Abstract
Background
The aim of this study was to measure the body composition in adults with newly diagnosed type 2 diabetes mellitus and to explore the effect of metformin therapy on the various components of body composition, insulin sensitivity, and glucose homeostasis.
Methods
This was an observational study consisted of 51 newly diagnosed people with type 2 diabetes on 1000 mg metformin twice daily for 6 months. The body composition of each subject was measured by dual energy X-ray absorptiometry at enrollment and 24 weeks after metformin mono-therapy. Sarcopenia was defined and compared based on the ratio of appendicular skeletal muscle and height squared, skeletal muscle index and residual methods.
Homeostasis model assessment-insulin resistance and Quantitative Insulin Sensitivity Check Index were used for estimating insulin sensitivity. The level of physical activity was assessed using self-administered International physical Activity questionnaire.
Results
Forty one subjects (80.4%) completed the study. The mean age of the participants was 52.67 ± 10.43 years. Metformin treatment was associated with a significant decrease in total fat mass (−1.6 kg, P = 0.000). By week 24, the lean to fat ratio increased (P = 0.04) with men showing greater significant changes. Twenty percent of the female participants were detected to have sarcopenia.
In addition, there was a significant improvement of glucose homeostasis and insulin sensitivity.
Conclusions
Metformin therapy results in significant improvement in body composition and insulin sensitivity of adults with newly diagnosed type 2 diabetes. Furthermore, sarcopenia begins in women with diabetes much earlier than expected as an age related phenomenon.
Keywords: Diabetes Mellitus, Type 2, Insulin Sensitivity, Insulin Resistance, Sarcopenia, Dual Energy X-ray Absorptiometry
Introduction
Type 2 diabetes is a prevalent disease with substantial morbidity and mortality [1,2]. It is now considered as a global health problem with serious economic burdens [3].
People with type 2 diabetes are usually overweight or obese [4]. Metformin, the most widely used biguanides for the treatment of type 2 diabetes mellitus [5], induces weight stabilization or small weight loss in adults with diabetes [6]. This might be due to reduction of insulin resistance and subsequent hyperinsulinemia [7,8]. It has also reported that in non-diabetic subjects with risk factors for type 2 diabetes mellitus, metformin modifies body composition by decreasing total fat content and increasing lean mass and water content [9].
Measuring body composition by dual energy X-ray absorptiometry (DEXA) is a noninvasive and valid method [10] that allows separation of the body mass into bone mass, fat mass (FM), and fat-free mass (FFM). It can also give estimation for the regional body composition [11].
Insulin resistance or insulin sensitivity were estimated using the homeostatic model assessment-insulin resistance (HOMA-IR) [12], and the Quantitative Insulin Sensitivity Check Index (QUICKI) methods [9].
The aim of this study was to investigate the body composition, the alterations happened by metformin, and their impact on insulin resistance, insulin sensitivity, and metabolic control in adults with newly diagnosed type 2 diabetes mellitus.
Method and materials
Study design
This was a 24-week, prospective, non-interventional, observational study of 51 newly diagnosed people with type 2 diabetes who had been initiated metformin to investigate the body composition, the alterations happened by metformin, and their impact on insulin resistance, insulin sensitivity, and metabolic control in routine clinical practice. G-power software was used to calculate the sample size. Assuming the mean and SD of fat weight [9] as one of the component of body composition that was important in this study, 38 patients will provide 90% power at a 2-sided with significance level of 0.05. Allowing for a 20% dropout rate, the number of subjects required for the study was 46 patients. Key eligibility requirements were fasting plasma glucose concentration of ≥7.0 mmol/L (≥126 mg/dl) or a 2-h plasma glucose concentration of 11.1 mmol/L (≥200 mg/dL) after 75 g glucose by mouth, or glycated hemoglobin (HbA1c) ≥6.5% (48 mmol/mol).
The diagnosis was confirmed by repeating one of the mentioned methods on a different day. Subjects with a diagnosis of cardiac, renal, pulmonary, endocrine and hepatic disease, or any other systematic intercurrent illness that might alter body composition were excluded. They were excluded if they had a GFR < 70 and/or an alanine aminotransferase (ALT) that exceeds 1.5 times the upper limit of normal. Those with prior treatment with oral glucose lowering drugs and/or insulin were also excluded.
