Abstract
An increasing number of chemical reactions are being employed for bio-orthogonal ligation of detection labels to protein-bound functional groups. Several of these strategies, however, are limited in their application to pure proteins and are ineffective in complex biological samples such as cell lysates. Here we present the palladium-catalyzed oxidative Heck reaction as a new and robust bio-orthogonal strategy for linking functionalized arylboronic acids to protein-bound alkenes in high yields and with excellent chemoselectivity even in the presence of complex protein mixtures from living cells. Advantageously, this reaction proceeds under aerobic conditions, whereas most other metal-catalyzed reactions require inert atmosphere.
Keywords: cross-coupling, Heck reaction, homogeneous catalysis, palladium, protein modifications
Bio-orthogonal ligation of protein-bound small organic molecules is a long-standing challenge with many implications for cell biology.[1] In combination with metabolic labeling, it has enabled the detection of post-translational modifications for which no antibodies are available.[1] In addition, bio-orthogonal ligations have shown excellent selectivity for the detection of specific endogenous protein modifications.[2] Regrettably, though, the wealth of organic chemistry reactions is poorly translated into new methods that are applicable in cell biology. In this study we present the oxidative Heck reaction as a strategy for bio-orthogonal ligation reactions between arylboronic acids and protein-bound alkenes.
The most widely used strategy for bio-orthogonal ligation is the linkage of azides to alkynes through copper-catalyzed Huisgen cycloaddition, known as the “click reaction”.[3] Despite their success as chemical reporters, however, terminal alkyne systems suffer from a lack of stability under physiological conditions, most probably due to the relatively acidic alkyne hydrogen atom.[4] Side reactions are alkyne homocoupling,[5] covalent inactivation of specific oxidative enzymes,[6] and the covalent binding of terminal alkynes to active site cysteine residues (thiol-yne reaction).[7] Some of these problems are avoided by use of the recently developed strain-promoted “click reaction”,[8] but the relatively large sizes of the strained alkynes limit their application in metabolic labeling.[8]
Nonconjugated alkenes appear to be ideal protein labels because of their low chemical reactivity and their low abundance in cellular proteins. Alkenes have been employed as protein labels for ligation through the photochemical thiol-ene reaction.[9] In addition, they can be ligated by reaction with tetrazoles or tetrazines after photochemical conversion of these reagents into highly reactive nitrile imine intermediates.[10] However, no metal-catalyzed couplings have been described so far. This is remarkable because the potential of metal-catalyzed couplings in protein labeling reactions has been demonstrated by recently developed bio-orthogonal ligation strategies employing ruthenium-catalyzed cross-metathesis of allyl-substituted cysteines,[11] palladium-catalyzed Suzuki–Miyaura reactions of phenyl iodides,[12, 13] and other reactions.[14]
We anticipated that the Pd-catalyzed oxidative Heck reaction—that is, coupling of an arylboronic acid to an alkene—could be developed into an excellent tool in protein ligation, provided that an active catalyst applicable in water could be identified.[15–17] Importantly, the oxidative Heck reaction, unlike most other metal-catalyzed reactions, does not require oxygen-free conditions; this makes this reaction robust for applications in cell lysates.
Although many previous studies focused on oxidative Heck reactions of activated alkenes such as cyclohexenones[16] and acrylate esters,[15] several recent studies have reported high yields and in several cases excellent regioselectivities in oxidative Heck reactions of isolated alkenes.[17–19] This sets the stage for development of this reaction into a protein ligation strategy for isolated alkene systems.
In order to use the oxidative Heck reaction for cross-coupling of arylboronic acids to protein-bound alkenes we employed Pd(OAc)2 in combination with the bis-imine of acenaphthenequinone and mesitylamine (BIAN). We had developed BIAN earlier as a superior ligand for oxidative Heck reactions of challenging substrates.[16] Using mass spectrometry we demonstrated selective and complete ligation of a fluorescently labeled phenylboronic acid derivative to protein-bound alkenes, even in the presence of complex protein mixtures from cell lysates.
