Abstract
The main drivers of acute inflammation are macrophages, which are known to have receptors for catecholamines. Based on their function, macrophages are broadly categorized as having either M1 (proinflammatory) or M2 phenotypes (anti-inflammatory). In this study, we investigated catecholamine-induced alterations in the phenotype of activated macrophages. In the presence of lipopolysaccharide (LPS), mouse peritoneal macrophages acquired an M1 phenotype. However, the copresence of LPS and either epinephrine or norepinephrine resulted in a strong M2 phenotype including high levels of arginase-1 and interleukin-10, and a reduced expression of M1 markers. Furthermore, epinephrine enhanced macrophage phagocytosis and promoted type 2 T-cell responses in vitro, which are known features of M2 macrophages. Analysis of M2 subtype-specific markers indicated that LPS and catecholamine-cotreated macrophages were not alternatively activated but were rather of the regulatory macrophage subtype. Interestingly, catecholamines signaled through the β2-adrenergic receptor but not the canonical cAMP/protein kinase A signaling pathway. Instead, the M2 pathway required an intact phosphoinositol 3-kinase pathway. Blockade of the β2-adrenergic receptor reduced survival and enhanced injury in mouse models of endotoxemia and LPS-induced acute lung injury, respectively. These results demonstrate a role for the β2-adrenergic receptor in promoting the M2 macrophage phenotype.
Key Words: Catecholamines, Cytokines, Macrophages, Inflammation
Introduction
Macrophages are a critical cell type for regulating inflammation. Activated macrophages can be broadly categorized into M1 or M2 phenotypes based on their phenotype and function. The M1 phenotype is elicited by the activation of macrophages with interferon (IFN)-γ or microbial products [e.g. lipopolysaccharide (LPS)], as well as other agonists. M1 macrophages are generally characterized by a high production of proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-12, and inflammatory chemokines, and they are important activators of Th1 type immune responses. M1 macrophages also produce high levels of antimicrobial reactive oxygen species and nitrogen intermediates [for reviews, see [1, 2, 3]].
M2 macrophages are an assorted collection of macrophage phenotypes that have been categorized as ‘not classically activated M1’ [4]. M2 macrophages can be elicited by exposure to IL-4, IL-13, or IL-10, as well as glucocorticoids [4]. In general, M2 macrophages share a phenotype that is characterized by a low production of proinflammatory cytokines/chemokines, the high expression of growth factors, and the promotion of Th2-type immune responses [4, 5]. M2 macrophages express high levels of arginase-1 which competes with inducible nitric oxide synthase (iNOS) for the same substrate, L-arginine [6, 7]. iNOS-mediated synthesis of NO from L-arginine is a well-described pathway for inflammatory (M1) macrophages [8]. The alternative arginine pathway, mediated by arginase-1, orients L-arginine conversion towards ornithine, proline, and polyamine and hence towards the promotion of cell growth and/or fibrosis [9]. Thus, the high expression of iNOS and arginase-1 helps to define M1 and M2 macrophages, respectively.
M2 macrophages have been categorized further into two phenotypes with both common and distinct characteristics [reviewed in [1]]. Alternatively activated macrophages (designated the M2a phenotype by some groups) [10] are elicited by exposure to IL-4 and IL-13 and can be identified by specific surface markers (e.g. mannose receptor) or products [e.g. resistin-like alpha (Retnla/FIZZ) or chitinase 3-like 3 (Ym1)] [10, 11]. Regulatory macrophages are generated by the activation of Fc gamma receptors (M2b designation) [12] or by exposure to glucocorticoids or IL-10 (M2c designation). Regulatory macrophages are distinct from alternatively activated macrophages because of their robust production of IL-10 and the lack of specific markers of alternatively activated macrophages, even though they similarly promote Th2 type immune responses. Therefore, M2 macrophages can be classified into distinct groups with etiological and functional differences.
Mediators of the neuroendocrine system (e.g. catecholamines) have been shown to modify inflammatory responses. On a cellular level, adrenergic receptors can modify TLR-activated cytokine production by macrophages. In general, β-adrenergic receptor agonists have been demonstrated to reduce inflammatory cytokine production in vitro [13, 14, 15, 16]. In addition, adrenergic receptors modulate inflammation in vivo. Specifically, we have previously demonstrated that the activation of β2-adrenergic receptors by synthetic agents (albuterol or formoterol) reduced the presence of inflammatory cytokines in bronchoalveolar lavage fluid (BALF) during experimental acute lung injury (ALI) [15]. This evidence led us to hypothesize that activation of the β2-adrenergic receptor on macrophages not only inhibits the M1 macrophage phenotype but also promotes the M2 macrophage phenotype.
In this report, we demonstrate that catecholamines promote the M2 macrophage phenotype through the β2-adrenergic receptor. While LPS-activated macrophages had a strong M1 phenotype, the copresence of LPS and catecholamines resulted in an anti-inflammatory M2 macrophage phenotype compared to LPS treatment alone. M2 macrophages induced by the copresence of LPS and catecholamines were of the regulatory macrophage phenotype (M2b/c). In vivo, blockade of the β2-adrenergic receptor during endotoxemia resulted in increased mortality, demonstrating a protective role for catecholamines in this model. Furthermore, the adoptive transfer of relatively few LPS/catecholamine-cotreated macrophages was protective during lethal endotoxemia. During LPS-induced ALI, blockade of the β2-adrenergic receptor resulted in increased inflammatory cytokine production, polymorphonuclear neutrophil infiltration, and exacerbated injury. Taken together, these results demonstrate a role for β2-adrenergic receptor activation by catecholamines in promoting the M2 regulatory macrophage phenotype.
Materials and Methods
Animals
All procedures were performed in accordance with US National Institutes of Health guidelines and were approved by the University of Michigan Committee for the Use and Care of Animals. Male age-matched C57BL/6, BALB/c, or DO11.10 (BALB/c background) mice were purchased from Jackson Laboratories (Bar Harbor, Me., USA). All experiments were performed on C57BL/6 mice unless otherwise stated. All animals were housed under specific pathogen-free conditions with free access to food and water.
