multiple comparison analysis (vs. control: *P<0.05; ***P<0.001 vs. KIR2DS+DAP12: <<P<0.01; <<<P<0.001).
To confirm that the observed changes in surface expression were dependent on the interaction with DAP12, the aspartic acid residue at amino acid position 50 of DAP12 was mutated to an alanine, as this amino acid is critical for a successful interaction between KIR2DS and DAP12 [24]. KIR2DS1 surface expression, when cotransfected with the mutant DAP12 (D50A), was comparable with the levels observed to KIR2DS1 surface expression following cotransfection with empty vector (Fig. 1C). Western blot analysis shows that the expression level of the mutant DAP12 in these experiments was comparable with WT DAP12 following transfection (Supplemental Fig. 1A). This Western blot also reveals that the endogenous protein levels of DAP12 in the NKL cells used for the study were undetectable; however, DAP12 mRNA was detectable by RT-PCR in the NKL cells but not in the Jurkat cell line (Supplemental Fig. 1B). Based on our results from Jurkat cells, we suspect that the receptors, when transfected with empty vector, are capable of being expressed at the cell surface, independent of DAP12. These data support previous observations of KIR2DS2 surface expression on DAP12-negative primary T cells [14, 15].
Coexpression of exogenous DAP12 in NKL cells with KIR2DS2 or KIR2DS4 produced similar twofold increases of relative surface expression for each of these receptors compared with surface expression in the absence of exogenous DAP12 (Fig. 1E and F). Expression of exogenous DAP12 alone did not induce endogenous expression of the KIR2DS molecules analyzed in this study (data not shown). The data from this model system suggest that KIR2DS molecules are capable of expression at the cell surface independent of DAP12, but exogenous DAP12 expression significantly enhances the number of receptors at the cell surface.
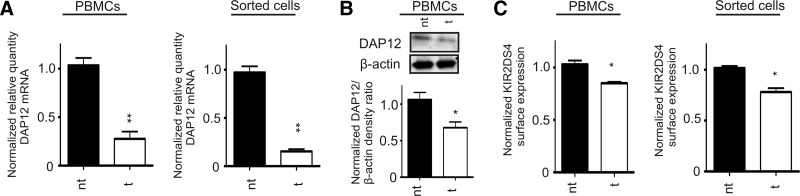
Resulting from the observed changes in surface expression of KIR2DS molecules in the presence of exogenous DAP12, we sought to determine the impact of DAP12 on KIR2DS surface expression on primary cells. Two individuals positive for the full-length allele, KIR2DS4*001, were chosen for analysis. as KIR2DS4 surface expression could be evaluated without concern for cross-reactivity of the KIR2DS4-specific antibody with another KIR molecule, unlike the antibodies specific for KIR2DS1 and KIR2DS2 that cross-react with KIR2DL1 and KIR2DL2/L3, respectively. Treatment of PBMCs or KIR2DS4 positively sorted cells from these PBMCs with DAP12-specific siRNA resulted in a significant reduction of DAP12 transcripts compared with nontargeting siRNA (Fig. 2A). However, the impact on DAP12 protein levels observed was not as dramatic, but there was a consistent 30% reduction of DAP12 protein in PBMCs following incubation with DAP12-targeting siRNA (Fig. 2B). Despite the low efficiency in reducing DAP12 protein, following treatment with DAP12-specific siRNA, a consistent 20–25% decrease of KIR2DS4 surface expression was observed in the whole PBMC and KIR2DS4 positively sorted populations from both individuals (Fig. 2C). It is likely that a more stable reduction in DAP12 would cause a greater decrease in surface expression. The correlated decrease of KIR2DS4 surface expression following knockdown of DAP12 protein in primary cells, however, does support the data obtained from the transient expression system, indicating an impact of DAP12 on KIR2DS surface expression.
Figure 2. KIR2DS4 surface expression is decreased after knockdown of DAP12 in primary cells.
Primary cells were treated with nontargeting (nt) or DAP12 targeting (t) siRNA. (A) The relative quantity of DAP12 mRNA transcripts was determined by rqRT-PCR using β-actin as an internal loading control from RNA isolated from PBMCs or KIR2DS4 positively sorted cells from these PBMCs. The relative quantity of DAP12 mRNA was normalized to levels expressed following treatment with the nontargeting siRNA. The data represent the mean (±sem) from five independent experiments performed in duplicate from two individuals. (B) Representative Western blot of DAP12 and densitometry analysis of PBMC whole cell lysates following treatment with nontargeting or DAP12-targeting siRNA. The density values obtained for β-actin were used to normalize the DAP12 density values. The data represent the mean (±sem) from five independent experiments performed in duplicate from two individuals. (C) KIR2DS4 surface expression from two donors was normalized to expression levels obtained following nontargeting siRNA treatment for whole PBMCs or KIR2DS4 positively sorted cells. The results were recorded from five independent experiments performed in duplicate and are represented by the normalized mean ± sem. Statistical analyses for all of the primary cell experiments were performed with a Mann-Whitney U-test (*P<0.05; **P<0.01).
DAP12 impacts trafficking of KIR2DS to the cell surface
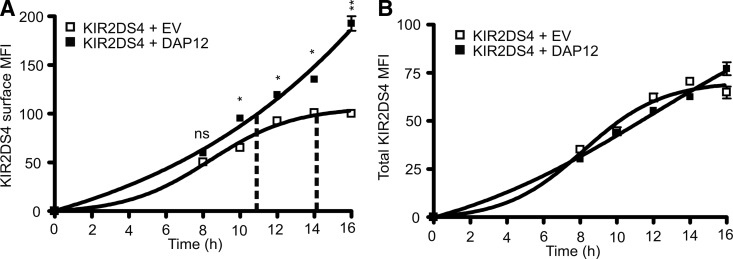
The effect on KIR2DS surface expression as a result of changes in DAP12 expression in the previous experiments could be attributed to effects in trafficking, as well as receptor stability at the cell surface. To further analyze the dynamics by which DAP12 enhances surface expression of KIR2DS molecules, KIR2DS4 surface expression on NKL cells was monitored over a post-transfection time-course in the presence or absence of exogenous DAP12. Whereas the level of KIR2DS4 surface expression remained similar at the 8-h time-point following transfection, independent of exogenous DAP12, the surface expression at the subsequent time-points was significantly higher when KIR2DS4 was coexpressed with exogenous DAP12 (Fig. 3A). The expression of the KIR2DS4 at the cell surface continued to increase at a high rate with exogenous DAP12 compared with a clear plateau effect observed for KIR2DS4 with empty vector. The maximum surface expression observed for KIR2DS4 with empty vector was obtained 14 h post-transfection, demarcated by the vertical, dashed lines. This same surface expression level was obtained between 10 and 12 h post-transfection when KIR2DS4 was transfected with DAP12. The total KIR2DS4 expression within these experiments was similar between empty vector and DAP12 conditions, dismissing the possibility that these results are a result of increased total KIR2DS4 levels within the cells (Fig. 3B). The data show that total KIR expression continues to increase, independent of exogenous DAP12; however, a continued increase in receptor surface expression is only achieved in the presence of exogenous DAP12. Conversely, in the absence of exogenous DAP12, total KIR expression continues to increase, but the level expressed at the surface reaches a plateau. These data suggest that DAP12 may be playing a role in enhancing the maturation and post-translational modifications of KIR2DS or that DAP12 has a role in impacting the recycling of internalized receptors back to the cell surface.