Patients
Fifty one subjects met the inclusion criteria and were enrolled in the study. Weight measurement was done using a calibrated digital scale (Seca gmbh & co. kg. Germany). A stadiometer (Seca gmbh & co. kg. Germany) calibrated before each measurement, was used for height measurement. Abdominal and hip circumferences were assessed by a trained nurse, using a cloth tape. The waist was defined at the midpoint between the highest point of the iliac crest and the lowest part of the costal margin in the midaxillary line, and the hip was measured at the level of the greater femoral trochanters. These measurements were used for calculating the body mass index (BMI), and the waist to hip ratio (WHR).
Body composition and DXA assessment procedures
Body composition was assessed by Hologic whole body DEXA systems (QDR4500A, Hologic, Waltham, MA, USA; software version 8.21) A total body scan was performed at baseline and again at the end of the study. A standardized procedure for patient positioning and utilization of the QDR software was used. Total body FFM, FM, lean mass (LM), and percent fat were analyzed using Hologic version 8.21 software for tissue area assessment. Total body FFM was defined as lean soft tissue mass plus total body bone mineral content. Android FM was defined as adipose deposition around the abdomen; whereas, gynoid FM was adipose tissue accumulating around the hips. Appendicular skeletal muscle (ASM) was calculated as the sum of skeletal muscle mass in arms and legs. ASM/h2 was computed as the indicator of relative ASM. The scanner was calibrated daily against a spine calibration block and step phantom block supplied by the manufacturer. In addition, a whole body phantom was scanned weekly to assess any machine drift over time.
Skeletal muscle index (SMI) was analyzed based on the equation established by Janssen et al. (SMI = 100 × skeletal muscle mass/body mass) [13].
The predicted percentage of fat by sex, BMI, and age was calculated using the formulas proposed by Gallagher et al. [14].
Percentage of FM = 76.0 – 1097.8 (BMI−1) – 20.6 (Sex) + 0.053 (Age) + 95.0 (Asian) (BMI−1) – 0.044 (Asian) (Age) + 154 (Sex) (BMI−1) + 0.034 (Sex) (Age)
These formulas reported a correlation coefficient of about 0.90 and standard error of estimation of about 4%. Variables were defined as sex = 1 for male and 0 for female; Asian = 1 for Asian and 0 for other races [14].
Physical activity assessment
At baseline, the level of physical activity was assessed with self-administered International Physical Activity Questionnaire (IPAQ). All of the participants were asked to have and maintain ≥ 30 min/day or 150 min per week of moderate-intensity such as walking or 75 min per week of vigorous aerobic physical activity or an equivalent combination of the two, based on recommendations of standards of medical care in diabetes 2012 [15]. Furthermore, each participant met a dietitian who administered a personalized isocaloric diet.
Interventions and treatment
After an overnight fast of at least 8 hours, blood samples were obtained for measurement of fasting blood sugar (FBS) using a glucose analyzer (YSI 2700 Select, YSI, Inc., Yellow Springs, OH), fasting insulin (IMX assay, Abbott Laboratories, North Chicago, IL), and HbA1c using ion exchange chromatography (DS5 Analyzer, Drew Scientific limited, Cumbria, United Kingdom).
HOMA-IR index was used to estimate insulin resistance: HOMA-IR index = Fasting insulin (μU/mL) × Fasting glucose (mg/dL)/405.
Insulin sensitivity was determined by the quantitative insulin sensitivity check index (QUICKI) calculated as
Once baseline assessments were completed, metformin (Iran, Arya Pharmaceutical Company) was prescribed to all of the eligible subjects with a starting dose of 1000 mg/day.
After 12 weeks, the participants were reevaluated regarding their lifestyle and adherence to the medication. The metformin dose was titrated, if needed, based on HbA1c obtained after 12 weeks of starting treatment. The participants were followed by another 12 weeks.
Sarcopenia classification
We compared the three methods used for identification of sarcopenia. Based on the definition by Baumgartner and colleagues, ASM/h2 was calculated as the ratio of ASM (kg) and height squared (m2) [16]. Participants were categorized as having sarcopenia based on ASM/h2 cut-points defined in previous studies (>2 SD below sex-specific means of normal reference population: 7.26 kg/m2 for men and 5.45 kg/m2 for women [16].
Considering SMI method, Class I sarcopenia was established in the participants whose SMI were within one to two SDs below the sex-specific mean of young adults. Class II sarcopenia was ascertained in the participants whose SMI were lower than two SDs below the sex-specific mean of young adults.
