Abstract
We present the development of a native chemical ligation handle that also functions as a masked electrophile that can be liberated during synthesis when required. This handle can thus be used for the synthesis of complex activity-based probes. We describe the use of this handle in the generation of linkage-specific activity-based deubiquitylating enzyme probes that contain substrate context and closely mimic the native ubiquitin isopeptide linkage. We have generated activity-based probes based on all seven isopeptide-linked diubiquitin topoisomers and demonstrated their structural integrity and ability to label DUBs in a linkage-specific manner.
Keywords: activity-based probes, native chemical ligation, protein modifications, solid-phase peptide synthesis, ubiquitin
Ubiquitin (Ub) is a 76-residue post-translational modifier that is commonly conjugated onto lysine residues of a protein substrate and that forms isopeptide-linked chains through self-conjugation onto any of its own seven lysine residues (K6, K11, K27, K29, K33, K48, and K63).[1] Deubiquitylating enzymes (DUBs) are proteases that remove Ub from a substrate and can be divided into a major group of cysteine proteases and a minor group of metalloproteases.[2] Because DUBs regulate many cellular processes by controlling the Ub landscape, they have become potential drug targets.[3]
An important class of reagents used to study DUB activity and substrate specificity are activity-based probes (ABPs).[4] For a decade, the design of these ABPs has been based on monoUb modified at the C terminus with an electrophilic group that targets the active site cysteine residue,[5] with recent improvements in the level of detection sensitivity and/or reactivity of the electrophilic group.[6] Although monoUb-based ABPs contribute(d) significantly to our understanding of DUBs, recent work has made it clear that the presence of substrate context is important for full understanding of how DUBs achieve their substrate specificity.[7] Very recently, three approaches that allow the introduction of substrate context into DUB ABPs have been reported.[8] We have previously reported a nonenzymatic strategy for isopeptide-linked Ub conjugation by native chemical ligation (NCL) between a Ub thioester and a γ- or δ-thiolysine ligation handle.[9] Because this allowed us to construct all seven isopeptide-linked diUb conjugates, we wondered whether we could design a NCL handle that would not only ligate Ub onto a polypeptide but also serve as a precursor for the electrophilic “warhead” designated to react with the DUB active site cysteine nucleophile. Here we report a general and scalable synthetic approach towards DUB ABPs that harbor substrate sequence context (Figure 1) based on (native) chemical ligation handle 1 (Figure 1). This handle allows us first to ligate Ub onto a polypeptide of interest and then, by selective elimination of the residual thiol moiety,[10] to transform it into an electrophile that can capture the catalytic Cys residue of a DUB. Our approach has two key aspects: 1) selective “unmasking” of the reactive electrophilic species in the last step of synthesis avoids potential side-reactions during chemical manipulations, and 2) the final product closely mimics the native isopeptide-linked conjugate in linker length and structure.[8] By our approach (Figure 1) we prepared linkage-specific DUB ABPs resembling all seven native diUb isopeptide connectivities (i.e., K6, K11, K27, K29, K33, K48, and K63) and verified their structural integrity with various DUBs.
Figure 1.
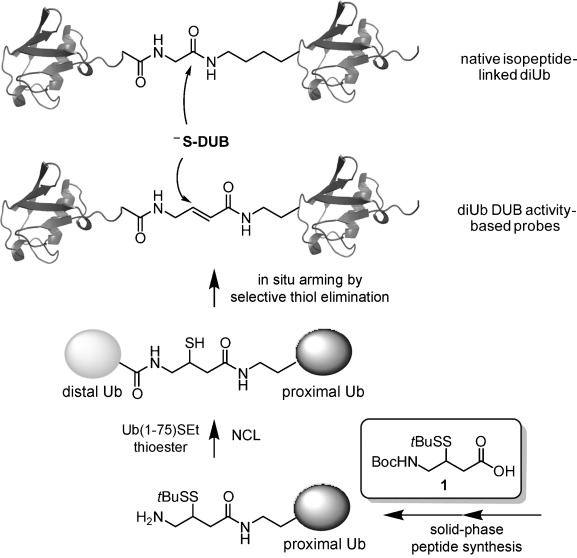
General outline of our approach towards DUB ABPs that harbor substrate sequence context. The ABP design contains a reactive Michael acceptor at the same position as the native isopeptide bond, enabling covalent capture of cysteine protease DUBs in a structurally relevant manner.