We selected the enzyme 4-oxalocrotonate tautomerase (4-OT) as a model protein for the generation of protein-bound alkenes in order to establish the oxidative Heck reaction for bio-orthogonal ligation. This enzyme contains no cysteine residues, and so we selected the mutant R61C (4-OT R61C) to include a cysteine residue in the solvent-exposed C terminus region of the protein.[20] Upon expression the enzyme proved to be a dimer, due to formation of disulfide bonds (see the Supporting Information). After reduction, alkenes were coupled through a maleimide linker to give the protein-bound terminal alkene 4-OT R61C-1, the cis internal alkene 4-OT R61C-2, the trans internal alkene 4-OT R61C-3, and, as a control, the protein 4-OT R61C-4 without the olefinic functional group (Scheme 1, Table 1).
Scheme 1.
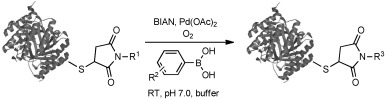
Oxidative Heck reactions with alkenes linked to the protein 4-OT R61C as shown in Table 1.
Table 1.
Aqueous oxidative Heck reactions with protein-bound alkenes according to Scheme 1 monitored by LC-MS
| R1 | R2 | R3 | Catalyst/boronic acid | Conversion |
|---|---|---|---|---|
| H | 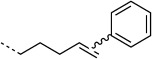 |
50:300 | full | |
| H | 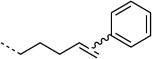 |
20:100 | full | |
| H | 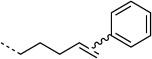 |
10:50 | ≈85 % | |
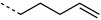 |
4-OMe |  |
20:100 | full |
| 4-OT R61C-1 | ||||
| 4-COOMe | 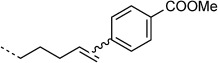 |
20:100 | ≈85 % | |
| 3-NH-dansyl | 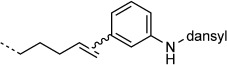 |
20:100 | full | |
 |
H | 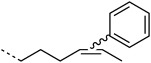 |
20:100 | ≈70 % |
| 4-OT R61C-2 | ||||
 |
H | 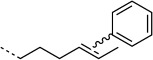 |
20:100 | ≈5 % |
| 4-OT R61C-3 | ||||
 |
H | 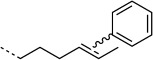 |
20:100 | no |
| 4-OT R61C-4 |
In an attempt to develop a water-soluble catalyst we considered application of the highly water-soluble 2-amino-4,6-dihydroxypyrimidine ligands as reported by Davis et al. in the context of Pd-catalyzed ligation reactions.[12] In our hands, however, the use of 2-amino-4,6-dihydroxypyrimidine as a ligand was not effective for this type of oxidative Heck reaction and led to low yields (20 %) in test reactions on small molecules. We therefore chose to rely on the BIAN ligand to prepare the catalyst.
Because of its limited water solubility the Pd(OAc)2/BIAN catalyst was prepared in an organic solvent (DMF), with the final buffer/DMF ratio in the reaction mixture being 6:1. This proved to be compatible with protein solutions. All reactions were performed at room temperature and at neutral pH under oxygen for a 24 h period. Conversion was monitored by LC-MS after removal of palladium by EDTA chelation in order to provide suitable mass spectra.
Gratifyingly, we found full conversion of the protein-bound alkene 4-OT R61C-1 into its arylated product with use of 50 or 20 equiv of the Pd(OAc)2/BIAN catalyst and 300 or 100 equiv of the phenylboronic acid (Table 1). Application of 10 equiv of the catalyst and 50 equiv of the phenylboronic acid did not result in full conversion (85 %). As a result, 20 equiv of Pd(OAc)2/BIAN and 100 equiv of the arylboronic acid were chosen as optimal for further experimentation.