The residuals method is based on the regression model recommended by Newman et al. [17]. Linear regression equations using height (m) and FM (kg) to predict ASM were determined for male and female, respectively. The residuals of the regression were applied to identify participants whose ASM were lower or higher than the predicted. The 20th percentile of the distribution of residuals was used as the cutoff points for sarcopenia. Separate models were fit for men (ASM (kg) = −30.239 + 30.105 × height (m) + 0.141 × fat mass (kg)) and women (ASM (kg) = −15.407 + 17.595× height (m) + 0.162 × fat mass (kg).
The study was carried out at Endocrine Research Centre, Institute of Endocrinology and Metabolism. Ethical approval was obtained from the institutional review board of Institute of Endocrinology and Metabolism affiliated to Iran University of Medical Sciences (525MT/2011). All participants signed a written informed consent. Participants were free to withdraw at will at any time. If they withdrew, the data collected were used for analysis until the point when consent was withdrawn. The primary endpoints were changes in body composition and BMI by the end of 24 weeks. Secondary endpoints were insulin resistance, insulin sensitivity, HbA1c, fasting insulin, and fasting blood sugar.
Statistical analysis
IBM SPSS for Windows Version 19 (IBM Corp., Armonk, NY, USA) was applied for the statistical analysis. The data were analyzed anonymously. Descriptive statistics (means and SDs) were used to describe key clinical and demographic characteristics. Normal assumptions were checked by looking at the Normal plot, or Frequency histogram with as well as Kolmogorov-Smirnov test. In case of normal distribution, we used parametric statistical test while we used non-parametric statistical tests in case of non-normal distribution. The relationship between age and ASM/h2, SMI, and total fat mass in men and women were illustrated by scatter plots and fit lines. Based on ASM/h2, SMI, and residuals methods, the prevalence of sarcopenia in men and women were calculated.
The comparison of the variables before and after the treatment was performed using the paired t-test or Wilcoxon. The relationships between variables were shown using Spearman’s Correlation coefficient. The ordinal variables were compared between two groups via the independent t-test or Mann–Whitney U-test. A P-value < 0.05 was considered statistically significant.
Results
Of the 51 participants, a total of 41 subjects (80.4%) had good adherence to the study protocol and completed the 6-month study. Baseline anthropometric and laboratory indices did not significantly differ between those who finished the study and those who withdrew (data not shown). The mean age was 52.67 ± 10.43 years (range 26 – 71 years) and 59% were female. BMI was higher in women than in men. Table 1 illustrates descriptive baseline characteristics of the participants.
Table 1.
Descriptive baseline characteristics of the participants
| Female (n = 30) | Male (n = 21) | P-Value | |
|---|---|---|---|
| Age (y) | 52.3 ± 10.58 | 53.2 ± 10.45 | 0.98 |
| Weight (kg) | 74.03 ± 10.82 | 81.09 ± 12 | 0.05 |
| BMI (kg/m 2 ) | 30.06 ± 4.3 | 27.78 ± 3.27 | 0.04 |
| Waist (cm) | 101.83 ± 9.18 | 101.52 ± 7.6 | 0.90 |
| WHR | 0.95 ± 0.05 | 0.96 ± 0.04 | 0.26 |
| HbA1c (%) | 7.95 ± 2.19 | 8.45 ± 1.92 | 0.46 |
| HOMA-IR | 3.33 ± 1.53 | 3.95 ± 3.44 | 0.74 |
| QUICKI | 0.33 ± 0.02 | 0.33 ± 0.03 | 0.74 |
Independent sample t-test and Mann Whitney U-test were used.
BMI: Body Mass Index
WHR: Waist to Hip Ratio
HbA1c: Glycosylated Hemoglobin
HOMA-IR: Homeostatic Model Assessment-Insulin Resistance
QUICKI: Quantitative Insulin Sensitivity Check Index
Table 2 describes the changes of anthropometric variables by the end of the study. A significant sex difference was also noted (P = 0.000), with women showing a greater mean decrease in BMI (−0.62 Kg/m2) at 24 week with Metformin treatment than men (−0.58 Kg/m2). BMI was significantly related to total, gynoid, and android FM at baseline and the end of the study (P = 0.000). Waist circumference and WHR did not differ significantly by the end of the study in men and women. Waist circumference was mostly correlated with total FM (P = 0.000) and android FM (P = 0.000).