Figure 3. KIR2DS4 surface expression continues to increase over time in the presence of exogenous DAP12.
(A) KIR2DS4 surface expression on NKL cells was analyzed post-transfection by flow cytometry at indicated time-points following cotransfection with empty vector (□) or DAP12 (■). The zero time-point indicates the time of simultaneous cotransfection of the NKL cells with KIR2DS4 along with empty vector or DAP12. Vertical, dashed lines represent when the maximum surface expression of KIR2DS4 was achieved with empty vector and the respective expression value obtained with DAP12. (B) The total expression of KIR2DS4 was also recorded at the same time-points as monitored by the C-terminal V5 tag. Assays were performed using three independent biological samples, and results were reproduced in two independent experiments. Statistical analysis was performed using a two-way repeated-measures ANOVA, followed by Bonferroni post-tests (*P<0.05; **P<0.01). The two groups did not statistically differ at any time-point analyzed for total expression values in B.
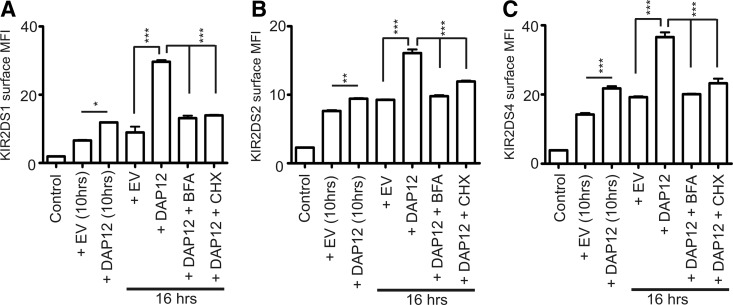
To determine if the continuous increase in KIR2DS4 surface expression observed during the time-course experiment was a result of the impact of DAP12 on the transport of newly synthesized receptors, NKL transfectants were treated with BFA to inhibit protein transport or CHX to inhibit protein synthesis, starting 10 h post-transfection. The results were consistent for all KIR2DS molecules analyzed (Fig. 4A–C). KIR2DS surface expression with exogenous DAP12 at 10 h post-transfection was significantly greater compared with KIR2DS cotransfected with an empty vector at this time-point. Consistent with the previous time-course analysis, a dramatic increase of surface expression was observed at the 16-h time-point in the presence of exogenous DAP12. Treatment of the KIR2DS and DAP12 cotransfectants with BFA or CHX, beginning 10 h post-transfection, prevented this dramatic increase of surface expression, resulting in surface-expression levels comparable with levels observed without exogenous DAP12. The inhibition of increased surface expression when KIR2DS and DAP12 transfectants were treated with CHX demonstrates that the enhanced level of receptor surface expression achieved with exogenous DAP12 is a result of expression of newly synthesized proteins at the cell surface and not a result of differences in recycling patterns of the receptor in the absence or presence of exogenous DAP12. These results support the implications of the previous time-course experiments and illustrate a role for DAP12 in efficiently trafficking newly synthesized KIR2DS molecules to the cell surface.
Figure 4. DAP12 trafficks newly synthesized KIR2DS molecules to the cell surface.
Surface expression of KIR2DS molecules was analyzed at 10 h and 16 h post-transfection of NKL cells with KIR and empty vector or DAP12. After the 10-h time-point, cells were left untreated or treated with BFA or CHX for 6 h and analyzed for KIR2DS surface expression. Flow cytometry analysis of surface expression was recorded for KIR2DS1 (A), KIR2DS2 (B), and KIR2DS4 (C). KIR2DS1 was used as a negative control for external staining for KIR2DS2 and KIR2DS4, whereas KIR2DS4 was used as the negative control for the KIR2DS1. Each experiment was performed with three independent biological samples resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. Results (mean±sem) were analyzed using a one-way ANOVA, followed by Bonferroni's multiple comparison analysis (*P<0.05; **P<0.01; ***P<0.001). All comparisons to the control were statistically significant.
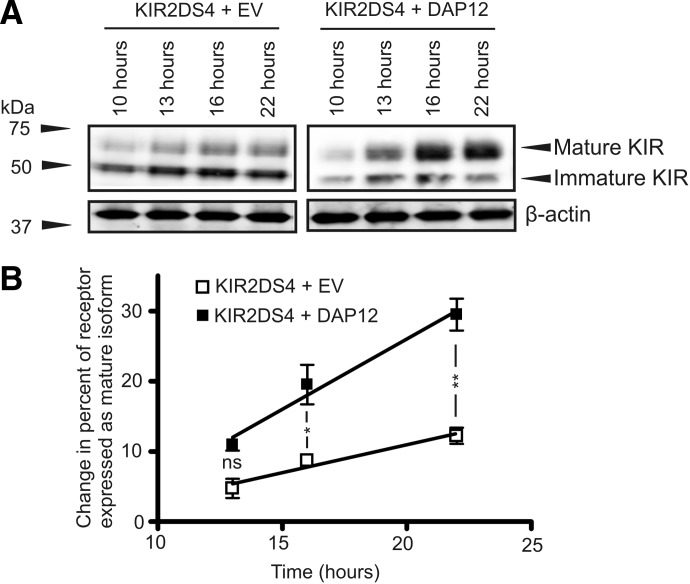
To further evaluate the potential impact of DAP12 on the post-translation processing of KIR2DS molecules, expression of two different isoforms of KIR2DS was analyzed over a time-course in the presence or absence of exogenous DAP12. We have previously described expression of two different isoforms of KIR2DS molecules in our transfected model based on molecular weight and susceptibility to Endo H [22]. The larger isoform represents the mature isoform of the protein that has undergone extended glycosylation in the Golgi, preventing digestion of this isoform by Endo H. This mature isoform is also the only form that is expressed at the cell surface, as described previously. The lower molecular weight isoform is an immature isoform that is susceptible to Endo H treatment, suggesting that this isoform has not been processed by the Golgi apparatus and is likely sequestered within the ER. NKL cells were cotransfected with KIR2DS4 and empty vector or DAP12, harvested, and lysed at multiple time-points post-transfection. Western blot analysis of these lysates show that when KIR2DS4 was expressed in the absence of DAP12, the level of immature isoform of the receptor is clearly the predominant isoform of the protein expressed and that the level of expression of this isoform appears to increase slightly over the time-course (Fig. 5A). A minimal level of increase was also observed for the mature isoform of KIR2DS4 in the absence of DAP12. These results are dramatically different from the pattern of KIR2DS4 isoform expression in the presence of exogenous DAP12. When KIR2DS4 was expressed with DAP12, the level of mature isoform increased constantly over the time-course, with an inverse decrease in expression of the immature isoform. The results for changes observed in mature isoform expression over the time-course were quantified from these Western blots (Fig. 5B). The quantification shows that the percent of the receptor expressed as the mature isoform, when cotransfected with empty vector, is only increased slightly over the time-course and achieves an increase of 10% at 22 h post-transfection compared with the level expressed at 10 h post-transfection. In comparison, a 10% change in percent of the isoform expressed as mature was achieved at the 13-h time-point when KIR2DS4 was expressed with exogenous DAP12. The change in the percentage of receptor expressed as the mature isoform continued to increase dramatically over the time-course in the presence of exogenous DAP12, ultimately ending with a 30% change in percent of the receptor expressed as mature at 22 h post-transfection compared with the 10-h time-point. The data show that not only does DAP12 increase the percentage of receptor expressed as the mature form but also shows that the rate at which the mature isoform is expressed is significantly greater compared with when the receptor is expressed without exogenous DAP12. These results suggest that DAP12 aids in efficiently trafficking KIR2DS4 from the ER, through the Golgi, and ultimately to the cell surface.