Subsequently, cis and trans alkenes were also subjected to the same reaction conditions. Interestingly, 4-OT R61C-2 showed significant conversion (70 %) whereas 4-OT R61C-3, in contrast, hardly reacted (5 %; Table 1). This demonstrates that the oxidative Heck reaction can also be employed to label cis internal alkenes and discriminates between cis and trans alkenes. This finding presents exciting opportunities for selective labeling and detection of cis (in the presence of trans) unsaturated fatty acids in biological systems.
To investigate the selectivity of the reaction for protein-bound alkenes, control experiments were performed with omission either of the catalyst or of the boronic acid (control experiments 1 and 2, Supporting Information). In both cases the protein 4-OT R61C-1 did not react. Importantly, protein 4-OT R61C-4, which does not contain an alkene system, did not react under any of the reaction conditions (Table 1). This demonstrates that the phenyl group selectively reacts with the protein-bound alkene and not with other functional groups in the protein.
Next, the influence of the electronic properties of arylboronic acid derivatives on their reactivity with protein-bound alkenes was investigated. It was found that 4-methoxyphenylboronic acid gave full conversion, whereas 4-methoxycarbonylphenylboronic acid gave only 85 % conversion. The same phenomenon was observed in reactions with small-molecule alkenes in the presence of the same catalyst (Scheme 2, Table 2, entries 1–3). Apparently the reaction is disfavored by the presence of electron-withdrawing groups on the phenylboronic acid.
Scheme 2.
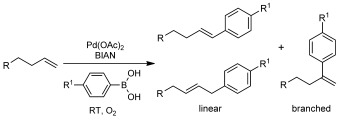
Oxidative Heck reactions with small-molecule alkenes and the isomers formed as shown in Table 2.
Table 2.
Oxidative Heck reactions between arylboronic acids and small-molecule alkenes according to Scheme 2
| Alkene | Boronic acid; R1 | Isolated yield [%] | Linear/branched | |
|---|---|---|---|---|
| 1 |  |
H | 67[a] | 2.1:1 |
| 2 |  |
–OCH3 | 64[a] | 1:1.1 |
| 3 |  |
–COOCH3 | – | – |
| 4 | 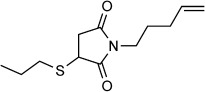 |
H | 81[b] | 2.3:1 |
Pd(OAc)2 (5 mol %), BIAN (7 mol %), phenylboronic acid (1.5 equiv), room temperature, 30 h.
Pd(OAc)2 (1 equiv), BIAN (1.4 equiv), phenylboronic acid (10 equiv), room temperature, 24 h.
Although irrelevant in most applications as bio-orthogonal ligation strategy, multiple regio- and stereoisomers can be formed in oxidative Heck reactions of terminal alkenes (e.g., linear versus branched, Scheme 2). However, the positions of the resulting double bonds could not be determined from the mass spectrometric analysis in the protein ligation reactions. In order to estimate which isomers are formed under Pd/BIAN catalysis conditions, small-molecule alkenes were linked to arylboronic acids (Table 2), and the product ratios were investigated. The ratio between the linear and branched isomers in the reactions with phenyl- and 4-methoxyphenylboronic acids varies between 3:1 and 1:1. In order to mimic the conditions used for the protein ligations further, the reaction was performed with the alkene part of protein 4-OT R61C-1 (Table 2, entry 4), with use of a stoichiometric amount of catalyst and a tenfold excess of the boronic acid in DMF for a 24 h period. The ratio between the linear and branched products was about 2:1, which suggests that similar isomers are formed in protein ligations. Apparently, chelate control as reported by White et al.[18] in the case of their regioselective oxidative Heck reactions does not play a significant role here. Despite its limited relevance for most protein ligations, chelate control to achieve higher regioselectivity remains an interesting challenge for further development of this reaction.