Materials
Epinephrine, norepinephrine, ICI 118,551 (all from Sigma, St. Louis, Mo., USA), 2′5′-dideoxyadenosine (2′5′-DDA; Santa Cruz Biotechnology, Santa Cruz, Calif., USA), SQ22536 (Santa Cruz Biotechnology), PKA inhibitor 14-22 amide (EMD Millipore, Billerica, Mass., USA), and wortmannin (Santa Cruz Biotechnology) were all used at the concentrations listed in the legends to figures 1, 2, 3, 4, 5. LPS (Escherichia coli o111:B4) was purchased from Sigma. α1- (prazosin; Sigma), α2- (RX821002; Tocris, Bristol, UK), β1- (betaxolol; Sigma), and β3- (SR59230A; Sigma) adrenergic receptor antagonists were also used in this studies. The optimal concentrations for these reagents were determined in preliminary experiments (data not shown). AlexaFluor647 anti-mouse CD206 was from BioLegend (San Diego, Calif., USA).
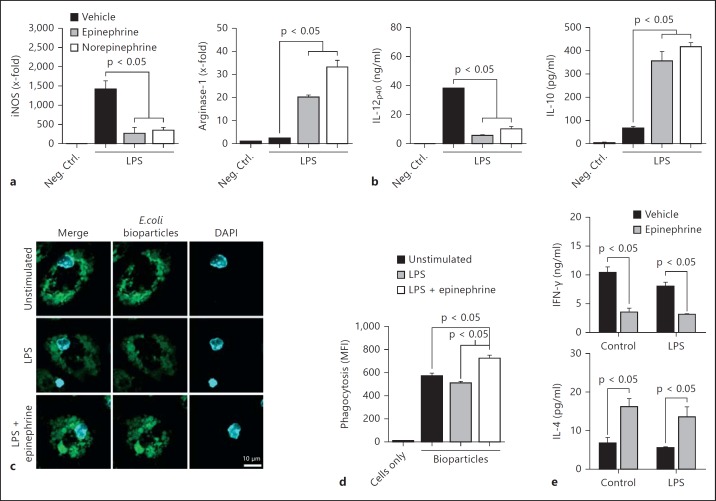
Fig. 1.
Catecholamines promoted the M2 macrophage activation phenotype. a-d Mouse peritoneal macrophages were incubated with LPS (100 ng/ml) in the absence or copresence of epinephrine (1 μM) or norepinephrine (1 μM) for 18 h. a Relative expression of mRNA transcripts for iNOS and arginase-1. b Culture supernatant levels of IL-12p40 and IL-10 determined by ELISA after 18 h. c Confocal microscopic analysis of macrophage phagocytosis of fluorescent E. coli bioparticles. d Flow cytometric analysis of the phagocytosis of fluorescent E. coli bioparticles. e Levels of IFN-γ or IL-4 in DO11.10 transgenic lymphocyte/macrophage culture supernatants. Macrophages were incubated in the presence or absence of LPS (100 ng/ml) and/or epinephrine (1 μM) for 1 h. Cells were washed, and 150 μg/ml ovalbumin was added for 30 min. Then, peripheral lymph node lymphocytes were added, and cell-free culture supernatants were harvested after 84 h. Levels of cytokines were determined by ELISA. MFI = Mean fluorescence intensity; Neg. Ctrl. = negative control.
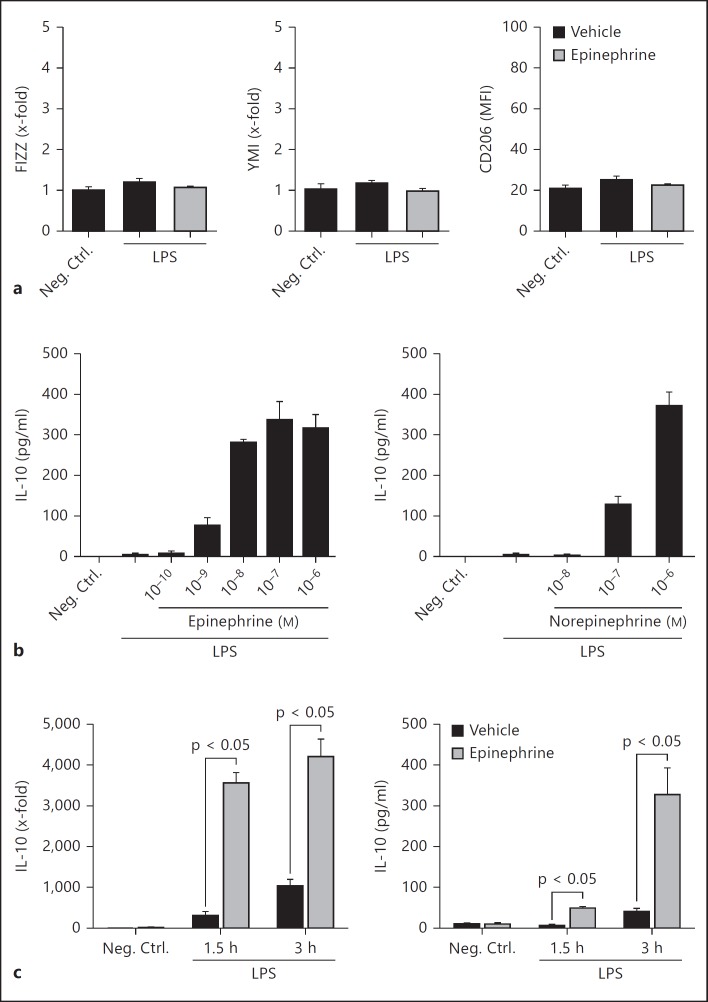
Fig. 2.