Figure 5. DAP12 enhances the process of post-translational modifications of KIR2DS4.
Expression of immature and mature isoforms of KIR2DS4 was analyzed post-transfection in the presence or absence of exogenous DAP12. (A) Representative Western blot analysis of KIR2DS4 isoform expression from whole lysates of tranfected NKL cells at the indicated time-points post-transfection in the presence or absence of exogenous DAP12 using a V5-specific antibody. β-Actin was used as a loading control. (B) Quantification of Western blots showing the change in percentage of receptor expressed as the mature isoform evaluated at each time-point compared with the values obtained 10 h post-transfection. The densitometry and quantification were performed on Western blot results from four independent experiments. The results were statistically analyzed by a two-way repeated-measures ANOVA followed by Bonferroni post-tests (*P<0.05; **P<0.01).
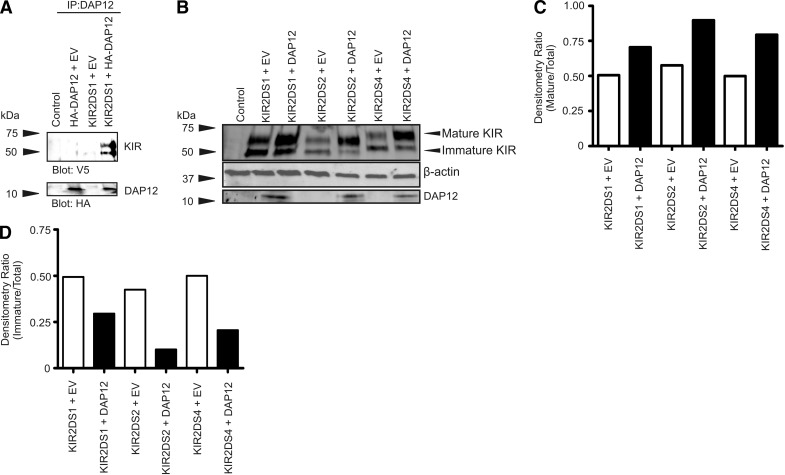
As the data support the role of DAP12 in trafficking, we hypothesized that the interaction between KIR2DS and DAP12 occurred early in the post-translational development of KIR, resulting in enhanced efficiency in maturation of the KIR2DS molecules. The association of DAP12 with the different isoforms of KIR2DS described above in transfected NKL cells was examined by Western blot after immunoprecipitation with a DAP12-specific antibody. Following the immunoprecipitation, two isoforms of KIR2DS1 were detected by Western blot (Fig. 6A). Coimmunoprecipitation of the Endo H-sensitive, immature isoform of KIR2DS1 suggests that the interaction between KIR2DS and DAP12 occurs very early in the maturation process, likely within the ER.
Figure 6. DAP12 interacts with KIR2DS molecules early in the maturation process.
(A) Western blot of immune complexes obtained following immunoprecipitation of lysates from NKL cells cotransfected with constructs encoding KIR2DS1 and HA-DAP12 with a DAP12-specific antibody. The membrane was probed using antibodies specific for the V5 (KIR) or HA (DAP12) epitope. (B) Western blot of NKL lysates using a V5-specific antibody following expression of KIR2DS molecules, with or without exogenous DAP12. The membrane was also analyzed for DAP12 expression. β-Actin served as the internal loading control. (C) Densitometry ratios calculated from the Western blot in B showing a ratio of mature KIR expression (upper part of top band in B) to total KIR expression. (D) Densitometry ratios calculated from the Western blot in B showing a ratio of immature KIR expression (lower part of top band in B) to total KIR expression. The Western blot and subsequent densitometry data represent the results of three independent experiments.
To further understand the impact of the early interaction of DAP12 on KIR2DS maturation, the relative quantities of immature and mature isoforms of each KIR2DS molecule in the absence and presence of exogenous DAP12 were determined. Single KIR2DS-encoding constructs were cotransfected into NKL cells with the DAP12 vector or the empty vector. Whole-cell lysates from these transfectants were examined by Western blot along with follow-up densitometry analysis to determine changes in expression of the different isoforms of the receptors (Fig. 6B–D). In the absence of exogenous DAP12, each receptor was present at approximately equal ratios of mature to immature isoforms of the protein (Fig. 6C and D). The presence of the mature isoform of all of the KIR2DS molecules analyzed was enhanced in the presence of exogenous DAP12 (Fig. 6C), coinciding with a decrease in the amount of the immature isoform of the receptors (Fig. 6D). These data support that the interaction of KIR2DS molecules with DAP12 in the ER aids in efficiently shuttling the receptors through the maturation process and ultimately to the cell surface.
