Summary
Neocortical circuits are assembled from subtypes of glutamatergic excitatory and GABAergic inhibitory neurons with divergent anatomical and molecular signatures and unique physiological properties. Excitatory neurons derive from progenitors in the pallium, whereas inhibitory neurons originate from progenitors in the subpallium. Both classes of neurons subsequently migrate along well-defined routes to their final target area, where they integrate into common neuronal circuits. Recent findings show that neuronal diversity within the lineages of excitatory and inhibitory neurons is in part already established at the level of progenitor cells prior to migration. This poses challenges for our understanding of how radial units of interconnected excitatory and inhibitory neurons are assembled from progenitors that are spatially segregated and diverse in nature.
Introduction
The mammalian neocortex is subdivided into areas with specialized functions such as the somatosensory, motor and visual cortices. Despite this functional diversification, certain features are remarkably similar among different cortical areas. Most prominently, all cortical areas show a characteristic laminar appearance that is caused by the assembly of diverse subtypes of excitatory projection neurons and inhibitory interneurons into well-defined cell layers (Figure 1). Within the two main classes of neocortical neurons, numerous subclasses can be identified. Projection neurons use glutamate as their neurotransmitter, and can be classified into different subtypes by their distinct laminar position and projection patterns. For example, most layer VI projection neurons project to the thalamus, while layer V neurons connect to basal ganglia, midbrain, hindbrain and spinal cord. By contrast, layer IV spiny stellate neurons receive most of the inputs from the thalamus and project locally within the neocortex, while layer II and III projection neurons form connections within the cortical hemispheres and between them (Fig. 1). It is worth noting, however, that neurons with similar projection patterns are often dispersed over several cell layers and, conversely, that the same layer contains projection neurons with distinct molecular signatures, which together suggest a great diversity of cortical projection neurons [1,2]. This also holds true for GABAergic interneurons, which can be classified into nearly 30 different subtypes based on molecular, morphological and physiological criteria [3]. Certain subtypes of inhibitory neurons tend to populate specific neocortical cell layers, while others are dispersed more widely across multiple layers. For example, the cell bodies of Martinotti cells are preferentially located in cortical layers II, III, V and VI, while double bouquet cells are predominantly found in layers II and III. Basked cells, in contrast, are widely distributed throughout all neocortical cell layers except for layer I (Figure 1).
Fig. 1. Subtypes of glutamatergic excitatory and GABAergic inhibitory and their laminar distribution within the neocortex.
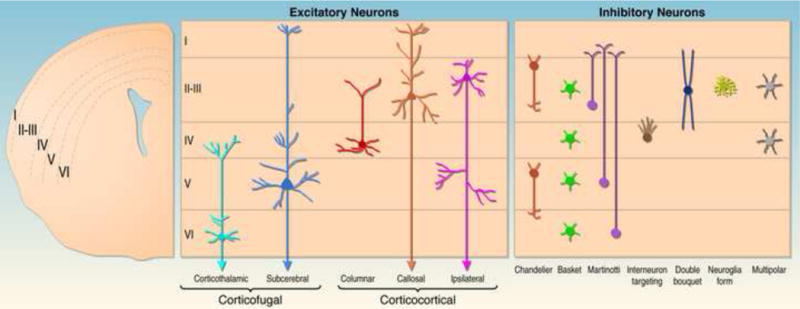
On the right a coronal hemisection of the brain is shown. Neocortical cell layers I–VI are indicated. The middle panel shows the position of major subtypes of excitatory neurons within neocortical cell layers and their projection pattern. Note that the diagram is a simplification outlining the major laminar distribution of neuronal subtypes. For example, the majority of callosal projection neurons is located in layers II and III, but a significant subset is also found in deeper layers. The left panel shows the position of several subtypes of inhibitory interneurons within neocortical cell layers.
One fundamental unresolved question in neurobiology concerns the mechanisms by which the different subtypes of excitatory and inhibitory neurons are generated from different progenitor cells and subsequently integrate into neuronal circuits. In the late 80s, Rakic and colleagues synthesized the available evidence from many researchers into the influential radial unit hypothesis [4]. This hypothesis states that despite the functional diversity of different neocortical areas, there is an underlying unifying theme. According to the hypothesis, the neocortex consists of ontogenetic columns that are generated from progenitor cells near the ventricle. In other words, neocortical progenitor cells are multipotent and give rise to any class of pyramidal cell [4]. The daughter cells of these progenitors migrate radially into the neocortical wall, such that neurons of the same ontogeny occupy progressively more superficial layers to form radial units with related function. These proliferative units form a proto-map that is subsequently refined by thalamic inputs to establish neocortical areas with distinct sizes, cellular compositions and functionalities [5]. Discoveries that have been made since the inception of this hypothesis require certain modifications to the initial idea. At the time when this radial unit hypothesis was formulated, it was not known that excitatory and inhibitory neurons are generated in different germinal zones [6]. It is now well established that projection neurons derive from progenitor cells in the ventricular zone (VZ) of the pallium from where they migrate radially into the emerging neocortical wall. In contrast, interneurons are born in the subpallium and migrate along tangential routes into the developing neocortex before shifting from tangential to radial migration to reach the different neocortical cell layers [7]. Furthermore, recent studies have revealed an unanticipated heterogeneity in the progenitor pools for excitatory and inhibitory neurons. Each radial unit therefore contains neurons that are derived from diverse progenitor pools in the pallium and subpallium. We will discuss here these recent findings on the mechanisms by which the different subtypes of excitatory and inhibitory interneurons are generated, and how this relates to the radial unit hypothesis.