Table 2.
Changes in anthropometric variables by the end of the study
| Characteristics | Female | P-value | Male | P-value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 24 | Week 0 | Week 24 | |||
| Weight (kg) | 74.03 ± 10.82 | 72.84 ± 10.76 | 0.00 | 81.09 ± 12 | 77.46 ± 15.07 | 0.09 |
| BMI (kg/m 2 ) | 30.06 ± 4.3 | 29.44 ± 4.6 | 0.00 | 27.78 ± 3.27 | 27.18 ± 4.4 | 0.14 |
| Waist (cm) | 101.83 ± 9.18 | 99 ± 12.05 | 0.18 | 101.52 ± 7.6 | 99.46 ± 10.65 | 0.35 |
| WHR | 0.95 ± 0.05 | 0.95 ± 0.14 | 0.35 | 0.96 ± 0.04 | 0.95 ± 0.04 | 0.93 |
Paired t-test and Wilcoxon test was used.
BMI: Body Mass Index
WHR: Waist to Hip Ratio
The level of physical activity, classified as low, moderate, or high was assessed for all of the participants. It showed no significant difference by the end of the study, although, by week 24, more subjects reported higher level of physical activity (P = 0.082).
Primary endpoints
At baseline and at the end of the study, a significant sex difference was shown with women having higher level of gynoid FM and total FM (P-values < 0.05).
By week 24, statistically significant reduction was observed in the total FM (−1.6 kg; 95% CI = −2.39 to 0.84; P =0.000) and percentage of FM (P = 0.000). There was also a significant increase in the proportion of lean/fat ratio in total study population (+0.10; 95% CI = 0.005 to 0.199; P = 0.04) that was more pronounced in men.
This was observed despite a reduction in LM (−1.07 kg, 95% CI = 1.78 to −0.35, P = 0.00) by the end of the study. ASM/h2 was also reduced by week 24 (−0.19 kg/m2; 95% CI = −0.35 to −0.029; P = 0.02).
SMI increased by week 24 (P = 0.02), with men showing a greater significant increase than women. Table 3 demonstrates body composition parameters at baseline and week 24.
Table 3.
Body composition parameters at baseline and 24 week by sex
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Week 0 (n = 30) | Week 24 (n = 27) | P-value | Week 0 (n = 21) | Week 24 (n = 14) | P-value | |
| Android fat mass (kg) | 2.80 ± 0.77 | 2.63 ± 0.78 | 0.03 | 2.35 ± 0.79 | 2.04 ± 0.78 | 0.001 |
| Gynoid fat mass (kg) | 4.60 ± 1.02 | 4.42 ± 1.02 | 0.02 | 3.37 ± 0.83 | 3.11 ± 0.90 | 0.004 |
| 0.61 ± 0.12 | 0.60 ± 0.13 | 0.45 | 0.70 ± 0.16 | 0.65 ± 0.12 | 0.015 | |
| Trunk/limb fat | 1.02 ± 0.15 | 1.01 ± 0.15 | 0.47 | 1.21 ± 0.27 | 1.18 ± 0.24 | 0.25 |
| Fat mass/h2 (kg/m2) | 12.45 ± 3.1 | 11.88 ± 2.98 | 0.027 | 7.68 ± 2.1 | 7.11 ± 2.03 | 0.022 |
| Appendicular lean mass/h2 (kg/m2) | 7.49 ± 0.91 | 7.18 ± 1.03 | 0.006 | 8.75 ± 0.74 | 8.70 ± 0.92 | 0.38 |
| Appendicular lean mass/W | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.29 | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.69 |
| SMI | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.6 | 0.038 ± 0.001 | 0.039 ± 0.001 | 0.005 |
| Total fat mass (kg) | 29.91 ± 6.91 | 28.68 ± 6.58 | 0.015 | 21.88 ± 5.87 | 20.12 ± 6.00 | 0.003 |
| Percentage of Fat (%) | 43.03 ± 5.35 | 41.54 ± 5.77 | 0.000 | 27.11 ± 3.61 | 25.82 ± 4.90 | 0.08 |
| Total lean mass (kg) | 41.95 ± 4.86 | 40.79 ± 5.12 | 0.014 | 53.6 ± 6.59 | 52.67 ± 7.60 | 0.17 |
| Lean/Fat ratio | 1.45 ± 0.31 | 1.48 ± 0.33 | 0.65 | 2.60 ± 0.69 | 2.8 ± 0.71 | 0.041 |
Paired t-test and Wilcoxon test was used.
h2: Height Squared
W: Weight
SMI: Skeletal Muscle Index
We also found an inverse relationship between ASM/h2, SMI, and age in both men and women. However, the total FM did not show similar trends (Figure 1).