The synthesis of key building block 1 (Scheme 1) started with the esterification and Boc protection of commercially available racemic 4-amino-3-hydroxybutanoic acid (2) to provide 4 in 70 % yield over two steps. Treatment of 4 with p-toluenesulfonyl chloride in pyridine afforded tosylate 5 in 93 % yield. Substitution of the tosylate with potassium thioacetate gave a low yield of the thioacetate 6, due to elimination of 5 to afford Boc-4-aminobut-2-enoic methyl ester (8 a). By use of the more sterically hindered DBU (1,8-diazabicycloundec-7-ene) salt of thioacetic acid, the desired thioacetate 6 was obtained in 83 % yield. Selective hydrolysis of the thioacetate with hydroxylamine[11] and simultaneous in situ protection of the thiol with S-tert-butyl methanesulfonothioate gave S-tert-butyl disulfide 7. Because of the base-sensitive character of the building block, the methyl ester of 7 was hydrolyzed with the mild and ester-selective reagent trimethyltin hydroxide,[12] affording 1 in 36 % yield from 6.
Scheme 1.
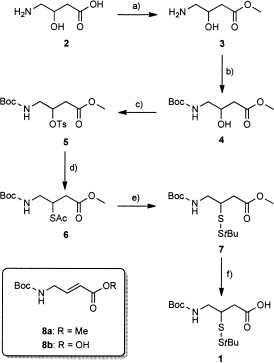
Synthesis of the ligation handle. Reagents and conditions: a) SOCl2, MeOH, 75 °C; b) Boc2O, Et3N, CH3CN, 70 % two steps; c) TosCl, pyridine, 93 %; d) HSAc, DBU, DMF, 60 °C, 83 %; e) H2NOH⋅HCl, Et3N, S-tert-butyl methanesulfonothioate, MeOH; f) Me3SnOH, DCE, 80 °C, 36 % two steps.
To validate our concept we decided to focus first on ABPs based on K11- and K48-linked diUb. In order to preserve the length of the native isopeptide linkage in our design, a lysine residue of interest in Ub was replaced with a diaminobutyric acid residue (Dab, Scheme 2). Using our previously reported linear Fmoc-based solid-phase peptide synthesis (SPPS) of the Ub polypeptide,[9a,b] we synthesized Ub Lys11Dab(Alloc) and Lys48Dab(Alloc) mutants. Next, the Dab(Alloc) residue was selectively deprotected with Pd(PPh3)4 and Ph3SiH, followed by on-resin coupling of ligation handle 1 by using benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) and DiPEA. Global deprotection with 90 % TFA and purification by HPLC gave the desired proximal Ub mutants 9 (Scheme 2) in ±15 % overall yield, based on a 100 μmol synthesis scale.
Scheme 2.
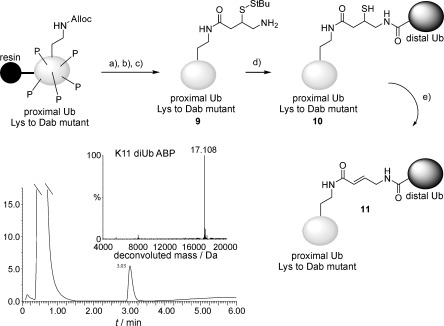
Synthesis of diUb probes. Reagents and conditions: a) Ph3SiH, Pd(PPh3)4, NMP; b) 1, PyBOP, DIPEA, NMP; c) TFA/iPr3SiH/phenol/H2O (90:2.5:2.5:5), 3 h, RT; d) 6 m Gnd⋅HCl, 0.15 m sodium phosphate buffer pH 7, 250 mm MPAA, Ub(1–75)SEt, overnight; e) 50 mm sodium phosphate buffer pH 8, 2,5-dibromohexanediamide, 37 °C, overnight.