DAP12 stabilizes KIR2DS molecules at the cell surface
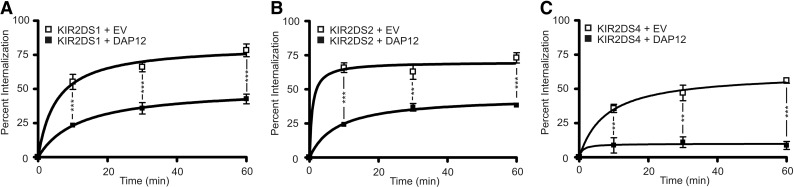
Thus far, the data have illustrated an important role for DAP12 in trafficking KIR2DS molecules. To determine if DAP12 impacts the stability of KIR2DS at the cell surface, the relative amount of internalized KIR2DS molecules was analyzed in the absence or presence of exogenous DAP12, as described in Materials and Methods. Whereas internalization of each receptor was observed independent of exogenous DAP12, a significantly higher proportion of receptors was internalized in the absence of exogenous DAP12 (Fig. 7A–C). For all of the receptors, the internalization occurred very rapidly, with the majority of receptors internalized within the first 10 min of antibody incubation. For KIR2DS1 and KIR2DS2, DAP12 approximately doubled the stability of receptors, whereas close to a fivefold increase in stability was observed for KIR2DS4. The data suggest that DAP12 may impact KIR2DS surface expression and function by stabilizing the receptors at the cell surface.
Figure 7. DAP12 stabilizes KIR2DS molecules at the cell surface.
NKL cells were cotransfected with specified KIR2DS constructs and empty vector (□) or DAP12 (■). Following incubation at 37°C with a PE-conjugated antibody specific for each receptor for 0, 10, 20, or 60 min, cells were removed and placed at 4°C. At each time-point, the remaining external antibody was stripped away with an acidic reagent, and the internalized receptor was analyzed by flow cytometry. The percentage of internalized receptors was calculated for NKL cells transfected with KIR2DS1 (A), KIR2DS2 (B), or KIR2DS4 (C), as described in Materials and Methods. Each experiment was performed with three independent biological samples resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. Statistical analysis was performed using a two-way repeated-measures ANOVA, followed by Bonferroni post-tests (**P<0.01; ***P<0.001).
Internalized KIR2DS molecules traffick to lysosomes independent of exogenous DAP12
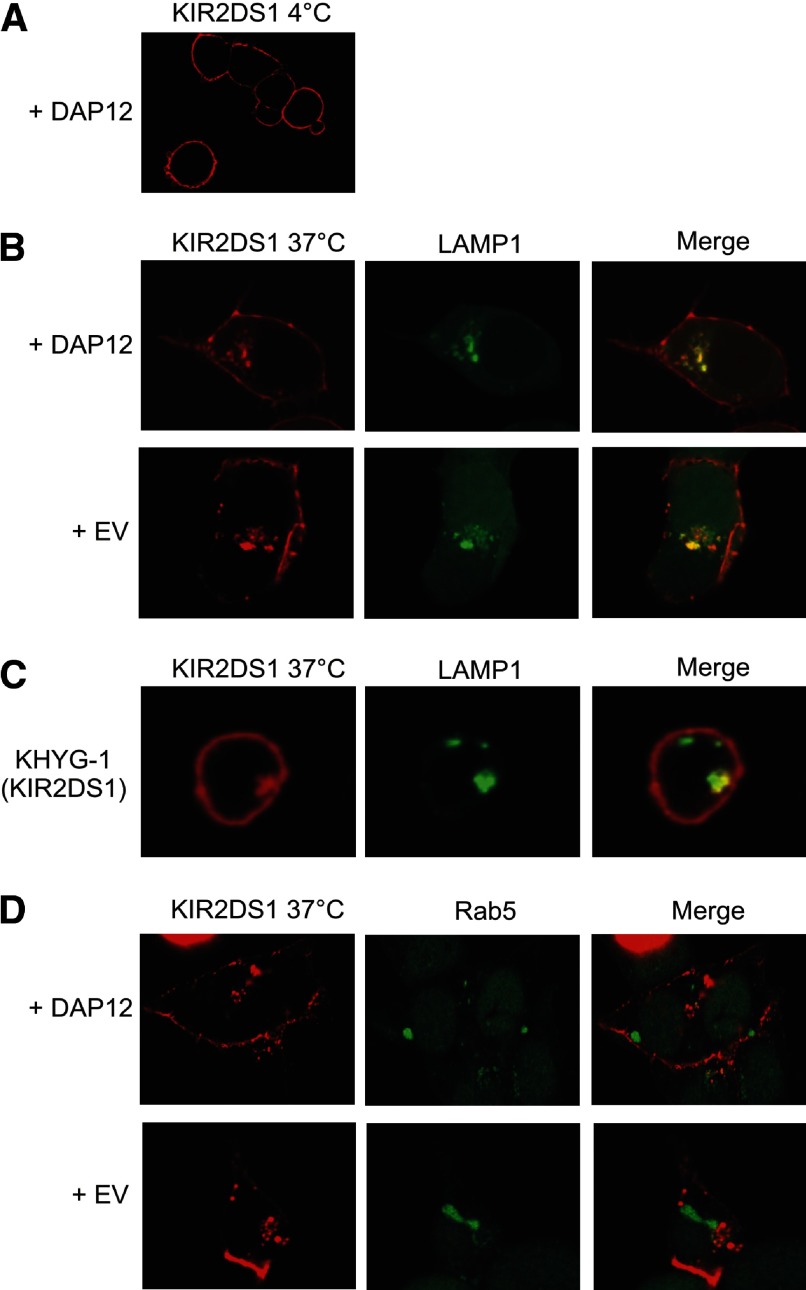
The rate of immune receptor internalization, as well as the ultimate localization of the receptor after internalization, can modulate immune receptor signaling and function. For instance, KIR2DL4 is internalized to and signals from early endosomes [25, 26]. Based on these findings, we sought to determine if KIR2DS molecules followed a similar pattern of internalization as KIR2DL4 and to determine if DAP12 impacted recruitment to specific internal compartments. To determine the location of KIR2DS after internalization, HEK293T cells were transfected with KIR2DS1 and DAP12 or empty vector, along with LAMP1-GFP- or Rab5-GFP-encoding vectors. The transfectants were incubated at 4°C or 37°C with a conjugated, KIR2DS- specific antibody for 2 h, followed by analysis via confocal microscopy. The 2-h incubation is the same time period used for analysis of KIR2DL4 internalization and function [25]. The transfectants probed with the antibody at 4°C exhibit clear membrane staining of KIR2DS1 (Fig. 8A). The images observed following incubation with the antibody at 37°C revealed the internalized KIR2DS1 localized to LAMP1-associated lysosomal compartments, independent of DAP12 expression (Fig. 8B). Recruitment of KIR2DS1 to LAMP1 compartments was also observed in the NK cell line, KHYG-1, following stable transduction of KIR2DS1 (Fig. 8C). KIR2DS1 molecules were found to localize to LAMP1 compartments, independent of antibody incubation, as cells that were fixed, permeabilized, and probed for KIR2DS1 showed colocalization of the receptor with LAMP1 as well (data not shown). In the presence or absence of exogenous DAP12, recruitment to early endosomes, marked by Rab5, was not observed for KIR2DS1 (Fig. 8D). Whereas the data support a role for DAP12 in anterograde transport of KIR2DS molecules to the cell surface, the confocal data suggest no impact of DAP12 on retrograde transport from the cell surface.