Figure 1.
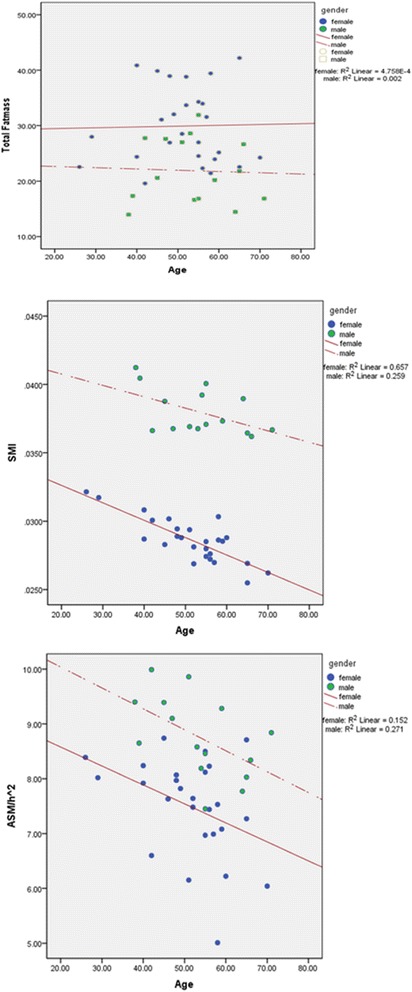
The relationship between age and ASM/h 2 , SMI, and total FM in men and women. ASM: Appendicular skeletal muscle. h2: height squared. SMI: Skeletal muscle index. FM: Fat Mass.
Figure 2 shows the relationship between insulin sensitivity index (QUICKI) and total FM, percentage FM, android FM, and gynoid FM.
Figure 2.
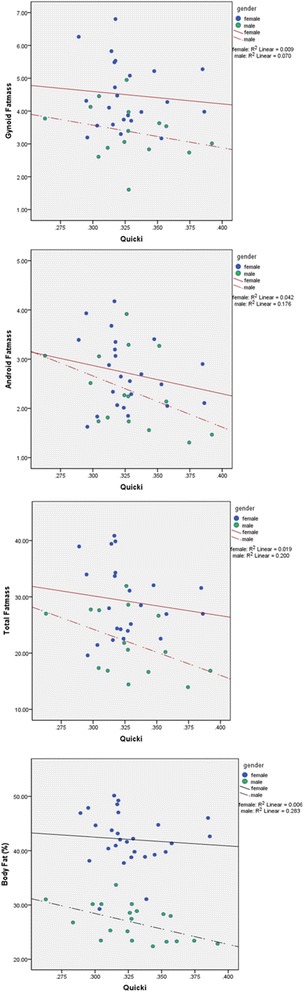
The relationship between QUICKI (at the beginning of the study) and total FM, Percentage of body fat, android FM, and gynoid FM in men and women. FM: Fat Mass.
In both men and women insulin sensitivity increased as fat mass decreased.
Secondary endpoints
Fasting blood sugar was improved significantly from 165.75 ± 60.75 mg/dL to 128.87 ± 40.1 (P = 0.0001). By week 24, statistically significant reductions in HbA1c (−1.34%; 95% CI = 1.99 to 0.68; P =0.000) and a significant increase in insulin sensitivity (QUICKI) (+0.01; 95% CI = 0.001 to 0.027; P = 0.03) was shown. However, ∆HOMA-IR was borderline statistically significant (P = 0.06).
Table 4 summarizes the changes in HbA1c, fasting serum insulin, insulin resistance (HOMA-IR), and sensitivity (QUICKI), by the end of the study.
Table 4.
Glycemic status, insulin resistance and sensitivity at baseline and week 24
| Week 0 | Week 24 | Mean difference ± SD | 95% Confidence Interval | P-value | |
|---|---|---|---|---|---|
| HbA1c (%) | 8.21±2.23 | 6.88±1.50 | -1.34±2.05 | (-1.99-0.68) | 0.000 |
| Fasting Insulin Level (μU/mL) | 8.37±4.20 | 7.97±4.49 | -0.4±5.49 | (-1.45 2.26) | 0.66 |
| HOMA-IR | 3.41±2.5 | 2.51±1.42 | -0.90±2.79 | (-1.84 0.45) | 0.063 |
| QUICKI | 0.33±0.03 | 0.34±0.03 | 0.01±0.03 | (0.001 0.027) | 0.032 |
Paired t-test and Wilcoxon test was used.