Lineage specification of excitatory neurons
Subtypes of neocortical excitatory neurons are generated in a precise temporal order from progenitor cells in the neocortical ventricular zone (VZ) and subventricular zone (SVZ). The first neurons to be generated populate the preplate, which forms between the VZ and the meninges. The preplate is subsequently split into the marginal zone and the subplate by waves of radially migrating projection neurons. These cells generate the different neocortical cell layers in an inside-out manner, starting with deep layer neurons (layers VI and V), and followed by upper layer neurons (layers IV, III and II). Finally, the progenitor cells of pyramidal cells are thought to generate astrocytes [1,2]. Heterochronic transplantation studies in ferrets, in vitro experiments, and retrovirus lineage tracing studies suggested that the different subtypes of excitatory projection neurons are generated from a common progenitor whose fate potential changes over time to generate different subtypes of projection neurons in a defined temporal order [2]. Further support for this “progressive restriction model” came from the study of neurogenesis in invertebrates. Doe and colleagues demonstrated that the competence of Drosophila neuroblasts to generate different neuronal subtypes changes over time due to the sequential expression of different transcription factors [8]. Taken together, the findings in mammals and invertebrates provided support for an evolutionary conserved mechanism of temporal fate restriction of a common neural progenitor type.
Recent studies, however, show that the pallial VZ and SVZ contain a range of progenitor types that are morphologically and molecularly distinct (Fig. 2). Radial glial cells (RGCs) are one of the most prominent classes of neocortical progenitors [9–11]. The cell bodies of RGCs are located in the VZ, and they form apical and basal processes that are anchored at the ventricular surface and the meninges, respectively. RGCs self-renew to amplify the RGC pool, but they also undergo asymmetric cell division to generate neurons [10,11], or more commonly two morphologically distinct class of progenitors, the intermediate progenitors (IPCs) [12–14] and short neural progenitors (SNPs) [15–17] that populate the SVZ and VZ, respectively. In mice and rats, IPCs and SNPs primarily undergo symmetric cell divisions to produce pairs of neuron, although some IPCs can also undergo a limited number of additional symmetric divisions prior to generating neurons [12,18]. In the neocortex of primates and especially in humans, the SVZ is vastly expanded in size [19,20]. It contains an additional progenitor type that has been named basal RGC (bRGC) or outer SVZ radial glia (oRG) [21,22], as well as IPCs that can undergo several rounds of symmetric and asymmetric divisions [21,22]. oRGs resemble RGC in the VZ but where initially shown to lack an apical process that is anchored at the ventricle [21,22], and they are rarely found in the rodent brain [23,24]. Recent studies in macaques suggest that oRGs can be further subdivided into several morphologically distinct classes [25••]. The increased complexity of the progenitor pool and expansion of the SVZ in some species has been proposed to underlie the evolutionary expansion of the neocortex [26].
Fig. 2. Subtypes of progenitor cells in the developing neocortex.
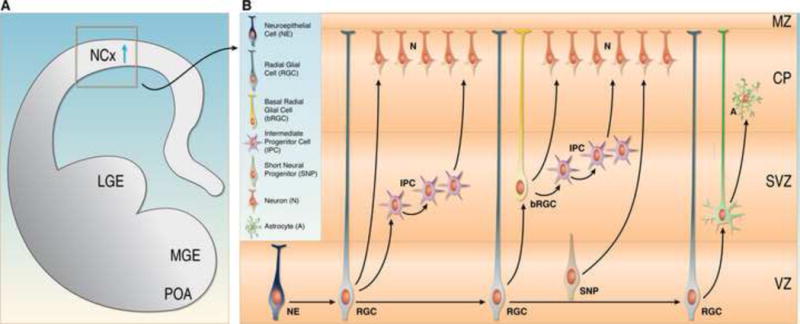
(A) Coronal hemisection of the developing brain. The neocortex (NCx), the lateral ganglionic eminence (LGE), the medial ganglionic eminence (MGE), and the preoptic area (POA) are indicated. The blue arrow shows the migration route of excitatory neurons from the ventricular zone into the developing cortical wall. (B) Enlargement of the region boxed in (A) indicating different progenitor types. Neuroepithelial cells (NE) are present early in development and give rise to radial glial cells (RGCs). RGCs self-renew and give rise to neurons (N). RGCs also generate intermediate progenitors (IPCs) and short neural progenitors (SNPs), which divide further to generate neurons. In addition, RGCs give rise to basal radial glial cells (bRGCs), which generate via IPs additional neurons. At the end of neurogenesis, RGCs and bRGCs transform into astrocyte progenitors, which then generate astrocytes (A).
