Abstract
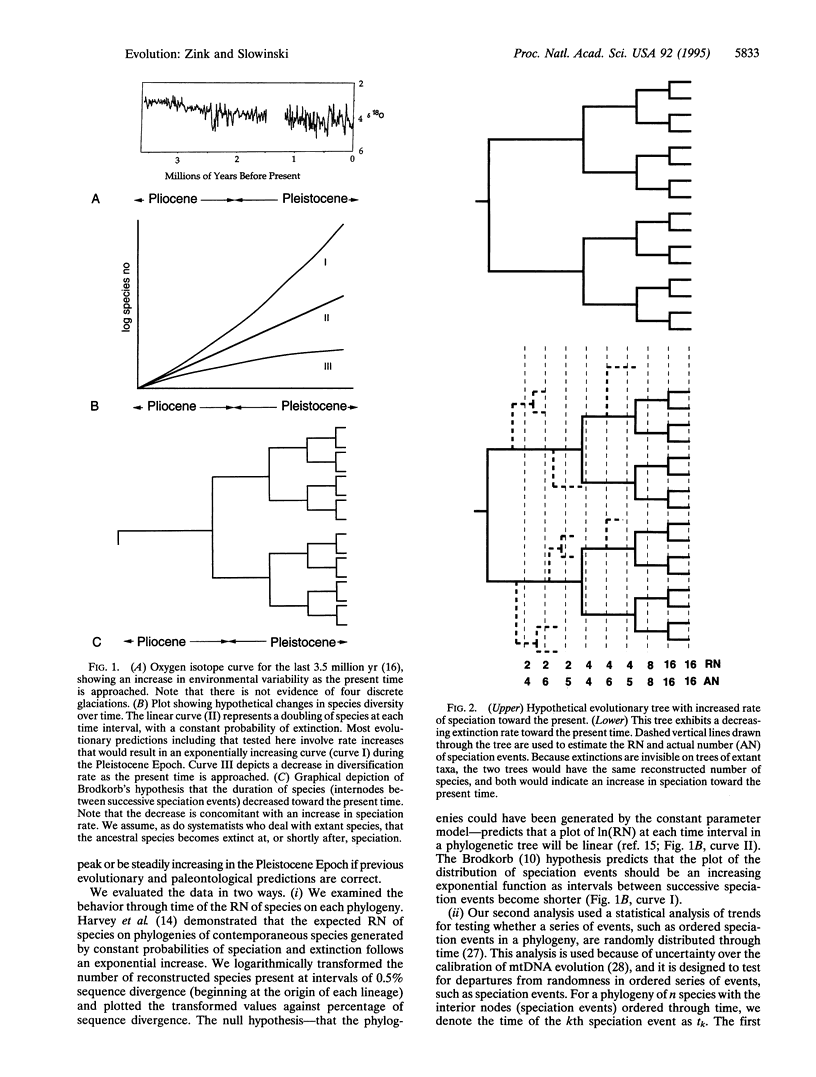
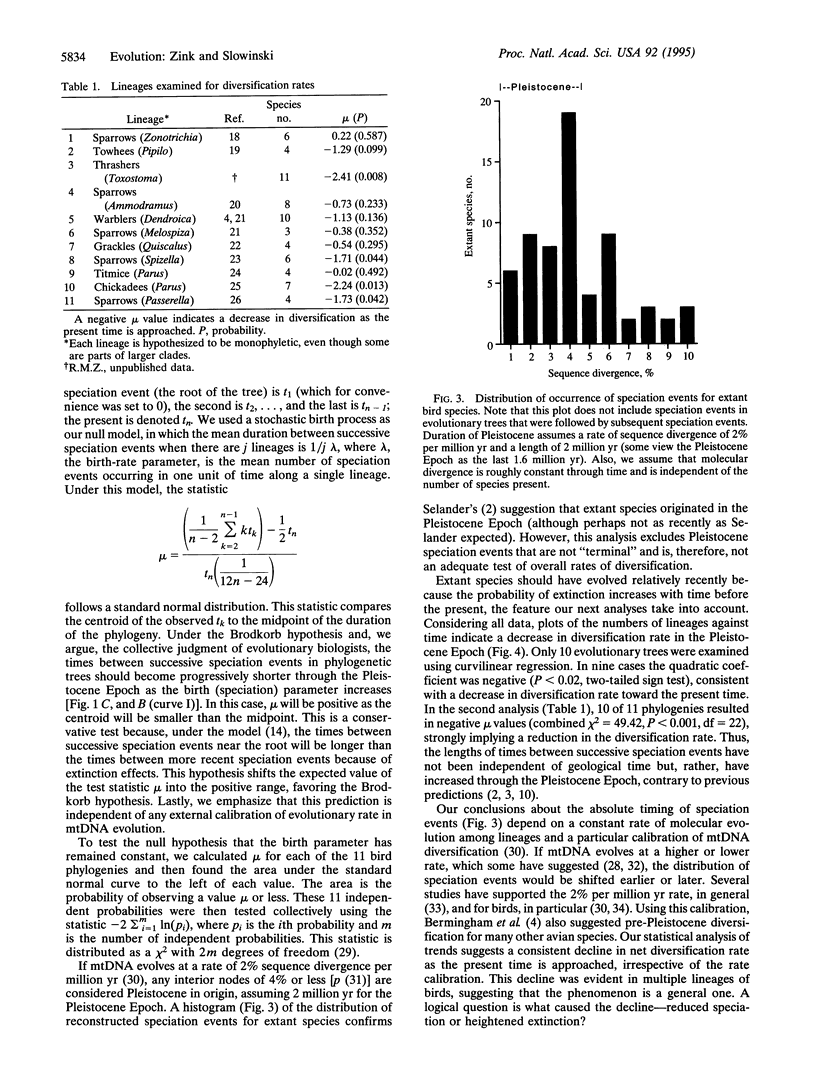
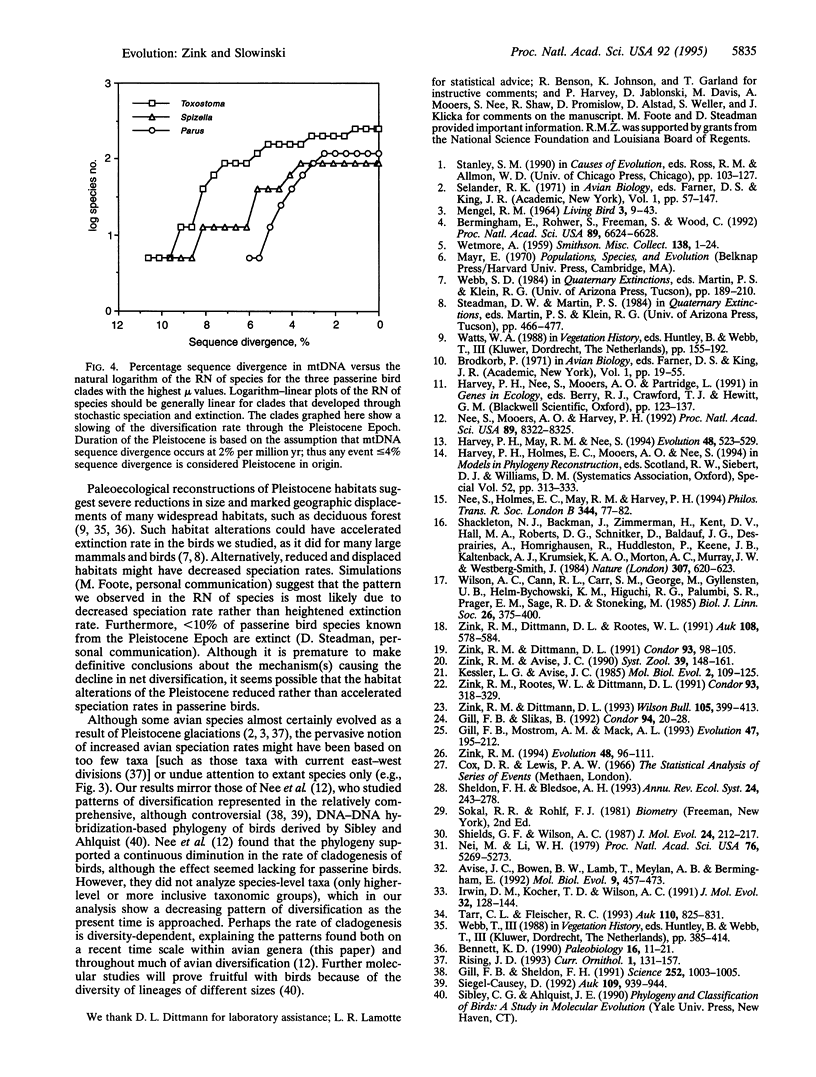
Pleistocene glaciations have been suggested as major events influencing speciation rates in vertebrates. Avian paleontological studies suggest that most extant species evolved in the Pleistocene Epoch and that species' durations decreased through the Pleistocene because of heightened speciation rates. Molecular systematic studies provide another data base for testing these predictions. In particular, rates of diversification can be determined from molecular phylogenetic trees. For example, an increasing rate of speciation (but constant extinction) requires shorter intervals between successive speciation events on a phylogenetic tree. Examination of the cumulative distribution of reconstructed speciation events in mtDNA phylogenies of 11 avian genera, however, reveals longer intervals between successive speciation events as the present time is approached, suggesting a decrease in net diversification rate through the Pleistocene Epoch. Thus, molecular systematic studies do not indicate a pulse of Pleistocene diversification in passerine birds but suggest, instead, that