The required Ub(1–75)SEt thioester, representing the distal part of our diUb probe design, was synthesized in a similar fashion and good overall yield by Fmoc SPPS (see the Supporting Information).[9a,b] Native chemical ligation of the (proximal) Ub mutant (20 mg mL−1, ±2.25 mm) and Ub(1–75)SEt (1.5 equiv, 30 mg mL−1, ±3.5 mm) thioester was performed in 6 m Gdn⋅HCl, 0.15 m sodium phosphate pH 7.2 with mercaptophenylacetic acid (MPAA, 250 mm) as ligation catalyst.[13] Judged by LC-MS, overnight incubation at 37 °C resulted in full consumption of the proximal Ub mutant 9 (Scheme 2) and formation of the ligation product as MPAA disulfide. A short treatment with TCEP followed by preparative HPLC gave the K11 and K48 diUb conjugates 10 (Scheme 2) in 31 and 48 % yield, respectively.
“Arming” (i.e., thiol elimination) of the warhead was achieved by overnight incubation of 10 (0.5 mg mL−1, 29 μm) with 100 equiv of 2,5-dibromohexanediamide at 37 °C in 50 mm sodium phosphate pH 8.[10] LC-MS analysis (Figure S1) showed formation of the expected sulfonium intermediate (Figure S1 B), which is transformed into the armed diUb ABP by basic elimination of the sulfonium group. Finally, HPLC purification afforded both the K11 and K48 diUb ABPs (11, Scheme 2) in 50 % yield from 10.
To investigate their reactivity toward DUBs, we treated the K11 and K48 diUb ABPs with ubiquitin-specific protease 8 (USP8; catalytic domain). Incubation with the K11 and K48 diUb probes showed efficient covalent labeling of USP8 (Figure 2 A). Next, we targeted the OTU DUB Cezanne, which exhibits selectivity toward K11-linked Ub chains.[14] Incubation of Cezanne with both probes indeed showed selective labeling of the K11 diUb probe over the K48 diUb probe (Figure 2 B), thus demonstrating the ability of these probes to capture active DUBs selectively and with distinct target preferences. As a control, we treated Cezanne with 9, the thiol precursor of the K11 diUb ABP, and, as expected, no covalent labeling was observed (Figure S2).
Figure 2.
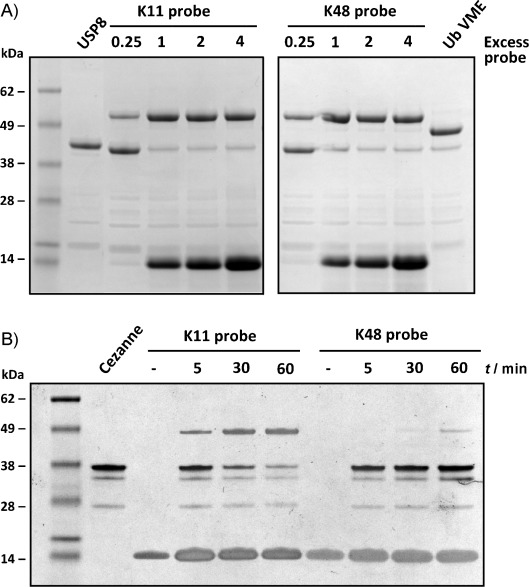
A) Labeling with DUB USP8 (CD). B) Selective reactivity of the K11 and K48 diUb VME probes towards Cezanne 1.