Until recently, it has remained unclear whether the morphologically distinct progenitors identified in the neocortical germinal zone have different lineage potentials. Initially it was thought that IPCs in the SVZ, which are molecularly distinct from RGCs in the VZ, generate upper layer neurons, while deep layer neurons would only derive from progenitors in the VZ [27–29]. However, IPCs are also present throughout early stages of neocortical neurogenesis, and only 10% of all neocortical neurons are directly derived from RGCs [30]. Since IPCs are generated from RGCs [12–14], these two cell types likely represent different progenitor states along a developmental time line rather than separate fate-restricted lineages. Similarly, in vitro and in vivo lineage tracing experiments have shown that the vast majority of neocortical progenitor cells either generate neurons or glia [2]. This heterogeneity may reflect the co-existence of several pre-specified progenitors for neurons and glia but it is more commonly thought to reflect spatiotemporal differences caused by a developmental gradient.
The most conclusive evidence for the co-existence of neocortical progenitors with different lineage potentials has recently been derived from genetic lineage-tracing experiments. Some genes such as Cux2 are expressed at early developmental stages in subsets of progenitors in the VZ and SVZ and at later stages in subsets of excitatory neurons. For Cux2, these are the vast majority of the Satb2+ callosal projection neurons that are predominantly present in upper layers, and to a minor degree in deep layers V and VI [27,29]. Genetic fate mapping experiments were conducted by crossing mice containing Cre-inducible lineage tracers with mice expressing Cre or tamoxifen-inducible CreERT2 from the Cux2 locus [31••]. The findings from these studies demonstrated that a subset of Cux2+ RGCs are specified to generate Satb2+ callosal projection neurons in upper and deep layers of the neocortex, whereas lower-layer neurons that project to subcortical targets are produced from RGCs of the Cux2− lineage. In addition, subpopulations of interneurons that migrate into the neocortex along tangential routes express Cux2 [27,31••]. Importantly, Cux2+ progenitors for neocortical projection neurons are primarily proliferative during phases of lower layer neurogenesis and start to generate significant numbers of upper layer neurons only at later developmental time points. When the progenitors were forced to prematurely leave the cell cycle, they prematurely generated upper layer neurons, providing further evidence that the progenitors are pre-specified to generate Satb2+ neurons. Taken together, these findings demonstrate the existence of Cux2+ RGC cells that are restricted in their fate potential even before the onset of neurogenesis to generate Satb2+ callosal projections neurons that for the most part reside in upper neocortical cell layers and to a minor part in deep layers [31••].
A recent study challenged the observation that Cux2+ RGCs are specified to generate Satb2+ callosal projection neurons. Using the same tamoxifen inducible Cux2-CreERT2 mouse line used previously by Franco and colleagues [31••], the authors described that neurons derived from the Cux2 lineage occupy both upper and deep neocortical layers at P0 [32]. However, this result is not unexpected, because the radial migration of projection neurons is not complete at birth, and indeed many cells within the Cux2-lineage still had the morphology of radially migrating neurons at P0 [32]. In addition, Cux2-Cre traces Satb2+ cells both in deep and upper neocortical cell layers, and it marks a subset of interneurons [31••,32]. Therefore, labeling of neurons in deep cortical cell layers by Cux2-CreERT2 lineage tracing is expected and especially prominent during developmental time points. To further support the conclusion that Cux2+ progenitors generate both callosal and subcerebral projection neurons, the authors used molecular markers and showed that some Cux2-derived projection neurons in deep layers express at P0 markers such as CTIP2 [32], a gene that is strongly expressed in layer V neurons that project to subcerebral targets [33]. However, during early stages of differentiation, neurons frequently co-express genes that at later stages preferentially label subtypes of projection neurons with different layers position and projection patterns [34–37]. Thus, the apparent discrepancy between the two studies might be largely explained by the fact that Guo and colleagues [32] analyzed maker expression and neuronal position prior to the establishment of the mature neocortical cell layers. Future studies should contribute to clarify this important issue.