Catecholamines induced the regulatory macrophage M2 phenotype. a Macrophages were treated with LPS (100 ng/ml) in the presence or absence of epinephrine (1 μM) for 18 h. The levels of FIZZ and YM1 (mRNA, RT-PCR) and CD206 (surface mean fluorescence intensity, flow cytometry) were determined. b Dose-response curve of epinephrine (left) or norepinephrine (right) in the presence of LPS (100 ng/ml). The levels of IL-10 in culture supernatants after 4 h were determined by ELISA. c Macrophages were treated with LPS (100 ng/ml) in the absence or copresence of epinephrine (1 μM). IL-10 mRNA (left) and protein in culture supernatants (right) were determined at different time points following stimulation. Neg. Ctrl. = Negative control.
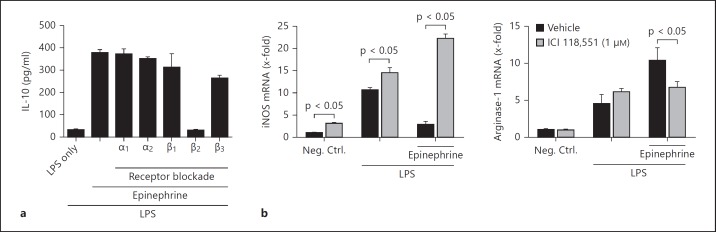
Fig. 3.
Epinephrine signals through the β2-adrenergic receptor. a Macrophages were incubated in the presence or absence of LPS (100 ng/ml), epinephrine (1 μM), and/or adrenergic receptor antagonists (1 μM each). The levels of IL-10 in culture supernatants were determined after 4 h. b Macrophages were incubated in the presence or absence of LPS (100 ng/ml), epinephrine (1 μM), and/or β2-adrenergic receptor antagonist ICI 118,551 for 18 h. The relative expression of mRNA transcripts for iNOS and arginase-1 was determined by RT-PCR. Neg. Ctrl. = Negative control.
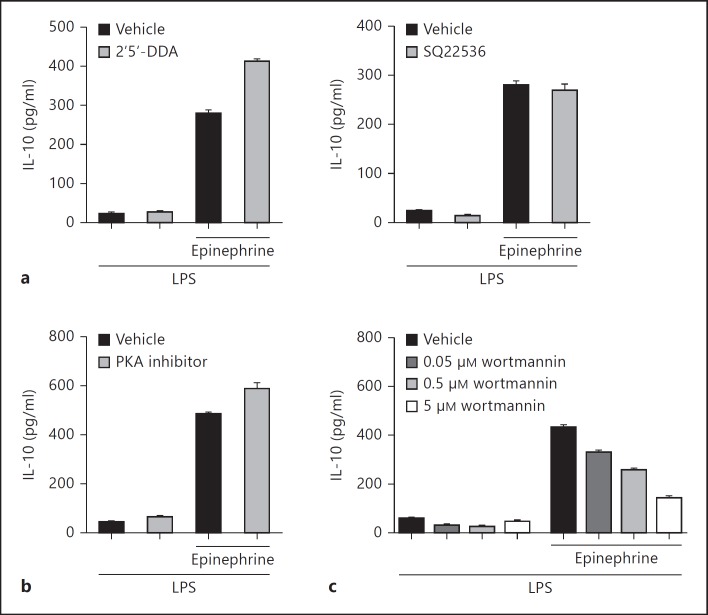
Fig. 4.
Catecholamine-enhanced IL-10 production is mediated through the PI3K pathway. Macrophages were incubated with LPS (100 ng/ml) in the absence or copresence of epinephrine (1 μM). IL-10 levels in the culture supernatants after 4 h were determined by ELISA. Cells were pretreated with inhibitors for 30 min prior to stimulation. a Adenylate cyclase activity was inhibited with 2′5′-DDA (20 μM) or SQ22536 (10 μM). b PKA activity was inhibited with PKA inhibitor 14-22 amide (5 μM). c PI3K activity was inhibited with various doses of wortmannin.
Fig. 5.
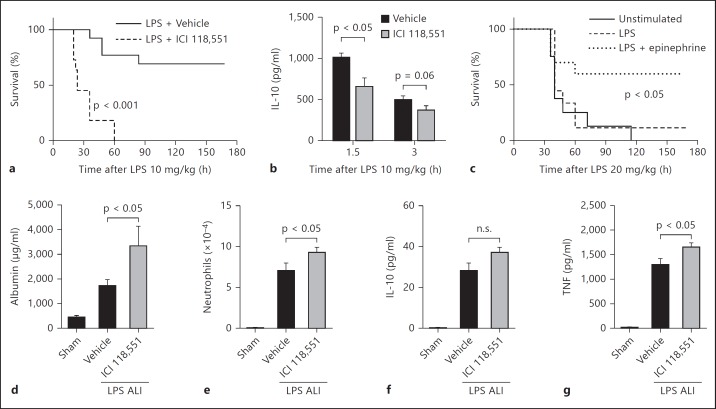
Activation of the β2-adrenergic receptor by endogenous catecholamines is protective during acute inflammation. a Survival curve following administration of LPS with or without ICI 118,551 (500 μg/mouse, n ≥ 11 mice per group). b Plasma levels of IL-10 during endotoxemia as described in a (n = 4 mice per group). c Macrophages were incubated in vitro in the presence or absence of LPS (100 ng/ml) and epinephrine (1 μM) for 1 h. Cells were harvested, washed, and adoptively transferred to mice (1 × 106 cells/mouse i.p.). Four hours after cell transfer, endotoxemia was performed (time 0, n ≥ 8 mice per group). d-g ALI was induced via i.t. administration of LPS (60 μg) and BALF was harvested 8 h later. Sham animals received only sterile saline. The β2-adrenergic receptor was blocked by administration of ICI 118,551 (25 mg/kg i.p. and 6 ng i.t.). BALF levels of albumin (d), neutrophil numbers (e), and IL-10 (f) and TNF (g) levels were determined (n ≥ 9 mice per group). n.s. = Not significant.