Figure 8. Confocal microscopy shows that internalized KIR2DS1 localizes to LAMP1-associated lysosomal compartments.
(A) KIR2DS1 was transfected with DAP12 into HEK293T cells and probed with a KIR2DS1-specific antibody at 4°C. (B) HEK293T cells were transfected with KIR2DS1 and empty vector or DAP12, along with a vector encoding LAMP1-GFP. The cells were probed with an APC-conjugated KIR2DS1 antibody for 2 h at 37°C, resulting in internalization of the receptor. The merged images to the right show the overlay of the KIR2DS1 and LAMP1 images. (C) KHYG-1 cells stably expressing KIR2DS1 were probed with a KIR2DS1-specific antibody at 37°C. The cells were then fixed, permeabilized, and stained using a LAMP1-specific antibody. (D) HEK293T cells were transfected with a vector encoding Rab5-GFP, along with KIR2DS1 with empty vector or DAP12. The merged images in the rightmost column display the overlay of the KIR2DS1 image with the LAMP1 or RAB5 images obtained. The image acquired after 4°C incubation was taken with a magnification of 100×, whereas the remaining 37°C images were obtained with a 200× magnification. The images represent results observed in 10 fields in each of three independent experiments.
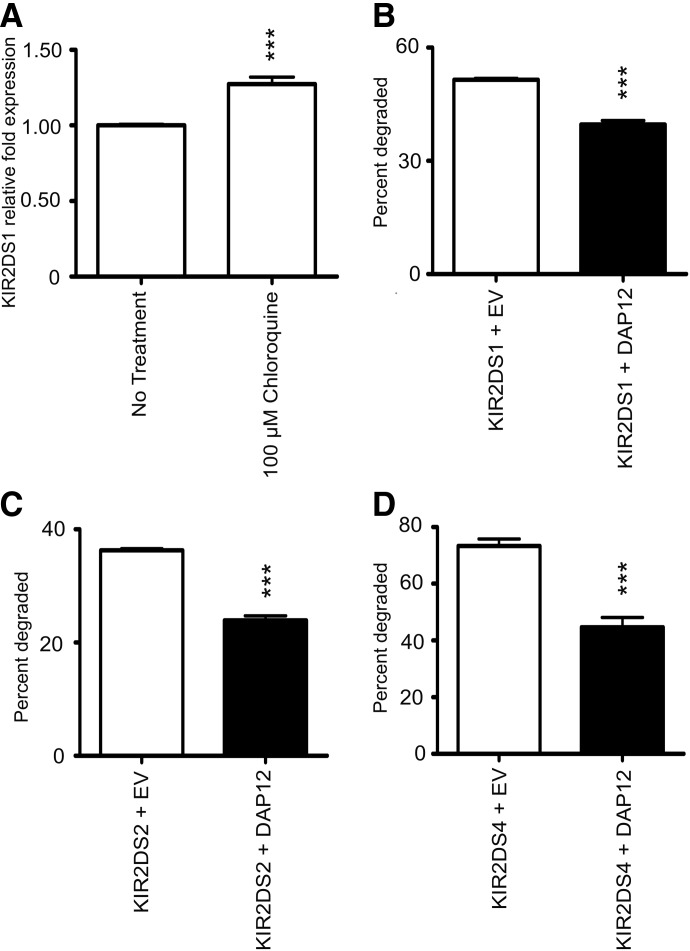
To determine if KIR2DS1 was degraded by the lysosomes, total expression levels of KIR2DS1 in our stable KHYG-1 cells were analyzed. The cells were left untreated or treated with choloroquine to prevent lysosomal degradation. Following a 2-h incubation with chloroquine, a 30% increase in total KIR2DS1 expression was observed, suggesting that upon internalization of KIR2DS1, the receptor localizes to the lysosomes for degradation (Fig. 9A). These data support KIR2DS degradation by the lysosomes but do not exclude the possibility of proteasomal degradation of these receptors. Based on our data showing that DAP12 stabilizes KIR2DS molecules at the cell surface, we hypothesized that DAP12 would impact the rate of degradation of the KIR2DS molecules. Degradation of KIR2DS molecules was analyzed following treatment with CHX, beginning 10 h post-transfection of NKL cells in the presence or absence of exogenous DAP12. Following the CHX treatment for 6 h, a significantly higher percentage of all KIR2DS molecules analyzed was degraded in the absence of exogenous DAP12 (Fig. 9B–D). These degradation experiments support the evidence from the internalization and confocal analyses, suggesting that whereas DAP12 does not affect the location after internalization, the adapter molecule does retard the rate at which the receptors are trafficked to lysosomes and subsequently slows the degradation rate of the receptors.
Figure 9. DAP12 retards lysosomal degradation of KIR2DS molecules.
(A) Total expression of KIR2DS1 in KHYG-1 cells stably expressing KIR2DS1 was recorded by flow cytometry using a FITC-conjugated, V5-specific antibody following treatment with chloroquine for 2 h. The results were normalized to expression levels of KIR2DS1 in untreated cells. (B–D) The percentage of KIR2DS1 (B), KIR2DS2 (C), and KIR2DS4 (D) degraded in NKL cells in the presence of absence of exogenous DAP12 was calculated as described in Materials and Methods. Each experiment was performed with three independent biological samples resulting from three independent transfections/condition. Each experiment was repeated and analyzed in two independent experiments. The data were statistically analyzed using unpaired Student's t-test (***P<0.001).
DISCUSSION
The KIR2DS are known to modify NK cell activity. These receptors have a role in NK cell education, activation against leukemic blasts, and melanoma and are genetically associated with the outcome of pregnancy, as well as relapse-free survival following hematopoietic stem cell transplant [12, 27–30]. DAP12 is the key adapter molecule that interacts with KIR2DS molecules, and upon ligand interaction with the receptor, DAP12 is responsible for transmitting signals through ITAMs located in its cytoplasmic domain [13]. Although KIR2DS molecules function through interactions at the cell surface, little is known regarding the biology of their maturation and transport to the surface. In this study, we hypothesized that DAP12 would have an impact on KIR2DS function beyond signaling. The data reveal novel impacts of DAP12 on efficiently trafficking KIR2DS molecules through post-translational modifications in the Golgi and out to the cell membrane. Our results also show a significant impact of DAP12 on stabilizing these receptors at the cell membrane. These mechanisms explain how DAP12 modulated KIR2DS surface expression in an overexpressed transfection model, as well as in knockdown experiments using primary PBMCs. These mechanisms demonstrate how changing levels of DAP12 expression can regulate KIR2DS surface expression and functional characteristics.