In summary, the new findings show that neuronal subtype specification occurs at least in part already at the level of progenitor cells, where some progenitor cells are specified to generate neurons for upper layers and deep layers, respectively. However, upper and deep layers contain various neuronal subtypes. Further specification events are therefore necessary to generate neuronal diversity within these two progenitor lineages. Some of this diversity is apparently established at the level of postmitotic neurons [1]. For example, the transcription factors Fezf2, Ctip2, Tbr1 and Satb2 show layer specific expression in differentiated cortical neurons. Fezf2 is expressed at high levels in layer V neurons and at lower levels in layer VI neurons, and also in a mosaic in the cortical VZ. Fezf2 is required for the specification of subcerebral projection neurons of layer V [38,39], while Tbr1, which is expressed in layer VI neurons is required to specify corticothalamic projection neurons [36,40,41]. In contrast, Satb2 is expressed in neurons that project across the corpus callosum and required for their specification [37]. Ctip2 seems to play a central role in controlling the specification program of many cortical projection neurons since it is activated by and acts downstream of Fezf2 during the specification of subcerebral projection neurons [33]. Conversely, Satb2 represses the expression of Ctip2 during the differentiation of callosal projection neurons [34].
Collectively, these findings indicate that lineage diversification occurs both at the level of progenitors and postmitotic neurons, which calls for a revision of the unitary “progressive restriction model” of neocortical neurogenesis. A new model that incorporates these new findings has been proposed and termed the “sequential progenitor-diversification model” [2]. This model involves three steps of cell-type diversification: (i) the specification of lineage-restricted progenitors for lower and upper layer neurons; (ii) further diversification of these progenitors at the level of progenitors and postmitotic neurons to generate subtypes of upper and lower layer neurons; (iii) the final execution of the differentiation program at the stage of postmitotic neurons.
Several important questions regarding the mechanisms of progenitor diversification remain. First, what is the origin of Cux2- and Cux2+ progenitors? Are they directly generated from neuroepithelial cells of the neural tube in early developing embryos and then maintained as mutually distinct progenitor pools (Fig. 3; Model 1: Segregated Lineages)? Alternatively, neuroepithelial cells might first generate a Cux2− lineage that is highly diverse in nature. Subsets of Cux2− cells might self-renew to amplify the numbers of Cux2− cells, others might divide to generate Cux2+ progenitors (and other progenitor types?), and still others might generate IPCs or neurons (Fig. 3; Model 2: Nested Lineages). Of course, these are only two extremes and other models can be envisioned. While Cux2 is a clear lineage marker for callosal projection neurons, it will be important to search for further markers that allow for the study of the Cux2− lineage in greater detail. Diversity in the Cux2− lineage could also be revealed by mosaic analysis using modified retroviruses or the MADM strategy [42]. If the Cux2− lineage contains progenitors with different fate potentials, mosaic analysis should reveal clones that span all cortical layers in addition to clones that are destined for upper layers and deep layers only. The relative proportion of these progenitors might shift over developmental time. Second, by what mechanisms do Cux2− and Cux2+ cells generate different subtypes of upper- and deep-layer neurons? As outlined above, specification events occur at the level of postmitotic neurons, but this is likely not the only mechanism and further diversification within the pool of progenitors might occur. Two scenarios might apply. Cux2− and/or Cux2+ cells might generate additional progenitor subtypes that co-exist at the same developmental time point but differentiate on lineage-specific time scales. Alternatively, Cux2− and Cux2+ cells might be progressively restricted in their potential to generate different subtypes of deep and upper layer neurons in temporal order as predicted by the original “progressive restriction model”.
Fig. 3. Models for the generation of subtypes of excitatory projection neurons.
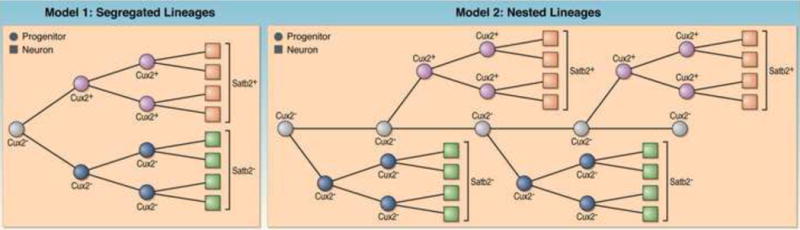
Two models are depicted that can explain how distinct progenitors generate Satb2+ callosal projection neurons and Satb2− subcerebral projection neurons. These are two extreme models and other models can be envisioned. Model 1: Segregated Lineages. An early progenitor generates two progenitor subtypes. These progenitors self-renew to amplify the progenitor pool and establish two independent progenitor lineages. One of the progenitor lineages is specified to produce Satb2− neurons that mostly populate deep layers, while the second progenitor lineage is specified to produce Satb2+ neurons that largely populate layers II–III but also reside in deeper layers. Model 2: Nested Lineages. A multipotent progenitor persists for an extended period of time and generates two progenitor subtypes. These progenitor subtypes proliferate to expand the two independent progenitor lineages. One of these progenitor subtypes generates Satb2+ neurons and the second one produces Satb2− neurons.