In line with our earlier work on the synthesis and application of fluorescent DUB ABPs,[6c] we decided to create fluorescent versions of the K11 and K48 diUb ABPs, allowing for a more sensitive and faster read-out of labeling experiments. K11 and K48 Cy5-diUb ABPs were synthesized by the synthetic protocol outlined in Scheme 2 but with (synthetic) Cy5Ub(1–75)SEt as the distal Ub component, and compared with the established N-terminally Cy5-labeled Ub-VME probe[6c] for profiling DUB activity. Mouse thymoma El-4 cell lysate was incubated with increasing probe concentrations (Figures 3 and S3), and labeled DUBs were visualized by in-gel fluorescence scanning. The diUb ABPs showed a more restrictive pattern than the monoUb-based Cy5Ub-VME in capturing active DUBs. In line with the Cezanne labeling experiments, Figure 3 also shows that the K11 and K48 Cy5diUb ABPs exhibit somewhat different labeling efficiencies of specific DUBs, especially at lower concentrations of probe.
Figure 3.
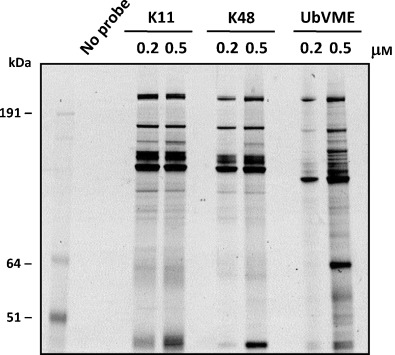
EL-4 cell lysate incubated with monoUb ABP Cy5Ub-VME and Cy5-labelled K11 and K48 Cy5diUb ABPs. Proteins were separated by SDS-PAGE and analyzed by in-gel fluorescence scanning.
At this stage we decided to make the remaining DiUb ABPs (i.e., K6-, K27-, K29-, K33-, and K63-linked; see the Supporting Information), by the protocol in Scheme 2. In contrast to the other linkages, the arming reaction of the K27- and K29- linked diUb ABP precursors 10 (Scheme 2) with 2,5-dibromohexanediamide took longer (±48 h) and gave rise in each case to a side product with an additional mass of 140 Da. This might correspond to basic elimination of the remaining bromide occurring as an alternative to the nucleophilic attack by the alkylated thiol.[10] The sluggish progress of this alkylation at the K27 and K29 positions under native conditions is not surprising, because K27 and K29 are the least exposed Lys residues in the Ub fold and also proved unreactive under native conditions during our earlier work synthesizing native linked diUb conjugates.[9a,b] Gratifyingly, the side product underwent thiol elimination and transformed into the desired ABP product on overnight incubation at pH 10 (Figure S16). The structural integrities and reactivities of all seven diUb DUB ABPs were verified by their treatment and covalent labeling of full-length USP7 (Figure S4 B), which like many of the USP DUB family is known to exhibit promiscuous activity toward all seven isopeptide-linked diUb conjugates.[14, 15]
In conclusion, we have presented the synthesis and application of a new ligation handle that enables the synthesis of DUB ABPs with substrate context and a reactive warhead at the correct position in relation to the native Ub isopeptide linkage. Our synthetic approach offers significant synthetic advantages over other methods[8] because the reactive electrophilic species required for capturing the active site Cys residue of DUBs is introduced in the final synthetic step. This allows not only for the synthesis of complex structures but also for the use of reagents that are not compatible with the presence of an electrophilic group. We have shown the efficiency of our approach by synthesizing DUB ABPs based on all seven diUb isopeptide topoisomers and demonstrated their ability to capture DUBs selectively and with distinct target preference. Our strategy of native chemical ligation and “in situ” arming through thiol elimination provides a general route to effective ABP probes that extend beyond their use in ubiquitin.