The observed impact that DAP12 has on KIR2DS surface expression is in agreement with the observed decrease in surface expression of the mouse-activating receptor LY49H in DAP12 genetically null mice [31]. Other adapter molecules in NK cells, such as DAP10, can also regulate surface expression of their cognate receptors [16]. The observed changes in surface expression of KIR2DS may impact NK cell responses, as increased surface expression levels of NK cell-activating receptors have been correlated with greater activation responses by NK cells [32]. Surface density may prove to be even more important for receptors such as KIR2DS, which bind their ligand with a relatively low affinity. Multimerization of immune receptors is a mechanism that enhances sensitivity of receptors for their ligands [33–35]. It is possible that increased surface expression in the presence of DAP12 may allow for more efficient dimerization of the KIR2DS molecules at the cell surface, increasing the affinities of these receptors for their respective ligands. In an in vitro setting, KIR2DL1 forms homodimers with greater affinity for its ligand through interactions with an N-terminal histidine [36, 37]. The KIR2DS molecules, with the exception of KIR2DS4, share the same N-terminal histidine and therefore, may also have the capacity to dimerize. Although we observed KIR2DS surface expression in the absence of DAP12, it is likely that the increased level of expression in the presence of the adapter molecule would provide greater probability of interactions occurring between receptor and ligand with potentially stronger affinity.
The data presented in this manuscript illustrate that the increased surface expression of KIR2DS molecules in the presence of DAP12 is a result of the adapter's impact on trafficking the receptors to the cell surface, as well as stabilizing the receptors at the surface. The increased efficiency of KIR2DS trafficking to the cell surface appears to begin within the ER, as immunoprecipitation of DAP12 revealed an interaction with an immature, not fully glycosylated, isoform of KIR2DS1. This result supports evidence from in vitro ER microsomes, suggesting that the interaction between DAP12 and KIR2DS molecules occurs very early after translation [24]. This early interaction impacts the efficiency by which newly synthesized receptors are trafficked out to the cell surface. The TCR/CD3 complex follows a similar pattern of assembly in the ER, followed by transport to the Golgi for complete processing of N-linked oligosaccharides [38]. Whereas the interaction of KIR2DS molecules with DAP12 aids in this process, it is likely multiple proteins interact with KIR2DS during the post-translation modification process, as is exemplified by maturation and trafficking of GPCRs (G protein-coupled receptors) [39]. Glycosylation of the stimulatory KIR appears to be an important process in their maturation, as these proteins have an expected mass of 31 kDa but following complete glycosylation in the Golgi, have a molecular weight between 55 and 60 kDa. The interaction between DAP12 and KIR2DS may impact the conformation of the receptor, making key residues available for interactions with other chaperone proteins and glycosyltransferases, ultimately leading to enhanced efficiency in the maturation process of these receptors.
Upon reaching the cell surface, the receptors are stabilized significantly when interacting with DAP12. The poor stability of KIR2DS molecules at the cell surface in the absence of DAP12 may be a result of the unfavorable presence of the positively charged lysine within the transmembrane region of the receptor. The interaction with DAP12 may increase stability by masking the positively charged lysine through a noncovalent bond with a negatively charged aspartic residue in the transmembrane region of DAP12. Another mechanism might be that DAP12 may cover motifs on the KIR2DS molecules that are otherwise targets for other proteins that can affect KIR internalization. Surface expression and recycling of receptors can be impacted by phosphorylation of serine/threoinine residues in the cytoplasmic domains of the receptors [40, 41]. CSKI and -II phosphorylate KIR3DL1 on serine residues 364 and 367, respectively [42]. Mutations of these residues to alanine that is not phosphorylated lead to an increase in surface expression of KIR3DL1. The KIR2DS molecules have the same CSKI and -II phosphorylation motifs, as well as multiple PKC phosphorylation sites within their short cytoplasmic domains. It is possible that DAP12 may prevent such kinases from recognizing and phosphorylating these residues, therefore preventing the negative impact that the phosphorylated residues have on surface expression observed for KIR3DL1.
Although the data illustrate a significant impact of DAP12 on anterograde trafficking of KIR2DS to the cell surface, DAP12 appears to have no impact on retrograde trafficking of KIR2DS molecules to internal compartments. The data show that KIR2DS molecules are trafficked to lysosomal compartments and targeted for degradation, independent of an interaction with DAP12. Following internalization from the cell surface, TCRs endure a similar fate as KIR2DS molecules and are degraded by the lysosomes [43]. The trafficking of KIR2DS molecules to lysosomes differs from the internal recruitment of KIR2DL4 to early endosomes. However, our results are consistent with those for KIR2DL4, as the recruitment of KIR2DL4 to endosomes is coordinated by its extracellular domains, suggesting the adapter molecule for KIR2DL4, FcεRIγ, does not control internal localization [26]. The difference in localization may be explained by the presence of different extracellular domains expressed by KIR2DS molecules and KIR2DL4. All KIR2DS molecules are categorized as type I KIR2D, expressing the D1 and D2 extracellular domains, whereas KIR2DL4 is a type II KIR2D, expressing the D0 and D2 domains. The distinct internal localization may be attributed to the presence of the D1 versus D0 domains or a combination effect of the presence of D1/D2 in KIR2DS molecules versus D0/D2 in KIR2DL4.
The results obtained in this study may also extend beyond the biology of KIR2DS maturation and surface expression. All of the elements analyzed within this study—surface expression, surface stability, and lozalization to specific internal compartments—are known to impact immune receptor signaling. KIR2DS2 signaling has been described in T cells in the presence and absence of DAP12 [14, 15]. With DAP12, upon stimulation of the receptor, the cell is capable of eliciting cytotoxic and proinflammatory responses. However, in DAP12-negative T cells, stimulation of KIR2DS2 results in incomplete signaling, culminating in only enhanced, proinflammatory responses under costimulation of the TCR. The results from this study reveal that KIR2DS molecules trafficked to lysosomes, independent of DAP12, and do not appear to reside within early endosomes as is the case for KIR2DL4, suggesting that the signal transduction through these receptors is performed at the cell surface. As described previously the enhanced surface expression of KIR2DS in the presence of DAP12 may increase efficiency of dimer formation and therefore, increase affinity of the receptors for ligand. Multimerization of immune receptors at the cell surface also impacts the strength of signal transduction after ligand binding [34, 35]. While at the surface, DAP12 stabilizes the receptor, possibly allowing for recruitment of proper signaling scaffolds to assemble upon ligand recognition, resulting in a complete and robust activation signal. The enhanced stability of the receptor may impact the directed release of cytolytic granules toward the target cell. The low surface expression and rapid internalization could be the underlying mechanisms of why KIR2DS2 exhibited minimal but incomplete signaling in the absence of DAP12.