To enable detection of the protein-bound alkenes by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) we moved on to the linkage of the fluorescent 3-(dansylamino)phenylboronic acid to 4-OT R61C-1 (Table 1). Full conversion was observed by LC-MS, and the protein-bound fluorophore was visualized by fluorescence detection on SDS-PAGE (Figure 1 C). Furthermore, the same reaction was performed with the soluble fraction of lysates from RAW264.7 macrophages enriched with 4-OT R61C-1. We were pleased to find that 4-OT R61C-1 was the only fluorescently labeled protein (Figure 1 E and F). LC-MS analysis of the fluorescently labeled protein band from the gel in Figure 1 E demonstrated more than 90 % ligation of 4-OT R61C-1 (6924 Da), giving the fluorescently labeled product of 7248 Da (Figure 1 B). In order to reduce the contrast between the labeled band and the background, less 4-OT R61C-1 was added to the same cell lysate, and the oxidative Heck reaction was performed. Pleasingly, also in this experiment no fluorescence in the other proteins was observed (Figure 1 G and H).
Figure 1.
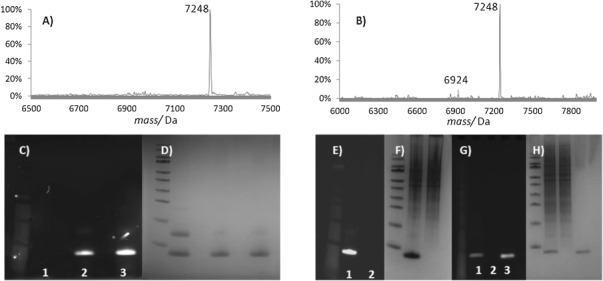
Fluorescent labeling of protein 4-OT R61C-1 with the aid of the oxidative Heck reaction. A) Mass spectrum demonstrating full conversion of pure 4-OT R61C-1. B) Mass spectrum demonstrating the conversion of 4-OT R61C-1 mixed with a complex protein mixture from cells (ratio 1:1). C) Fluorescence imaging on SDS-PAGE of labeled 4-OT R61C-1. 1) 5 μg of unlabeled protein 4-OT R61C (the dimer is also visible), and 2) 2 μg, and 3) 3 μg of labeled 4-OT R61C-1. D) Coomassie Brilliant Blue staining of C. E) Fluorescence imaging on SDS-PAGE of 4-OT R61C-1 labeled in the presence of a cell lysate (protein ratio 1:1). Reaction 1) in the presence of 4-OT R61C-1, and 2) in the absence of 4-OT R61C-1. F) Coomassie Brilliant Blue staining of E. G) Fluorescence imaging on SDS-PAGE of 4-OT R61C-1 labeled in the presence of a cell lysate (protein ratio 1:10). Reaction 1) in the presence of 4-OT R61C-1, 2) in the absence of 4-OT R61C-1, and 3) in the presence of an equivalent amount of fluorescently labeled 4-OT R61C after direct coupling with N-[2-(dansylamino)ethyl]maleimide. H) Coomassie staining of G.
In order to estimate the ligation yield, we loaded an equivalent amount of 4-OT R61C that was directly fluorescently labeled with N-[2-(dansylamino)ethyl]maleimide as a reference. The intensity of fluorescently labeled 4-OT R61C-1 produced by the oxidative Heck reaction (Figure 1 G, lane 1) was slightly lower than that of the reference (Figure 1 G, lane 3). This indicates that ligation at low alkene concentrations is qualitative rather than quantitative.
These experiments demonstrate that the oxidative Heck reaction is very selective and suitable for bio-orthogonal ligation to protein-bound alkenes even in the presence of a complex protein mixture from cells.
In conclusion, the oxidative Heck reaction has been added to the toolkit of bio-orthogonal protein ligations. Importantly, this ligation reaction runs to completion with protein-bound alkenes and proceeds under aerobic conditions. Control experiments demonstrate exclusive ligation to protein-bound alkenes and not to other protein functional groups. Pleasingly, this ligation works effectively in cell lysates and is selective for alkene-labeled proteins, which demonstrates the excellent performance of this ligation reaction in biological samples.