In summary, a routine native chemical ligation strategy for the synthesis of virtually any ubiquitylated polypeptide-based DUB ABP is now available. Studies directed towards exploiting their potential as cocrystallization tools in elucidating substrate binding of DUB are currently being investigated. In addition, the reagents reported here allow for proteomics studies of protease specificity[8d] and site-specific genetic incorporation of the diaminobutyric acid-Nγ-(4-amino-3-mercaptobutanoyl) ligation handle in recombinant proteins with an evolved tRNA synthetase.[16]
Acknowledgments
We thank Lara Krooshof and Raymond Kooij for experimental assistance, Dris El Atmioui and Henk Hilkmann for solid-phase peptide synthesis, Prof. Titia Sixma, Dr. Marcello Clerici, and Michael Uckelmann (Division of Biochemistry, NKI) and Tycho Mevissen and Dr. David Komander (MRC-LMB, Cambridge, UK) for providing DUBs. This research was sponsored by a grant from the European Research Council (to H.O.) and a European Union Marie Curie Reintegration Grant (to F.E.).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1a.Hochstrasser M. Nature. 2009;458:422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b.Kerscher O, Felberbaum R, Hochstrasser M. Annu. Rev. Cell Dev. Biol. 2006;22 doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 1c.Glickman MH, Ciechanover A. Physiol. Rev. 2002;82 doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 1d.Komander D, Rape M. Annu. Rev. Biochem. 2012;81 doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 1e.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Annu. Rev. Biochem. 2009;78 doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Tsou WL, Sheedlo MJ, Morrow ME, Blount JR, McGregor KM, Das C, Todi SV. PLoS One. 2012;7:e43112. doi: 10.1371/journal.pone.0043112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3a.Mattern MR, Wu J, Nicholson B. Biochim. Biophys. Acta Mol. Cell Res. 2012;1823:2014–2021. doi: 10.1016/j.bbamcr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b.Shanmugham A, Ovaa H. Curr. Opin. Drug Discovery Dev. 2008;11 [PubMed] [Google Scholar]
- 3c.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Nat. Rev. Drug Discovery. 2011;10 doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Cravatt BF, Wright AT, Kozarich JW. Annu. Rev. Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 4b.Sadaghiani AM, Verhelst SH, Bogyo M. Curr. Opin. Chem. Biol. 2007;11 doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 4c.Ovaa H. Nat. Rev. Cancer. 2007;7 doi: 10.1038/nrc2128. [DOI] [PubMed] [Google Scholar]
- 5a.Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. EMBO J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chem. Biol. 2002;9 doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 5c.Ovaa H, Kessler BM, Rolén U, Galardy PJ, Ploegh HL, Masucci MG. Proc. Natl. Acad. Sci. USA. 2004;101 doi: 10.1073/pnas.0308411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5d.Hemelaar J, Borodovsky A, Kessler BM, Reverter D, Cook J, Kolli N, Gan-Erdene T, Wilkinson KD, Gill G, Lima CD, Ploegh HL, Ovaa H. Mol. Cell. Biol. 2004;24 doi: 10.1128/MCB.24.1.84-95.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Ekkebus R, van Kasteren SI, Kulathu Y, Scholten A, Berlin I, Geurink PP, de Jong A, Goerdayal S, Neefjes J, Heck AJR, Komander D, Ovaa H. J. Am. Chem. Soc. 2013;135:2867–2870. doi: 10.1021/ja309802n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b.Sommer S, Weikart ND, Linne U, Mootz HD. Bioorg. Med. Chem. 2013;21 doi: 10.1016/j.bmc.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 6c.de Jong A, Merkx R, Berlin I, Rodenko B, Wijdeven RH, El Atmioui D, Yalcin Z, Robson CN, Neefjes JJ, Ovaa H. ChemBioChem. 2012;13 doi: 10.1002/cbic.201200497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6d.Love KR, Pandya RK, Spooner E, Ploegh HL. ACS Chem. Biol. 2009;4 doi: 10.1021/cb9000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6e.Claessen JHL, Witte MD, Yoder NC, Zhu AY, Spooner E, Ploegh HL. ChemBioChem. 2013;14 doi: 10.1002/cbic.201200701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6f.McGouran JF, Kramer HB, Mackeen MM, di Gleria K, Altun M, Kessler BM. Org. Biomol. Chem. 2012;10 doi: 10.1039/c2ob25258a. [DOI] [PubMed] [Google Scholar]