HbA1c: Glycosylated Hemoglobin
HOMA-IR: Homeostatic Model Assessment-Insulin Resistance
QUICKI: Quantitative Insulin Sensitivity Check Index
Exploratory endpoints
Based on ASM/h2 definition for sarcopenia, only one of the participants could be diagnosed as afflicted with sarcopenia. However, using the sarcopenia-residuals, 20% of the women were considered to have sarcopenia. Considering the low numbers of the male participants, we could not apply the formula for the men. The SMI method picked up a similar percentage of Class I (one SD below the mean value of young adults) sarcopenia for the women. We did not find Class II (two SDs below the mean value of young adults) sarcopenia in our study population.
The women with sarcopenia were older and their ASM/h2 was lower than non-sarcopenic women at baseline (P = 0.001) and week 24 (P = 0.004). However, there were not significant differences regarding metabolic indices and anthropometric variables at baseline and week 24 between sarcopenic and non-sarcopenic women.
Discussion
Diabetes mellitus may be associated with increased risk of sarcopenia which can result in physical disability and metabolic disorders [18,19]. On the other hand, metformin therapy can improve the parameters of body composition and insulin dynamics in people who are at risk for type 2 diabetes [9]. The current study measured the body composition in adults with newly diagnosed type 2 diabetes mellitus and explored the effect of metformin therapy on various components of body composition, insulin sensitivity, and glucose homeostasis. The results showed significant changes in body composition, insulin sensitivity and glucose homeostasis after 24 weeks of treatment with metformin. The level of physical activity remained unchanged suggesting that the alterations happened in body composition of the participants were mainly due to the effect of the treatment.
We found a slight decrease in body weight in both sexes though the changes of BMI remained statistically significant only for the females probably due to predominance of the women participated in the study. The weight reducing effect of metformin has been demonstrated previously as a result of decreased food intake in a dose-dependent manner [6] It modifies body composition in subjects with type 2 diabetes by reducing total body and abdominal fat [6,20].
In a study conducted by Kim et al., it was shown that men with diabetes had decreased lean body mass and increased body fat mass, even with similar BMI compared with nondiabetic subjects. Based on the Framingham and the National Health and Nutrition Examination study III, the greater fat mass is associated with mobility limitations and fat mass may be more important for mobility than lean mass in older adults [21,22]. In addition, it has been shown that in women with sarcopenia, lean mass influenced mobility after accounting for body size and fat mass [23]. It was also shown that regardless of gender, people with diabetes had decreased SMI values in KSOS study while SMI values increased after 6-month metformin therapy in our study.
The insulin resistance level was reduced with metformin, but borderline statistical significance was observed. Furthermore, statistically significant change has been reported in insulin sensitivity as a result of decreased fasting plasma insulin and glucose level by metformin therapy. Compared to the glucose clamp technique, that is considered to be the gold standard method for directly measuring insulin sensitivity in vivo, QUICKI is a simple alternative with excellent reproducibility. The correlation between QUICKI and glucose clamp is significantly better than the correlation between HOMA-IR and clamp technique [24]. However, there was no statistical difference in insulin level by the end of the study. The increased insulin sensitivity observed by metformin therapy was in parallel to the improvement in glucose homeostasis and HbA1c reduction of 1.33% by the week 24. Our findings are in agreement with several previous studies [9,25].
Considering the results of KSOS, the prevalence of sarcopenia in patients with diabetes was significantly higher than non-diabetic subjects. In subjects older than 60 years, a significant difference in prevalence of sarcopenia between groups with and without diabetes was observed in both gender while in the middle-aged group (age 40 –59 years) this difference was observed only in women. The result of our study is consistent with KSOS that middle-aged women exhibited high prevalence of sarcopenia.