Lineage specification of inhibitory neurons
The different classes of neocortical interneurons are generated in three spatially segregated regions of the subpallium: the medial and caudal ganglionic eminences (MGE and CGE, respectively), and the preoptic area (POA). This later region is anatomically contiguous with the MGE, with which it shares many features. Most neocortical interneurons derive from the ganglionic eminences, and a minor fraction (<10%) from the POA [43]. The MGE is the origin of most neocortical interneurons (~60%), most notably fast spiking chandelier cells and basket cells, many of which express the calcium binding protein Parvalbumin (PV+), and Somatostatin-containing (SST+) interneurons, most of which have the electrophysiological properties and typical morphology of Martinotti cells. The CGE produces interneurons with bipolar or double-bouquet morphologies, which frequently express Calretinin (CR+) and/or Vasointestinal peptide (VIP+), and rapidly adapting interneurons with multipolar morphologies. Finally, the POA gives rise to a small but diverse group of interneurons including multipolar NPY+ interneurons, PV+ basket cells and SST+ interneurons. CGE and POA-derived interneurons are found in all cortical layers, while MGE-derived interneurons are excluded from layer I. CGE-derived interneurons are particularly abundant in layers IV, III and II of the neocortex. So, in contrast to the progenitors of projection neurons, which are homogenously distributed throughout the entire pallium, the progenitor cells of neocortical interneurons are spatially segregated within the subpallium [43].
Progenitor cells in the subpallium are also organized into distinct VZ and SVZ compartments. Many progenitor cells in the VZ of the ganglionic eminences have the typical features of RGCs: they have a bipolar morphology with basal and apical processes, undergo interkinetic movements and divide in the apical surface, and express markers such as GLAST and BLBP (Fig. 4A,B) [44•, 45••,46]. In addition, mitotically active IPCs with a multipolar morphology have been identified within the SVZ of the ganglionic eminences. As in the neocortex, these cells seem to arise from the asymmetric division of RGCs [45••]. Interestingly, recent studies have revealed the existence of additional progenitor cells within the ganglionic eminences. For instance, the VZ of the LGE also contains “short neural precursor cells” (SNPs) lacking a long basal process [44•], equivalent to SNPs described in the pallium [17]. In addition, the ganglionic eminences are home to many subapical progenitor cells (SAPs), which are morphologically similar to RGCs but divide at subapical positions (Fig. 4A,B) [44•]. Non-apical divisions (SAPs and IPCs) represent 60–70% of the mitosis in the ganglionic eminences, which indicate that proliferative divisions and clonal expansion is central for the generation of interneuron lineages.
Fig. 4. Generation of subtypes of interneurons.
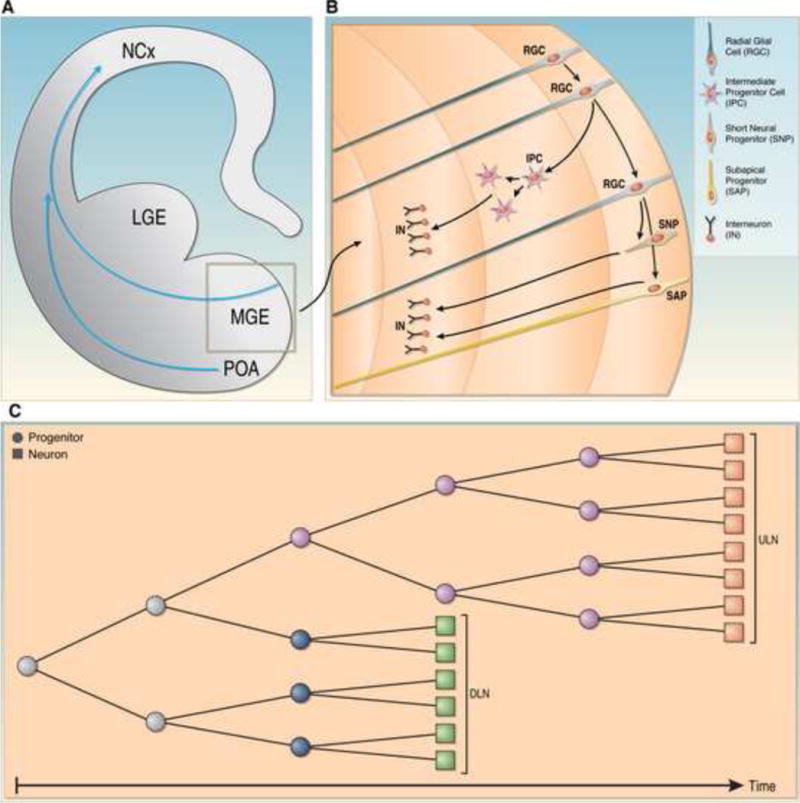
(A) Coronal hemisection of the developing brain. The blue arrow indicates the migration route of interneurons from the medial ganglionic eminence (MGE) to the neocortex (NCx). The lateral ganglionic eminence (LGE) is also depicted. (B) Enlargement of the region boxed in (A) indicating different progenitor types. Radial glial cells (RGCs) that were derived from neuroepithelial cells self renew and generate intermediate progenitors (IPCs), short neural progenitors (SNPs) and subapical progenitors (SAPs), all of which further proliferate to generate interneurons (INs). (C) Model for the generation of interneurons that populate deep layers (DLN) and upper layers (ULN) of the neocortex. In this model, distinct progenitors exist for DLN and ULN but their relative abundance changes over developmental time. Note that most of the data regarding the diversity of the progenitor pool for interneurons were obtained with studies on the LGE, which is not depicted here.