- 6g.Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KG, Konietzny R, Fischer R, Kogan E, Mackeen MM, McGouran J, Khoronenkova SV, Parsons JL, Dianov GL, Nicholson B, Kessler BM. Chem. Biol. 2011;18 doi: 10.1016/j.chembiol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 7a.Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, Wauer T, Hospenthal MK, Gyrd-Hansen M, Krappmann D, Hofmann K, Komander D. Cell. 2013;153:1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b.Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K, Nureki O, Iwai K, Komada M, Fukai S. Nature. 2008;455 doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- 8a.Haj-Yahya N, Hemantha HP, Meledin R, Bondalapati S, Seenaiah M, Brik A. Org. Lett. 2014;16:540–543. doi: 10.1021/ol403416w. [DOI] [PubMed] [Google Scholar]
- 8b.Li G, Liang Q, Gong P, Tencer AH, Zhuang Z. Chem. Commun. 2014;50 doi: 10.1039/c3cc47382a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c.Iphöfer A, Kummer A, Nimtz M, Ritter A, Arnold T, Frank R, van den Heuvel J, Kessler BM, Jänsch L, Franke R. ChemBioChem. 2012;13 doi: 10.1002/cbic.201200261. [DOI] [PubMed] [Google Scholar]
- 8d.McGouran JF, Gaertner SR, Altun M, Kramer HB, Kessler BM. Chem. Biol. 2013;20 doi: 10.1016/j.chembiol.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.El Oualid F, Merkx R, Ekkebus R, Hameed DS, Smit JJ, de Jong A, Hilkmann H, Sixma TK, Ovaa H. Angew. Chem. Int. Ed. 2010;49:10149–10153. doi: 10.1002/anie.201005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2010;122 [Google Scholar]
- 9b.Ovaa H, El Oualid F. 2009. (Stichting Het Nederlands Kanker Instituut), WO2010/131 962.
- 9c.Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. J. Am. Chem. Soc. 2009;131 doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]
- 9d.Ajish Kumar KS, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew. Chem. Int. Ed. 2009;48 doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2009;121 [Google Scholar]
- 9e.Haj-Yahya M, Kumar KSA, Erlich LA, Brik A. Biopolymers. 2010;94 doi: 10.1002/bip.21384. [DOI] [PubMed] [Google Scholar]
- 9f.Spasser L, Brik A. Angew. Chem. Int. Ed. 2012;51 doi: 10.1002/anie.201200020. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124 [Google Scholar]
- 9g.Weller CE, Pilkerton ME, Chatterjee C. Biopolymers. 2014;101 doi: 10.1002/bip.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Angew. Chem. Int. Ed. 2012;51:1835–1839. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124 [Google Scholar]
- 10b.Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernández-González M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Chem. Sci. 2011;2 [Google Scholar]
- 11.Kalia J, Raines RT. ChemBioChem. 2006;7:1375–1383. doi: 10.1002/cbic.200600150. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew. Chem. Int. Ed. 2005;44:1378–1382. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2005;117 As expected, harsher hydrolysis conditions (i.e., NaOH- or LiOH-mediated) resulted mainly in elimination product 8 b. [Google Scholar]
- 13.Johnson ECB, Kent SBH. J. Am. Chem. Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 14a.Mevissen TE, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F, Freund SM, Ovaa H, Komander D. Cell. 2013;154:169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b.Komander D, Clague MJ, Urbé S. Nat. Rev. Mol. Cell Biol. 2009;10 doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 15.Faesen AC, Luna-Vargas MP, Geurink PP, Clerici M, Merkx R, van Dijk WJ, Hameed DS, El Oualid F, Ovaa H, Sixma TK. Chem. Biol. 2011;18:1550–1561. doi: 10.1016/j.chembiol.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Liu CC, Schultz PG. Annu. Rev. Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