The data presented within this study provide detail to the biological impact of the interaction between KIR2DS and DAP12 and provide potential mechanisms whereby KIR2DS function could be compromised when DAP12 expression is decreased in vivo. The mechanisms described here may be evident in Nasu-Hakola patients. These patients carry loss-of-function mutations or deletions of DAP12 [44]. Our results infer the expression levels of KIR2DS on the cell surfaces of these patients may be impacted significantly by the loss of DAP12 expression. Different cytokine environments used to culture NK cells ex vivo have also been shown to diminish expression levels of DAP12 [18]. Tumor microenvironments where cytokine concentrations are enhanced to aid in tumor growth could impact DAP12 expression in vivo. As surface expression levels, receptor surface stability, and receptor localization all impact immune receptor function, the mechanisms revealed in this study may shed light on the impact of KIR2DS function and signaling capabilities in environments where DAP12 expression is compromised.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by funding from the U.S. Office of Naval Research N000014-11-1-0590 to the C. W. Bill Young Marrow Donor Recruitment and Research Program. These experiments were performed with the aid of the tissue culture, flow cytometry and cell sorting, biostatistics and bioinformatics, and microscopy and imaging shared resources of the Lombardi Comprehensive Cancer Center, supported by the National Cancer Institute, Cancer Center Support Grant.
We thank William Frazier, Karen Creswell, Kepher Makambi, and Noriko Steiner for helpful discussions and technical assistance.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- BFA
- brefeldin A
- CHX
- cycloheximide
- CSKI/II
- casein kinase I/II
- DAP12
- DNAX activation protein 12
- Endo H
- endoglycosidase H
- HEK293
- human embryo kidney 293
- KIR
- killer Ig-like receptor
- KIR2DS
- two-domain-stimulatory killer Ig-like receptor
- LAMP1
- lysosome-associated membrane protein 1
- MFI
- mean fluorescence intensity
- rqRT-PCR
- relative quantity RT-PCR
- SHP-1/2
- Src homology 2-containing tyrosine phosphatase 1/2
- siRNA
- small interfering RNA
AUTHORSHIP
T.J.M. performed all experiments and data analysis and wrote the manuscript. P.E.P. contributed critical analysis of data, ideas for experiments, and to the writing of the manuscript. C.K.H. was the senior investigator of the project and contributed mentorship, critical analysis for data, ideas for experiments, as well as to the writing of the manuscript.
DISCLOSURES
The authors declare no conflict of interest. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Navy, the Department of Defense, or the United States government.
REFERENCES
- 1. Winter C. C., Gumperz J. E., Parham P., Long E. O., Wagtmann N. (1998) Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 161, 571–577 [PubMed] [Google Scholar]
- 2. Litwin V., Gumperz J., Parham P., Phillips J. H., Lanier L. L. (1994) NKB1: a natural killer cell receptor involved in the recognition of polymorphic HLA-B molecules. J. Exp. Med. 180, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burshtyn D. N., Scharenberg A. M., Wagtmann N., Rajagopalan S., Berrada K., Yi T., Kinet J. P., Long E. O. (1996) Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 4, 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olcese L., Lang P., Vély F., Cambiaggi A., Marguet D., Blery M., Hippen K. L., Biassoni R., Moretta A., Moretta L., Cambier J. C., Vivier E. (1996) Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J. Immunol. 156, 4531–4534 [PubMed] [Google Scholar]
- 5. Anfossi N., Guia S., Falk C. S., Roetnyck S., Stewart C. A., Breso V., Frassati C., Reviron D., Middleton D., Romagne F., Ugolini S., Vivier E. (2006) Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 [DOI] [PubMed] [Google Scholar]
- 6. Ljunggren H. G., Kärre K. (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 [DOI] [PubMed] [Google Scholar]
- 7. Biassoni R., Pessino A., Malaspina A., Cantoni C., Bottino C., Sivori S., Moretta L., Moretta A. (1997) Role of amino acid position 70 in the binding affinity of p50.1 and p58.1 receptors for HLA-Cw4 molecules. Eur. J. Immunol. 27, 3095–3099 [DOI] [PubMed] [Google Scholar]
- 8. Chewning J. H., Gudme C. N., Hsu K. C., Selvakumar A., Dupont B. (2007) KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J. Immunol. 179, 854–868 [DOI] [PubMed] [Google Scholar]
- 9. Stewart C. A., Laugier-Anfossi F., Vély F., Saulquin X., Riedmuller J., Tisserant A., Gauthier L., Romagne F., Ferraci G., Arosa F. A., Moretta A., Sun P. D., Ugolini S., Vivier E. (2005) Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc. Natl. Acad. Sci. USA 102, 13224–13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graef T., Moesta A. K., Norman P. J., Abi-Rached L., Vago L., Older Aguilar A. M., Gleimer M., Hammond J. A., Guethlein L. A., Bushnell D. A., Robinson P. J., Parham P. (2009) KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J. Exp. Med. 206, 2557–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz G., Markel G., Mizrahi S., Arnon T. I., Mandelboim O. (2001) Recognition of HLA-Cw4 but not HLA-Cw6 by the NK cell receptor killer cell Ig-like receptor two-domain short tail number 4. J. Immunol. 166, 7260–7267 [DOI] [PubMed] [Google Scholar]
- 12. Katz G., Gazit R., Arnon T. I., Gonen-Gross T., Tarcic G., Markel G., Gruda R., Achdout H., Drize O., Merim S., Mandelboim O. (2004) MHC class I-independent recognition of NK-activating receptor KIR2DS4. J. Immunol. 173, 1819–1825 [DOI] [PubMed] [Google Scholar]
- 13. Lanier L. L., Corliss B. C., Wu J., Leong C., Phillips J. H. (1998) Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 [DOI] [PubMed] [Google Scholar]
- 14. Snyder M. R., Lucas M., Vivier E., Weyand C. M., Goronzy J. J. (2003) Selective activation of the c-Jun NH2-terminal protein kinase signaling pathway by stimulatory KIR in the absence of KARAP/DAP12 in CD4+ T cells. J. Exp. Med. 197, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snyder M. R., Nakajima T., Leibson P. J., Weyand C. M., Goronzy J. J. (2004) Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J. Immunol. 173, 3725–3731 [DOI] [PubMed] [Google Scholar]
- 16. Burgess S. J., Marusina A. I., Pathmanathan I., Borrego F., Coligan J. E. (2006) IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J. Immunol. 176, 1490–1497 [DOI] [PubMed] [Google Scholar]