It is presently unclear whether morphologically distinct progenitor cells in the ganglionic eminences have different lineage potentials. The classical view favors a “progressive restriction model” for the generation of neocortical interneurons, primarily because MGE-derived interneurons are temporally produced in an inside-out manner, with deep layer interneurons (layers VI and V) generated first and followed by upper layer interneurons (layers IV, III and II). However, methodological limitations until now have prevented experimental confirmation of this hypothesis. In contrast to neocortical projection neurons, whose spatial relationships with progenitor cells are maintained during development, interneurons disperse tangentially through the neocortex before adopting their final position. This poses a fundamental problem for establishing clonal relationships, because interneurons from different lineages would inevitably intermingle in the neocortex. In addition, analysis of interneuron lineages requires progenitor specificity, because different classes of interneurons derive from distinct progenitor pools. Brown and colleagues (2011) [45••] overcame this problem by performing clonal analyses with a mammalian retroviral vector pseudotyped with the ASLV-A envelope glycoprotein (EnvA), which they injected in mice in which only MGE/POA progenitor cells express the avian tumor virus A (TVA) receptor [47]. Using this approach, they discovered that clonally related MGE-derived interneurons tend to cluster in the neocortex [45••]. This observation has been subsequently confirmed for distinct classes of neocortical interneurons using a similar approach based on the use of conditional retroviruses whose activity depends on the expression of Cre [48••].
The analysis of the distribution of clonally related interneurons, however, revealed some apparent discrepancies between both studies. Brown and colleagues reported that interneurons derived from MGE/POA progenitors infected at E12.5 primarily adopt a vertical organization in the neocortex, consistent with a “progressive restriction model” in which a single progenitor cell would give rise to interneurons adopting progressively more superficial positions within the neocortex as time go by [45••]. In contrast, Ciceri and colleagues found that infection of MGE/POA progenitor cells at E11.5 and E14.5 labels clusters of interneurons that are largely segregated into deep (VI and V) and superficial (IV, III and II) layers, respectively [48••]. Interestingly, Brown and colleagues also found that a fraction of clones labeled at E12.5 give rises to interneuron clusters that are largely confined to one or two adjacent layers of the neocortex [45••]. These observations suggest that the subpallium may contain neocortical interneuron progenitors with different lineage potentials, at least in relation to the laminar distribution of their progeny. How can these studies be reconciled? One possibility is that the subpallium may contain several progenitor cells whose relative abundance varies during development (Fig. 4C). Interneuron precursors committed to produce primarily infragranular cells might be particularly abundant at early stages of neurogenesis, while other progenitor cells may only become neurogenic at later stages. Moreover, the “sequential progenitor-diversification model” may also apply to neocortical interneuron, because interneuron lineages are often organized in vertical arrays even though they are typically restricted to deep or superficial layers [45••,48••].
It remains to be elucidated whether the different lineage potential of interneuron precursors correspond to morphologically distinct progenitor cells. It has been suggested that SNPs, SAPs and IPCs all derive from RGCs [44•], which would imply that progenitor diversity must exist within the population of RGCs. Another intriguing question relates to the mechanism through which clonally related interneurons cluster in close proximity within the neocortex. Most interneurons within laminar-restricted clusters are synchronously generated [48••], which could contribute to explain their final distribution (i.e. interneurons would share guidance cues). This observation is also consistent with the great abundance of proliferative divisions in the ganglionic eminences, which would contribute to expand interneuron lineages prior to migration.