Experimental Section
See the Supporting Information.
Acknowledgments
This work was financially supported by a VIDI grant (016.122.302) to F.J.D. from the Netherlands Organization for Scientific Research (NWO).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Grammel M, Hang HC. Nat. Chem. Biol. 2013;9:475–484. doi: 10.1038/nchembio.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisastra R, Poelstra K, Bischoff R, Maarsingh H, Haisma HJ, Dekker FJ. ChemBioChem. 2011;12:2016–2020. doi: 10.1002/cbic.201100148. [DOI] [PubMed] [Google Scholar]
- 3.Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 4.Yang YY, Ascano JM, Hang HC. J. Am. Chem. Soc. 2010;132:3640–3641. doi: 10.1021/ja908871t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hein CD, Liu XM, Wang D. Pharm. Res. 2008;25:2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blobaum AL. Drug Metab. Dispos. 2006;34:1–7. doi: 10.1124/dmd.105.004747. [DOI] [PubMed] [Google Scholar]
- 7.Ekkebus R, van Kasteren SI, Kulathu Y, Scholten A, Berlin I, Geurink PP, de Jong A, Goerdayal S, Neefjes J, Heck AJR, Komander D, Ovaa H. J. Am. Chem. Soc. 2013;135:2867–2870. doi: 10.1021/ja309802n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hest JC, van Delft FL. ChemBioChem. 2011;12:1309–1312. doi: 10.1002/cbic.201100206. [DOI] [PubMed] [Google Scholar]
- 9.Hoyle CE, Bowman CN. Angew. Chem. 2010;122:1584–1617. [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49 [Google Scholar]
- 10.Lim RK, Lin Q. Acc. Chem. Res. 2011;44:828–839. doi: 10.1021/ar200021p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalker JM, Lin YA, Boutureira O, Davis BG. Chem. Commun. 2009:3714–3716. doi: 10.1039/b908004j. [DOI] [PubMed] [Google Scholar]
- 12.Chalker JM, Wood CS, Davis BG. J. Am. Chem. Soc. 2009;131:16346–16347. doi: 10.1021/ja907150m. [DOI] [PubMed] [Google Scholar]
- 13.Dumas A, Spicer CD, Gao Z, Takehana T, Lin YA, Yasukohchi T, Davis BG. Angew. Chem. 2013;125:4008–4013. doi: 10.1002/anie.201208626. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52 [Google Scholar]
- 14.Lin YA, Chalker JM, Davis BG. ChemBioChem. 2009;10:959–969. doi: 10.1002/cbic.200900002. [DOI] [PubMed] [Google Scholar]
- 15.Yoo KS, Yoon CH, Mishra RK, Jung YC, Yi SW, Jung KW. J. Am. Chem. Soc. 2006;128:16384–16393. doi: 10.1021/ja063710z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottumukkala L, Teichert JF, Heijnen D, Eisink N, van Dijk S, Ferrer C, van den Hoogenband A, Minnaard AJ. J. Org. Chem. 2011;76:3498–3501. doi: 10.1021/jo101942f. [DOI] [PubMed] [Google Scholar]
- 17.Werner EW, Sigman MS. J. Am. Chem. Soc. 2010;132:13981–13983. doi: 10.1021/ja1060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delcamp JH, Brucks AP, White MC. J. Am. Chem. Soc. 2008;130:11270–11271. doi: 10.1021/ja804120r. [DOI] [PubMed] [Google Scholar]
- 19.Werner EW, Sigman MS. J. Am. Chem. Soc. 2011;133:9692–9695. doi: 10.1021/ja203164p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor B, Czerwinksi RM, Johnson WH, Whitman CP, Hackert ML. Biochemistry. 1998;37:14692–14700. doi: 10.1021/bi981607j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