Peritoneal Macrophages
Peritoneal macrophages were elicited by intraperitoneal (i.p.) injection of 1.5 ml of 2.4% thioglycollate (Life Technologies, Grand Island, N.Y., USA). Macrophages were harvested 4 days later via i.p. instillation and retraction of 8 ml sterile ice-cold PBS. Cells were purified by adherence and cultured in RPMI media (Life Technologies) supplemented with 0.1% BSA (Sigma). In vitro experiments were performed in triplicate for ≥2 independent experiments, and representative results are shown.
RNA Isolation and RT-PCR
RNA was isolated using TRIzol reagent (Sigma) and treated with DNAse (Life Technologies) to remove any contaminating genomic DNA. cDNA was generated (oligo dT primers) and RT-PCR (SYBR) was performed using reagents from Life Technologies on a 7500 Real-Time PCR System (Applied Biosystems, Foster City, Calif., USA). Results were analyzed via the 2-ddCt method for relative quantitation and normalized to GAPDH. The primers (Life Technologies) used for amplification were as follows: GAPDH, 5′ CTTCAACAGCAACTCCCACTCTTCC 3′ (forward) and 5′ GGTGGTCCAGGGTTTCTTACTCC 3′ (reverse); IL-10, 5′ CCAGTTTTACCTGGTAGAAGTGATGCC 3′ (forward) and 5′ GTCTAGGTCCTGGAGTCCAGCAGAC 3′ (reverse); arginase-1, 5′ ATGGAAGAGACCTTCAGCTACCTGC 3′ (forward) and 5′ GCTGTCTTCCCAAGAGTTGGG 3′ (reverse); iNOS, 5′ ACATCGACCCGTCCACAGTAT 3′ (forward) and 5′ CAGAGGGGTAGGCTTGTCTCTGG 3′ (reverse); FIZZ, 5′ TCCAGCTAACTATCCCTCCACTGT 3′ (forward) and 5′ GGCCCATCTGTTCATAGTCTTGA 3′ (reverse); YM1, 5′ GGGCATACCTTTATCCTGAGTGACC 3′ (forward) and 5′ CCACTGAAGTCATCCATGTCCAGG 3′ (reverse).
Enzyme-Linked Immunosorbent Assays
Cytokine/chemokine enzyme-linked immunosorbent assays (ELISAs) were from R&D Systems (Minneapolis, Minn., USA) and mouse albumin ELISAs were from Bethyl Laboratories (Montgomery, Tex., USA). ELISAs were performed as per the manufacturer's recommendations.
Phagocytosis Assays
Unstimulated, LPS-treated, or LPS and epinephrine-cotreated macrophages were incubated with pHrodo Green E. coli Bioparticles® (1 mg/ml; Life Technologies) and incubated for 1 h (flow cytometry) or 1.5 h (confocal microscopy). For flow cytometric analysis, macrophages were washed and analyzed on a BD LSR-II flow cytometer equipped with FACSDiva software (both from BD Biosciences, San Jose, Calif., USA). Data was analyzed using FlowJo software (TreeStar, Ashland, Oreg., USA). More than 5 × 104 macrophages were analyzed from each sample. For confocal microscopic analysis, macrophages cultured on glass coverslips were washed and mounted on slides with ProLong Gold™ antifade reagent containing DAPI (Life Technologies). Slides were analyzed on a Nikon A-1 confocal system with Nikon Elements software. Digital monochromatic images were acquired under identical conditions and pseudo-colored.
Lymphocyte Stimulation Assays
Macrophages (2 × 105) from BALB/c mice were stimulated for 1 h in the presence or absence of epinephrine (1 μM) and/or LPS (100 ng/ml). Cells were washed, and ovalbumin (150 μg/ml; Sigma) was added for 30 min prior to the addition of peripheral lymph node lymphocytes (5 × 105; 600 μl total volume) from DO11.10 transgenic mice. Cultures were incubated for 84 h and cell-free supernatants were analyzed by ELISA for the presence of IFN-γ and IL-4. Samples generated under the same conditions, but containing only macrophages, had <5 pg/ml IFN-γ and no detectable IL-4.
Endotoxemia Studies
Endotoxemia was performed via i.p. administration of LPS (E. coli o111:B4) at 10 mg/kg of body weight or 20 mg/kg of body weight, depending on the experiment. ICI 118,551 was administered at a dose of 500 μg/mouse i.p. Plasma was harvested by bleeding from the retro-orbital venous plexus under isoflurane anesthesia. For survival studies, mice were observed at least every 12 h for 7 days. For adoptive transfer studies, peritoneal macrophages were treated in vitro with LPS in the presence or absence of epinephrine for 1 h. Cells were harvested, washed, and adoptively transferred to mice (1 × 106 cells in 200 μl PBS i.p.). Four hours after the cell transfer, endotoxemia was performed (20 mg/kg LPS i.p.).
Acute Lung injury
LPS-induced ALI was performed as previously described [15]. Briefly, following anesthetization with ketamine, mice received 60 μg LPS (E. coli o111:B4) intratracheally (i.t.) during inspiration in a volume of 30 μl saline. Sham ALI control mice received sterile saline. BALF was harvested via the slow instillation and retraction of 1 ml PBS. BALF was aliquoted and stored at −80°C until use. BALF neutrophils were enumerated on a hemocytometer following lysis of erythrocytes. For ICI 118,551 treatment, mice received 25 mg/kg i.p. 1 h before LPS and 6 ng i.t. during LPS instillation. Vehicle control mice received sterile saline.
Statistical Analysis
Data are expressed as means ± SEM. Data were analyzed using GraphPad Prism 6 graphing and statistical analysis software (GraphPad Inc., La Jolla, Calif., USA). Significant differences between multiple groups were determined by one-way ANOVA followed by Tukey's multiple comparisons test. Survival data were analyzed using log-rank (Mantel-Cox) tests. Where appropriate, significant differences between individual sample means were determined by Student's t test. p < 0.05 was considered statistically significant.