Matching excitatory and inhibitory neurons
Functional circuits in the neocortex rely on the assembly of excitatory and inhibitory neurons, and so an intriguing question that remains to be answered is how interneurons adopt their final position in the neocortex and how this may relate to the existence of different lineages of projection neurons. MGE-derived interneurons distribute through the neocortex following an inside-out pattern that roughly matches the positioning of projection neurons [49–51], which led to the original suggestion that interneurons may use similar mechanisms than pyramidal cells to reach their layer [52,53]. However, several lines of evidence suggest that interneurons converge into specific layers of the neocortex following an alternative mechanism. First, interneurons invade their corresponding layer well after projection neurons have settled [54,55]. Second, disruption of the normal layering of projection neurons affects the distribution of MGE-derived interneurons [55, 56•, 57]. These studies suggest that projection neurons directly influence the distribution of MGE-derived interneurons, probably through the production of layer-specific signals [58]. It is presently unclear whether this model also applies to CGE-derived interneurons, which distribute preferentially through superficial layers of the neocortex independently of their birthdate [59,60].
The recent lineage analyses for projection neurons and interneurons provide a new perspective to the “chemical matching” hypothesis for the development of excitatory and inhibitory cell assemblies. One possibility is that Cux2− and Cux2+ lineages are specified to provide different signals for interneurons populating deep and superficial layers of the neocortex. Since these interneurons seem to derive from largely independent lineages, responsiveness to the appropriate cues might also be specified at the level of progenitor cells. In other words, MGE-derived interneurons might be generated to primarily mirror the laminar organization of projection neuron lineages.
Implications for the radial unit model
The discovery of spatially distinct progenitor domains with several subtypes of progenitors for excitatory and inhibitory neurons reveals a complexity in neurogenesis that was not anticipated when the radial unit hypothesis was formulated. It is consistent with the results from subsequent retroviral lineage-tracing studies, which have shown that some clones appear spatially restricted as clusters while other clones are more dispersed [61,62]. The new findings also raise fundamental questions as to the mechanisms by which radial units are constructed and how cells within these units establish specific synaptic connections to form cortical microcircuits. Clearly, multiple cell types from several sources need to be integrated into a functional unit suggesting that active exchange of signals between the neurons that form these units likely occurs. Such signal exchange will likely be critical to coordinate the preferential establishment of synaptic connections between clonally related projection neurons [63] and between projection neurons and interneurons. The observation that projection neurons directly affect the behavior and distribution of MGE-derived interneurons provides one example of a possible mechanism, where different neuronal subtypes might express molecular address codes that determine interaction specificity between them. In addition, molecular signals likely control the tangential spread of neurons. In support of this model, ephrin-A/EphA signaling has recently been shown to promote the tangential intermingling of projection neurons during their radial migration [64], while ephrin-B1 restricts tangential migration [65•]. Intriguingly, in the visual system ephrin-A forward and ephrin-B reverse signaling cooperate to control the topographic mapping of axonal projections [66], suggesting similarities in signaling mechanism that are used to construct neocortical circuits and topographic maps. Finally, neurons have to choose between neurons with similar molecular signatures suggesting that competitive interactions between them shape radial units where neurons that “fire together, wire together”. Notably, in the barrel cortex, the laminar and columnar development depends on thalamocortical neurotransmission [67•], indicating that aspects of layer-dependent patterning of neocortical cytoarchitecture depend also on external inputs. Thalamic inputs will likely also be important to establish cortical areas with distinct sizes, cellular compositions and functionalities as originally proposed by the radial unit hypothesis.
Highlights.