diversification rates were lower in the Pleistocene than for the preceding period. Documented habitat shifts likely led to the decreased rate of diversification, although from molecular evidence we cannot discern whether speciation rates decreased or extinction rates increased.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Bowen B. W., Lamb T., Meylan A. B., Bermingham E. Mitochondrial DNA evolution at a turtle's pace: evidence for low genetic variability and reduced microevolutionary rate in the Testudines. Mol Biol Evol. 1992 May;9(3):457–473. doi: 10.1093/oxfordjournals.molbev.a040735. [DOI] [PubMed] [Google Scholar]
- Bermingham E., Rohwer S., Freeman S., Wood C. Vicariance biogeography in the Pleistocene and speciation in North American wood warblers: a test of Mengel's model. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6624–6628. doi: 10.1073/pnas.89.14.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill F. B., Sheldon F. H. The birds reclassified. Science. 1991 May 17;252(5008):1003–1005. doi: 10.1126/science.252.5008.1003. [DOI] [PubMed] [Google Scholar]
- Irwin D. M., Kocher T. D., Wilson A. C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991 Feb;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Kessler L. G., Avise J. C. A comparative description of mitochondrial DNA differentiation in selected avian and other vertebrate genera. Mol Biol Evol. 1985 Mar;2(2):109–125. doi: 10.1093/oxfordjournals.molbev.a040339. [DOI] [PubMed] [Google Scholar]
- Nee S., Holmes E. C., May R. M., Harvey P. H. Extinction rates can be estimated from molecular phylogenies. Philos Trans R Soc Lond B Biol Sci. 1994 Apr 29;344(1307):77–82. doi: 10.1098/rstb.1994.0054. [DOI] [PubMed] [Google Scholar]
- Nee S., Mooers A. O., Harvey P. H. Tempo and mode of evolution revealed from molecular phylogenies. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8322–8326. doi: 10.1073/pnas.89.17.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamathi A. M., Leake B., Flaskerud J., Lewis C., Bennett C. Outcomes of specialized and traditional AIDS counseling programs for impoverished women of color. Res Nurs Health. 1993 Feb;16(1):11–21. doi: 10.1002/nur.4770160104. [DOI] [PubMed] [Google Scholar]
- Shields G. F., Wilson A. C. Calibration of mitochondrial DNA evolution in geese. J Mol Evol. 1987;24(3):212–217. doi: 10.1007/BF02111234. [DOI] [PubMed] [Google Scholar]