Results
Catecholamines Promote the M2 Macrophage Phenotype
Markers used to distinguish between M1 and M2 macrophages are the high expression levels of iNOS and arginase-1, respectively [4]. We determined the relative levels of iNOS and arginase-1 mRNA following macrophage treatment with LPS (100 ng/ml) in the absence or copresence of catecholamines (1 μM). Treatment with LPS alone induced an M1 phenotype including strong iNOS expression and modest levels of arginase-1 (fig. 1a). However, the copresence of LPS and either catecholamine induced a strong M2 phenotype. Specifically, the level of iNOS mRNA was reduced by 75–81% upon treatment with LPS and either catecholamine compared to LPS alone (fig. 1a, left). Furthermore, the level of arginase-1 mRNA increased 8- to 14-fold with the copresence of catecholamines (fig. 1a, right). Catecholamines in the absence of LPS did not modulate iNOS or arginase-1 (data not shown). Therefore, macrophages incubated with catecholamines in the presence of LPS displayed reduced iNOS expression and enhanced arginase-1 expression, a phenotype akin to M2 macrophages.
The preferential secretion of IL-12 and IL-10 by M1 and M2 macrophages, respectively, helps define macrophage polarization phenotypes [10]. LPS-treated macrophages released high levels of the p40 subunit of IL-12 (fig. 1b, left). Macrophages treated with LPS in the copresence of either catecholamine had significantly reduced levels of IL-12p40 in the culture supernatant (fig. 1b, left). Conversely, LPS-treated macrophages released low levels of IL-10 into the culture supernatant, but catecholamine and LPS cotreatment resulted in 5- to 6-fold higher levels of IL-10 (fig. 1b, right). Therefore, in the presence of LPS and catecholamines, macrophages adopted a cytokine repertoire similar to M2 macrophages.
In general, M2 macrophages display greater phagocytic activity than M1 macrophages [17, 18, 19]. Untreated, LPS-treated, and LPS and epinephrine-cotreated macrophages all phagocytosed fluorescent bioparticles generated from E. coli (fig. 1c). Qualitatively, macrophages treated with LPS in the copresence of epinephrine displayed higher levels of fluorescence intensity, indicating increased phagocytic activity (fig. 1c). In order to quantitate this difference, bioparticle fluorescence was determined via flow cytometry. LPS-treated macrophages in the copresence of epinephrine had a 42% increase in phagocytic activity compared to LPS-treated cells (fig. 1d).
M2 macrophages are known to promote Th2 type immune responses characterized by reduced IFN-γ secretion and enhanced IL-4 secretion by T cells [20]. During in vitro studies using DO11.10 transgenic lymphocytes (ovalbumin-specific T-cell receptor), macrophages treated with epinephrine (and cultured in the presence of ovalbumin) reduced IFN-γ secretion and enhanced IL-4 secretion by lymphocytes (fig. 1e). This effect was independent of LPS stimulation (fig. 1e). Therefore, epinephrine induced a shift towards a Th2 type immune response in vitro. Taken together, these results suggest that catecholamines induced a phenotypic switch from M1 (LPS-treated) to M2 (LPS/catecholamine-treated) macrophages.
LPS and Epinephrine Copresence Induces Regulatory Macrophages
M2 macrophages can be divided into alternatively activated and regulatory subtypes. Alternatively activated macrophages are identified by high expression levels of FIZZ, Ym1, and the mannose receptor (CD206) [1]. The determination of mRNA or surface protein levels of these markers did not change in the copresence of LPS and epinephrine (fig. 2a), or with epinephrine treatment alone (data not shown), indicating that catecholamines do not induce alternatively activated macrophages.
Definitive markers for regulatory macrophages have not been established, but these macrophages are known producers of high levels of IL-10 [10]. Treatment of LPS-activated macrophages with catecholamines enhanced IL-10 production in a dose-dependent manner (fig. 2b). The EC50 for epinephrine and norepinephrine was 5 and 320 nM, respectively. The copresence of LPS and epinephrine had immediate and robust effects on IL-10 transcription (fig. 2c, left) and protein secretion into the culture supernatant (fig. 2c, right). Treatment of cells with epinephrine or norepinephrine in the absence of LPS did not enhance the low basal level of IL-10 production (data not shown). Taken together, these results suggest that the copresence of LPS and catecholamines induced macrophage polarization towards the M2 regulatory macrophage phenotype.
Catecholamines Modulate LPS-Induced Macrophage Cytokine Production
M1 and M2 macrophages are known to produce different cytokine milieus [10]. In our studies, the treatment of macrophages with either epinephrine or norepinephrine, in the copresence of LPS, resulted in a significant shift from M1 (LPS only) to M2 (LPS + catecholamine) cytokine/chemokine production. Specifically, cotreatment of macrophages with LPS and either catecholamine (1 μM) reduced the production of TNF, macrophage inflammatory protein (MIP)-1α, keratinocyte chemoattractant [KC, chemokine (C-X-C motif) ligand 1 (CXCL1)], and MIP-2 (CXCL2) compared to LPS treatment alone (table 1). Catecholamines did not significantly affect the production of IL-6, but the production of granulocyte colony-stimulating factor (G-CSF) was greatly enhanced compared to LPS treatment alone (table 1). Importantly, catecholamine (10-12-10-5M) neither enhanced nor inhibited the low baseline production of any cytokine in the absence of LPS (data not shown). Similar effects of catecholamines on LPS-induced cytokine production were observed when mouse bone marrow-derived macrophages or the MH-S cell line (sv40-transformed mouse alveolar macrophages) was used (data not shown). Furthermore, similar results were observed when other TLR agonists (TLR2, zymosan; TLR3, poly I:C) were used in lieu of LPS (data not shown). These results indicate that catecholamines modulated TLR-induced cytokine/chemokine production from macrophages, resulting in reduced inflammatory cytokine secretion.