As shown in several previous studies, in general population men lose greater skeletal muscle mass with aging even with greater skeletal muscle mass compared to women [26,27]; however, women with diabetes are particularly considered high risk for loss of skeletal muscle mass [28]. These results suggest that type 2 diabetes may be an important risk factor for sarcopenia, particularly in women, considering its future impacts on quality of life, physical disability and mortality. Although we could not apply the definitions for men because of the low numbers of the male participants in our study, it seems this relationship in men could mainly be observed in elderly population.
There is still a lack of consensus on definition of sarcopenia [29]. Considering the three methods to define sarcopenia, different rates have been estimated for sarcopenia in our study. Based on the definition on ASM/h2, only one of the participants could be diagnosed as afflicted with sarcopenia. It has been reported that appendicular skeletal muscle, i.e. ASM/h2 would lead to a lower cut points for sarcopenia for the adults Asians [30] while based on the sarcopenia-residuals and the SMI definitions for sarcopenia out of five female participants, one had sarcopenia.
Taking the results of this study into consideration, further studies are needed to define a comprehensive method to define sarcopenia in people with type 2 diabetes considering body size, gender and ethnicity. Furthermore, it is needed to explore if female with diabetes are more prone to develop sarcopenia.
In conclusion, administration of metformin for six months had favorable effects on body composition, insulin sensitivity, and glucose homeostasis in adults with newly diagnosed type 2 diabetes and subsequently was expected to postpone the incidence of sarcopenia especially in women with type 2 diabetes who are at higher risk for loss of skeletal muscle mass.
Limitations
Some limitations of this study include the limited number of the participants over the age of 60, small sample size, and lack of objective data about nutritional pattern of the participants.
Acknowledgements
We appreciate all the people who contributed to this study. Authors would also like to thank Ms. Nastaran Hasani who performed the total body scan in Pars Hospital, Tehran, Iran.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
This study was conceived by MEK, MM and RA and its methods developed by all authors. Data were collected by RA, ZB and LN and analysed by AEV. The manuscript was drafted by MM and RA, critically revised by MEK. All authors read and approved the final manuscript.
Contributor Information
Rokhsareh Aghili, Email: r-aghili@razi.tums.ac.ir.
Mojtaba Malek, Email: malek.m@iums.ac.ir.
Ameneh Ebrahim Valojerdi, Email: ebrahimv86@gmail.com.
Zahra Banazadeh, Email: zahrabanazadeh@yahoo.com.
Laily Najafi, Email: lailynajafi@yahoo.com.
Mohammad Ebrahim Khamseh, Email: khamseh.m@iums.ac.ir.
References
- 1.McAlpine RR, Morris AD, Emslie-Smith A, James P, Evans JM. The annual incidence of diabetic complications in a population of patients with type 1 and type 2 diabetes. Diabet Med. 2005;22:348–352. doi: 10.1111/j.1464-5491.2004.01391.x. [DOI] [PubMed] [Google Scholar]
- 2.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Davies M, Khunti K. Insulin management in overweight or obese type 2 diabetes patients: the role of insulin glargine. Diabetes Obes Metab. 2008;10(Suppl 2):42–49. doi: 10.1111/j.1463-1326.2008.00844.x. [DOI] [PubMed] [Google Scholar]
- 5.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149–157 e142. doi: 10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Meneghini LF, Orozco-Beltran D, Khunti K, Caputo S, Damci T, Liebl A, Ross SA. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011;96:3337–3353. doi: 10.1210/jc.2011-1074. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Moctezuma JR, Robles-Lopez G, Lopez-Carmona JM, Gutierrez-Rosas MJ. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes Obes Metab. 2005;7:189–192. doi: 10.1111/j.1463-1326.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher D, Song MY. Evaluation of body composition: practical guidelines. Prim Care. 2003;30:249–265. doi: 10.1016/S0095-4543(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 11.Tylavsky F, Lohman T, Blunt BA, Schoeller DA, Fuerst T, Cauley JA, Nevitt MC, Visser M, Harris TB. QDR 4500A DXA overestimates fat-free mass compared with criterion methods. J Appl Physiol. 2003;94:959–965. doi: 10.1152/japplphysiol.00732.2002. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi K. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khamseh ME, Malek M, Aghili R, Emami Z. Sarcopenia and diabetes: pathogenesis and consequences. Br J Diabetes Vasc Dis. 2011;11:230–234. doi: 10.1177/1474651411413644. [DOI] [Google Scholar]
- 20.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 21.Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2013;68:168–174. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 23.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 25.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 26.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 28.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB, Aging Health Study Body Composition Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ., 3rd Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60:213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]