- 17. Park Y. P., Choi S. C., Kiesler P., Gil-Krzewska A., Borrego F., Weck J., Krzeski K., Coligan J. E. (2011) Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the γc cytokines and TGF-β1. Blood 118, 3019–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Rham C., Ferrari-Lacraz S., Jendly S., Schneiter G., Dayer J. M., Villard J. (2007) The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res. Ther. 9, R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carr W. H., Rosen D. B., Arase H., Nixon D. F., Michaelsson J., Lanier L. L. (2007) Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J. Immunol. 178, 647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou L., Chen M., Jiang B., Kariyawasam K., Ng J., Hurley C. K. (2009) In contrast to other stimulatory natural killer cell immunoglobulin-like receptor loci, several KIR2DS5 alleles predominate in African Americans. Hum. Immunol. 70, 733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steiner N. K., Dakshanamurthy S., VandenBussche C. J., Hurley C. K. (2008) Extracellular domain alterations impact surface expression of stimulatory natural killer cell receptor KIR2DS5. Immunogenetics 60, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VandenBussche C. J., Mulrooney T. J., Frazier W. R., Dakshanamurthy S., Hurley C. K. (2009) Dramatically reduced surface expression of NK cell receptor KIR2DS3 is attributed to multiple residues throughout the molecule. Genes Immun. 10, 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hou P., Araujo E., Zhao T., Zhang M., Massenburg D., Veselits M., Doyle C., Dinner A. R., Clark M. R. (2006) B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 4, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng J., Call M. E., Wucherpfenning K. W. (2006) The assembly of diverse immune receptors is focused on a polar membrane-embedded interaction site. PLoS Biol. 4, e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajagopalan S., Bryceson Y. T., Kuppusamy S. P., Geraghty D. E., van der Meer A., Joosten I., Long E. O. (2006) Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 4, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajagopalan S., Moyle M. W., Joosten I., Long E. O. (2010) DNA-PKcs controls an endosomal signaling pathway for a proinflammoatory response by natural killer cells. Sci. Signal. 3, ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fauriat C., Ivarsson M. A., Ljunggren H. G., Malmberg K. J., Michaëlsson J. (2010) Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood 115, 1166–1174 [DOI] [PubMed] [Google Scholar]
- 28. Hiby S. E., Apps R., Sharkey A., Farrel L., Gardner L., Mulder A., Claas F. H., Walker J., Redman C., Morgan L., Tower C., Regan L., Moore G., Carrington M., Moffet A. (2010) Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sivori S., Carlomagno S., Falco M., Romeo E., Moretta L., Moretta A. (2011) Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood 117, 4284–4292 [DOI] [PubMed] [Google Scholar]
- 30. Venstrom J. M., Pittari G., Gooley T. A., Chewning J. H., Spellman S., Haagenson M., Gallagher M. M., Malkki M., Petersdorf E., Dupont B., Hsu K. C. (2012) HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 367, 805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orr M. T., Sun J. C., Hesslein D. G., Arase H., Phillips J. H., Takari T., Lanier L. L. (2009) Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J. Exp. Med. 206, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sivori S., Parolini S., Falco M., Biassoni R., Bottino C., Morreta L., Morreta A. (2000) 2B4 functions as a co-receptor in human NK cell activation. Eur. J. Immunol. 30, 787–793 [DOI] [PubMed] [Google Scholar]
- 33. Garrity D., Call M. E., Feng J., Wucherpfenning K. W. (2005) The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc. Natl. Acad. Sci. USA 102, 7641–7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irvine D. J., Purboo M. A., Krogsgaard M., Davis M. M. (2002) Direct observation of ligand recognition by T cells. Nature 419, 845–849 [DOI] [PubMed] [Google Scholar]
- 35. Sykulev Y., Joo M., Vturina I., Tsomides T. J., Eisen H. N. (1996) Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 [DOI] [PubMed] [Google Scholar]
- 36. Fan Q. R., Long E. O., Wiley D. C. (2000) A disulfide-linked natural killer cell receptor dimer has higher affinity for HLA-C than wild-type monomer. Eur. J. Immunol. 30, 2692–2697 [DOI] [PubMed] [Google Scholar]
- 37. Fan Q. R., Long E. O., Wiley D. C. (2000) Cobalt-mediated dimerization of the human natural killer cell inhibitory receptor. J. Biol. Chem. 275, 23700–23706 [DOI] [PubMed] [Google Scholar]
- 38. Alarcon B., Berkhout B., Breitmeyer J., Terhorst C. (1988) Assembly of the human T cell receptor-CD3 complex takes place in the endoplasmic reticulum and involves intermediary complexes between the CD3-γ.δ.ε core and single T cell receptor α or β chains. J. Biol. Chem. 263, 2953–2961 [PubMed] [Google Scholar]
- 39. Dong C., Filipeanu C. M., Duvernay M. T., Wu G. (2007) Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 1768, 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dietrich J., Bäckström T., Lauritsen J. P., Kastrup J., Christensen M. D., von Bulow F., Palmer E., Geisler C. (1998) The phosphorylation state of CD3γ influences T cell responsiveness and controls T cell receptor cycling. J. Biol. Chem. 273, 24232–24238 [DOI] [PubMed] [Google Scholar]
- 41. Von Essen M., Nielsen M. W., Bonefeld C. M., Boding L., Larsen J. M., Leitges M., Baier G., Odum N., Geisler C. (2006) Protein kinase C (PKC) α and PKC θ are the major PKC isotypes involved in TCR down-regulation. J. Immunol. 176, 7502–7510 [DOI] [PubMed] [Google Scholar]
- 42. Alvarez-Arias D. A., Campbell K. S. (2007) Protein kinase C regulates expression and function of inhibitory killer cell Ig-like receptors in NK cells. J. Immunol. 179, 5281–5290 [DOI] [PubMed] [Google Scholar]
- 43. Valitutti S., Muller S., Salio M., Lanzavecchia A. (1997) Degradation of T cell receptor (TCR)-CD3-ζ complexes after antigenic stimulation. J. Exp. Med. 185, 1859–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paloneva J., Kestilä M., Wu J., Salminen A., Böhling T., Ruotsalainen V., Hakola P., Bakker A. B., Phillips J. H., Pekkarinen P., Lanier L. L., Timonen T., Peltonen L. (2000) Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 25, 357–361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.