Table 1.
Effects of catecholamines on LPS-stimulated macrophage cytokine secretion
| Cytokine | Neg. Ctrl. | LPS | LPS + epinephrine | LPS + norepinephrine |
|---|---|---|---|---|
| TNF | 10±5.3 | 6,486±392 | 3,158±87* (–51%) | 2,796±143* (–57%) |
| IL-6 | n.d. | 613±53 | 492±20 (–20%) | 496±32 (–19%) |
| KC | 32±2.2 | 22,047±516 | 13,532±879* (–39%) | 14,887±320* (–32%) |
| MIP-2 | n.d. | 30,689±1,023 | 17,475±873* (–43%) | 20,216±504* (–34%) |
| MIP-1 | 69±11 | 36,532±1,973 | 17,823±2,506* (–51%) | 18,360±2,628* (–50%) |
| G-CSF | n.d. | 22±1.1 | 323±30* (+1,386%) | 383±32* (+1,658%) |
The levels of cytokines/chemokines (pg/ml) in culture supernatants were determined by ELISA. Data are representative of 3 independent experiments. Thioglycollate-elicited mouse peritoneal macrophages were treated for 4 h with LPS (100 ng/ml) in the presence or absence of epinephrine (1 μM) or Norepi. (1 µM). n.d. = Not detectable; Neg. Ctrl. = negative control.
Differences between LPS and LPS + catecholamine groups were significant at p < 0.05.
Epinephrine Signals through the β2-Adrenergic Receptor
While all 5 adrenergic receptors (α1, α2, α3, β1, and β2) can bind either catecholamine, they do so with different affinities and signaling properties. In addition, there can be major differences in adrenergic receptor expression depending on the cell type being used. We investigated which adrenergic receptor or receptors were responsible for the effects of epinephrine on macrophage cytokine production. We focused on epinephrine because the EC50 was >50-fold higher than for norepinephrine (fig. 2b). Adrenergic receptors were blocked using specific inhibitors prior to exposure to LPS in the presence or absence of epinephrine (1 μM). Results showed that blockade of the β2-adrenergic receptor with the specific β2-antagonist ICI 118,551 completely reversed the effects of epinephrine on IL-10 production (fig. 3a). The other adrenergic receptor antagonists did not significantly affect epinephrine-enhanced IL-10 production (fig. 3a).
Blockade of the β2-adrenergic receptor also inhibited changes in iNOS and arginase-1 expression. As in figure 1, the copresence of LPS and epinephrine reduced iNOS expression and enhanced arginase-1 expression compared to LPS treatment alone (fig. 3b, black bars). However, the presence of the specific β2-adrenergic receptor antagonist ICI 118,551 completely reversed the effects of epinephrine on the LPS-activated macrophage expression of iNOS and arginase-1 (fig. 3b). Surprisingly, β2-adrenergic receptor blockade enhanced iNOS expression even in the absence of exogenous epinephrine (fig. 3b, left). This observation may be due to the endogenous production of catecholamines by macrophages [21] or the known inverse agonist (blockade of spontaneous receptor signaling) properties of ICI 118,551 [22]. Taken together, these results indicate that catecholamines promoted the M2 regulatory macrophage phenotype through the β2-adrenergic receptor.
Epinephrine Modulates Cytokine Production through the Phosphoinositide 3-Kinase Pathway
β2-Adrenergic receptor activation by catecholamines in many cell types results in the canonical signaling pathway involving the activation of adenylate cyclase, increased [cAMP]i, and the activation of protein kinase A (PKA). Therefore, we investigated whether macrophage β2-adrenergic receptor activation modulates cytokine production through the cAMP/PKA pathway. Macrophages were treated with LPS in the presence or absence of epinephrine and the adenylate cyclase inhibitor 2′5′-DDA. Results showed that inhibition of adenylate cyclase did not reverse the effects of epinephrine on IL-10 production (fig. 4a, left). Similar results were observed when another adenylate cyclase inhibitor, SQ22536, was used (fig. 4a, right). Furthermore, specific inhibition of PKA (with PKA inhibitor 14-22 amide) in similar experiments did not reduce epinephrine-enhanced IL-10 production (fig. 4b). Therefore, for macrophages, the canonical β2-adrenergic signaling pathway did not have a role in catecholamine-mediated alteration of IL-10 production.
Given recent reports showing a role for phosphoinositide 3-kinase (PI3K) in β2-adrenergic receptor signaling [23, 24], we investigated whether PI3K mediates the effects of β2-receptor activation in macrophages. In a dose-dependent manner, inhibition of PI3K by wortmannin reversed the effects of epinephrine on IL-10 production (fig. 4c). Inhibition of other common signaling pathways (e.g. mitogen-activated protein kinases) did not influence epinephrine-induced IL-10 modulation (data not shown). Taken together, these results suggest that β2-adrenergic receptor activation by epinephrine enhanced macrophage IL-10 production through the PI3K pathway.