-
–
Fate-restricted progenitors in the pallium produce subtypes of excitatory projection neurons
-
–
Fate-restricted progenitors in the subpallium generate subtypes of interneurons
-
–
Pallium and subpallium may contain subsets of multipotent cortical progenitors
-
–
Radial units contain neurons that are generated from diverse and spatially segregated progenitors
Acknowledgments
We are grateful to members of the Marín and Müller labs for discussions on this subject. We thank Cristina Gil-Sanz for comments on the manuscript. We apologize to colleagues whose work is not cited in this review. Regrettably, space was too limited to cite all significant original articles. Our work on this topic is supported by grants from the Spanish Ministry of Economy and Competiveness (MINECO) SAF2011–28845 and CONSOLIDER CSD2007–00023, and the European Research Council (ERC-2011-AdG 293683) to OM, and the NIH (NS046456; HD070494), the Dorris Neuroscience Center, the Skaggs Institute for Chemical Biology, and the California Institute of Regenerative Medicine to UM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron. 2013;77:19–34. doi: 10.1016/j.neuron.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 5.Rakic P. The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev. 2007;55:204–219. doi: 10.1016/j.brainresrev.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 7.Marín O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci. 2013;38:2019–2029. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- 8.Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- 9.Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 10.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 11.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 12.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 13.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 14.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyler WA, Haydar TF. Multiplex genetic fate mapping reveals a novel route of neocortical neurogenesis, which is altered in the Ts65Dn mouse model of Down syndrome. J Neurosci. 2013;33:5106–5119. doi: 10.1523/JNEUROSCI.5380-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu SX, Goebbels S, Nakamura K, Nakamura K, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 22.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS One. 2012;7:e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Betizeau M, Cortay V, Dorothee P, Pfister S, Gautier E, Bellemin-Menard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80:442–467. doi: 10.1016/j.neuron.2013.09.032. This study provides evidence that in the macaque, the outer subventricular zone contains at least four distinct radial glia-like morphotypes, and that cortical neurons are generated through complex lineages by a mosaic of progenitors. [DOI] [PubMed] [Google Scholar]
- 26.Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 27.Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 28.Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- 29.Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 30.Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Muller U. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. This study challenges classical views of cortical neurogenesis by providing evidence that the neocortex may contain different classes of progenitor cells that are specialized in generating infra- and supragranular projection neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JL, Chen B. Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron. 2013;80:1167–1174. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan K, Leone DP, Bateson RK, Dobreva G, Kohwi Y, Kohwi-Shigematsu T, Grosschedl R, McConnell SK. A network of genetic repression and derepression specifies projection fates in the developing neocortex. Proc Natl Acad Sci USA. 2012;109:19071–19078. doi: 10.1073/pnas.1216793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, Niquille M, Yoshida M, Yoshida Y, Lebrand C, Mann F, et al. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, Bammler TK, Rubenstein JL, Hevner RF. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han W, Kwan KY, Shim S, Lam MM, Shin Y, Xu X, Zhu Y, Li M, Sestan N. TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. Proc Natl Acad Sci USA. 2011;108:3041–3046. doi: 10.1073/pnas.1016723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, Rubenstein JL, Chen B. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 2011;31:549–564. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–2141. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- 44•.Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun. 2013;4:2125. doi: 10.1038/ncomms3125. A thorough analysis of progenitor cell diversity in the subpallium identifies subapical progenitor cells in the ganglionic eminences of several mammalian species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. This important study addresses for the first time the spatial organization of cortical interneuron lineages. The findings suggest that sibling interneurons have a strong tendency to cluster in the neocortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halliday AL, Cepko CL. Generation and migration of cells in the developing striatum. Neuron. 1992;9:15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- 47.Beier KT, Samson ME, Matsuda T, Cepko CL. Conditional expression of the TVA receptor allows clonal analysis of descendents from Cre-expressing progenitor cells. Dev Biol. 2011;353:309–320. doi: 10.1016/j.ydbio.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marín O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. This study reinforces the idea that interneuron lineages have a strong tendency to cluster in the neocortex, as shown in [45••]. In addition, this study suggests that different lineages might generate infra- and supragranular interneurons. [DOI] [PubMed] [Google Scholar]
- 49.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairén A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- 51.Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- 52.Hammond V, So E, Gunnersen J, Valcanis H, Kalloniatis M, Tan SS. Layer positioning of late-born cortical interneurons is dependent on Reelin but not p35 signaling. J Neurosci. 2006;26:1646–1655. doi: 10.1523/JNEUROSCI.3651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pla R, Borrell V, Flames N, Marín O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. In line with previous work [55, 57], this study provides direct evidence that at least some classes of cortical interneurons adopt their final laminar distribution in response to cues provided by pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 58.Bartolini G, Ciceri G, Marin O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79:849–864. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- 62.Walsh C, Cepko CL. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993;362:632–635. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- 63.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Dimidschstein J, Passante L, Dufour A, van den Ameele J, Tiberi L, Hrechdakian T, Adams R, Klein R, Lie DC, Jossin Y, et al. Ephrin-b1 controls the columnar distribution of cortical pyramidal neurons by restricting their tangential migration. Neuron. 2013;79:1123–1135. doi: 10.1016/j.neuron.2013.07.015. Together with [64], this study provides evidence that ephrin signaling restricts the tangential motility of pyramidal neurons and contributes to the formation of ontogenic columns in the neocortex. [DOI] [PubMed] [Google Scholar]
- 66.Clandinin TR, Feldheim DA. Making a visual map: mechanisms and molecules. Curr Opin Neurobiol. 2009;19:174–180. doi: 10.1016/j.conb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79:970–986. doi: 10.1016/j.neuron.2013.06.043. This study demonstrates that glutamatergic synaptic transmission from thalamocortical neurons is required for the development of the laminar organization of the somatosensory cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]