β2-Adrenergic Receptor Activation by Endogenous Catecholamines Is Anti-Inflammatory during Acute Inflammation
Stimulation of the β2-adrenergic receptor by exogenous agonists has protective effects during acute inflammation in vivo [15]. However, the role of β2-adrenergic receptor activation by endogenous catecholamines during acute inflammatory reactions is not clear. Catecholamines are ubiquitous in vivo and are upregulated during inflammatory conditions [21]. Given our in vitro results, we hypothesized that blockade of β2-adrenergic receptors would enhance inflammation in vivo. We investigated the role of endogenous catecholamine-induced β2-adrenergic receptor activation in two models of acute inflammation in vivo: endotoxemia and ALI. During endotoxemia, administration of LPS (10 mg/kg i.p.) with the β2-antagonist ICI 118,551 (500 μg/mouse i.p.) resulted in 100% mortality while LPS alone resulted in only 30% mortality (fig. 5a). In addition, β2-adrenergic receptor blockade during endotoxemia was associated with reduced levels of IL-10 found in plasma (fig. 5b). Administration of ICI 118,551 will block β2-adrenergic receptors on a myriad of cell types, not just macrophages. Therefore, the increased mortality in this model may be due to the effects of ICI 118,551 on other cell types. To help determine whether catecholamine-induced regulatory macrophages had protective effects during endotoxemia, macrophages were treated with LPS in the presence or absence of epinephrine in vitro and then adoptively transferred to mice (1 × 106 cells per mouse i.p.) 4 h prior to the induction of lethal endotoxemia (20 mg/kg LPS i.p.). Results showed that mice that received macrophages pretreated with LPS and epinephrine were modestly protected from endotoxemia compared to unstimulated or LPS-treated macrophages (fig. 5c). Taken together, these results suggest that activation of the β2-adrenergic receptor by endogenous catecholamines is protective during endotoxemia. Furthermore, we demonstrated the protective effects of catecholamine-induced regulatory macrophages in this model.
To determine the role of the β2-adrenergic receptor in a different model of acute inflammation, ICI 118,551 was administered during LPS-induced ALI. Blockade of β2-adrenergic receptors resulted in a 2-fold increase in the albumin levels found in BALF, indicating amplified blood/alveolar barrier breakdown (fig. 5d). The number of neutrophils in BALF was modestly increased by β2-adrenergic receptor blockade (fig. 5e). Surprisingly, the low levels of IL-10 found in BALF were not changed by β2-adrenergic receptor blockade (fig. 5f). However, the levels of TNF in BALF were modestly enhanced by β2-adrenergic receptor blockade (fig. 5g). These patterns are consistent with our recent report showing that exogenous β2-adrenergic receptor agonists suppress the intensity of ALI [15]. Taken together, these results demonstrate that β2-adrenergic receptor activation by endogenous catecholamines is protective during acute inflammatory reactions.
Discussion
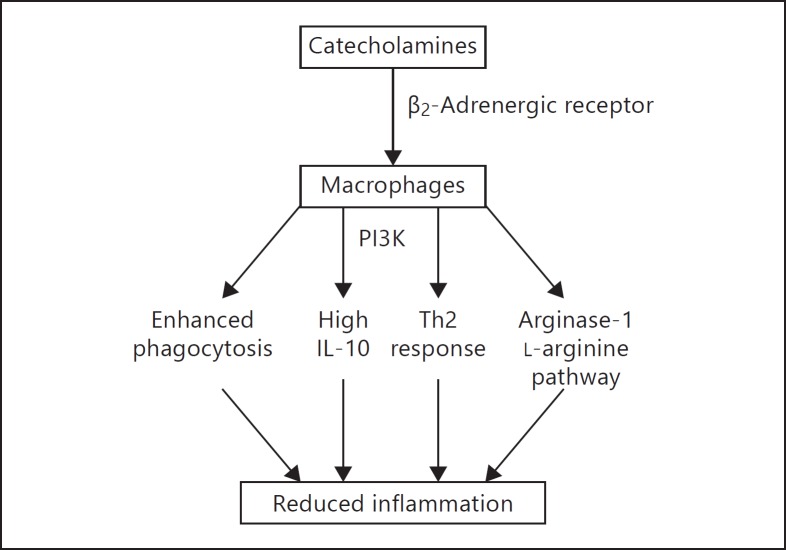
In this study, we demonstrated that catecholamines profoundly affect the macrophage phenotype through the β2-adrenergic receptor. Furthermore, β2-adrenergic receptor activation by endogenous catecholamines was shown to have a protective function during acute inflammation in vivo. Much is known about the modulatory effects of the adrenergic system on cytokine production. Early studies by van der Poll et al. [25, 26, 27] demonstrated that catecholamines inhibit the production of TNF, IL-6, and IL-1β in human whole blood models using high concentrations of catecholamines. In addition, several subsequent studies have shown that synthetic α-adrenergic receptor agonists enhance the LPS-induced production of TNF by macrophages, while β-adrenergic receptor agonists reduce TNF production [13, 14, 15, 16]. In this report, we extended these findings to demonstrate that catecholamine-induced activation of the β2-adrenergic receptor did not simply modify the production of a few inflammatory cytokines but rather modified the entire macrophage phenotype, resulting in an M2 regulatory macrophage phenotype. Evidence for this is the differential regulation of cytokines by catecholamines. Proinflammatory cytokines (TNF, IL-12, and CXCL chemokines) were downregulated by catecholamines while IL-10 and G-CSF were enhanced. Furthermore, catecholamines induced phenotypic changes consistent with the M2 phenotype, including enhanced phagocytosis, the promotion of Th2 type immune responses, and enhanced arginase-1 expression (summarized in fig. 6). Therefore, this study demonstrates for the first time that catecholamines promote the M2 regulatory macrophage phenotype through the β2-adrenergic receptor.
Fig. 6.
Summary of the effects of catecholamines on the promotion of anti-inflammatory (M2) macrophage functions resulting in reduced inflammation.
In addition to the cellular effects of catecholamines, we demonstrated a protective role for endogenous catecholamines during acute inflammation in vivo. Specifically, blockade of β2-adrenergic receptors by ICI 118,551 reduced survival during endotoxemia, which was associated with reduced levels of IL-10 in plasma. Adoptive transfer of a relatively small number of catecholamine-induced regulatory macrophages was protective during lethal endotoxemia. During ALI, blockade of β2-adrenergic receptors by ICI 118,551 enhanced the level of TNF found in BALF and exacerbated lung injury. However, previous studies using the same β2-antagonist reported no effect during ALI [21]. The differences in these studies may be due to the route (i.p. vs. i.p. and i.t.) and dose of the inhibitor. Due to this, the previous study may not have sufficiently blocked receptors in the alveolar compartment. Importantly, blockade of α2-adrenergic receptors has been shown to reduce the injury severity during ALI [21, 28]. Therefore, it is apparent that α2- and β2-adrenergic receptors act in a complimentary manner. Since catecholamines can bind both receptors, the relative contributions of these receptors during inflammatory reactions in vivo need to be addressed in the future.
It is important to note that β2-adrenergic receptor blockade did not enhance IL-10 production during ALI. We have previously reported that alveolar macrophages respond to synthetic β2-agonists by enhancing the production of IL-10 in the presence of LPS [15]. Why we could not detect a difference in IL-10 presence in BALF when β2-adrenergic receptors were blocked was not entirely apparent. It is known that other cell types in the lung during ALI also produce IL-10 (e.g. alveolar type II epithelium [29] and neutrophils [30]), which may mask any changes induced by β2-adrenergic receptor inhibition. In addition, the time point chosen for analysis (6 h after LPS instillation) may not be optimal to detect differences in the low levels of IL-10 that are produced during ALI. However, the anti-inflammatory properties of endogenous catecholamine-induced β2-receptor activation during ALI were clear, as evidenced by increased TNF production, increased neutrophil infiltration, and increased epithelial/endothelial barrier breakdown in the presence of the β2-receptor inhibitor.
It is known that sustained PI3K activity is necessary for M2 activation in response to IL-4 [31, 32]. Indeed, PI3K is essential in driving arginase-1 expression [33, 34]. Here, we demonstrated that the M2 phenotype induced by β2-adrenergic receptor activation also requires PI3K, in agreement with these reports. It is important to note that characterization of the catecholamine-induced regulatory M2 macrophage phenotype may be overly simplistic. Indeed, the M2 phenotype encompasses a full spectrum of activated macrophage phenotypes [1]. Based on the evidence in this report, we propose that catecholamines primarily promote the regulatory macrophage phenotype, characterized by high levels of IL-10 production. The regulatory macrophage phenotype requires 2 stimuli for efficient IL-10 release in vitro. The first signal (e.g. prostaglandins) often has no stimulatory ability by itself [35, 36]. However, when the initial signal is combined with TLR agonism it results in the synthesis of high levels of IL-10, the robust production of which may be the most reliable marker for regulatory macrophages [1, 12]. Here, we showed that this ‘recipe’ for high IL-10 production holds true for catecholamines.
Stress responses (e.g. glucocorticoids) can affect several macrophage functions including inhibition of the transcription of proinflammatory mediators [37]. The resulting macrophages are often of the regulatory type, which can skew T cell responses toward Th2, induce regulatory T cells, or fail to present antigen to T cells altogether [1, 38]. Here, we showed that catecholamines act as inducers of regulatory macrophages. Robust levels of glucocorticoids and catecholamines are released during periods of high immunological stress, like during sepsis [39]. Therefore, the resulting regulatory macrophages, and the high levels of IL-10 they produce, can render the host prone to infection. Conversely, regulatory macrophages can dampen vigorous but damaging inflammation. Therefore, modulation of the adrenergic system may be an important therapeutic route for enhancing protection against pathogens, or protecting the host from overly robust inflammatory responses. However, it is important to note that there is not a single stimulus that influences macrophage phenotypes in vivo. Instead, many different stimuli, including TLR agonists, IFN-γ, glucocorticoids, prostaglandins, etc., all contribute to the resulting phenotype of the activated macrophage. This diversity of signals is what generates the ability for fine-tuning of the resulting macrophage functions and products.
In summary, we report a novel function of the β2-adrenergic receptor on macrophages. Specifically, activation of the β2-adrenergic receptor by catecholamines induced regulatory M2 macrophages through the PI3K pathway. These IL-10-producing macrophages express high levels of arginase-1 mRNA, display enhanced phagocytosis, and skew T cell responses towards Th2. In addition, β2-adrenergic receptor blockade in vivo was detrimental in models of systemic and localized acute inflammation, thus demonstrating a protective role for endogenous catecholamine-induced β2-adrenergic receptor activation in vivo.
Disclosure Statement
The authors have no competing financial interests.
Acknowledgements
The authors thank Beverly Schumann and Sue Scott for their excellent assistance in the preparation of this paper. This work was supported by grants from the National Institutes of Health: GM-29507 and GM-61656 (P.A.W.) and NHLBI-T32-HL007517-29 (J.J.G.).
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 5.Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol Chem Hoppe Seyler. 1995;376:327–343. doi: 10.1515/bchm3.1995.376.6.327. [DOI] [PubMed] [Google Scholar]
- 9.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 11.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-α production from macrophages in vitro. J Immunol. 1994;152:3024–3031. [PubMed] [Google Scholar]
- 14.Huang JL, Zhang YL, Wang CC, Zhou JR, Ma Q, Wang X, Shen XH, Jiang CL. Enhanced phosphorylation of MAPKs by NE promotes TNF-α production by macrophage through α adrenergic receptor. Inflammation. 2012;35:527–534. doi: 10.1007/s10753-011-9342-4. [DOI] [PubMed] [Google Scholar]
- 15.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly LE, Tudhope SJ, Fenwick PS, Barnes PJ. Effects of formoterol and salmeterol on cytokine release from monocyte-derived macrophages. Eur Respir J. 2010;36:178–186. doi: 10.1183/09031936.00158008. [DOI] [PubMed] [Google Scholar]
- 17.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol. 2009;182:4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 18.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS One. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- 21.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 22.Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, Hyek MF, Kenakin TP, Allen LF, Lefkowitz RJ. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 23.Gregg CJ, Steppan J, Gonzalez DR, Champion HC, Phan AC, Nyhan D, Shoukas AA, Hare JM, Barouch LA, Berkowitz DE. β2-Adrenergic receptor-coupled phosphoinositide 3-kinase constrains camp-dependent increases in cardiac inotropy through phosphodiesterase 4 activation. Anesth Analg. 2010;111:870–877. doi: 10.1213/ANE.0b013e3181ee8312. [DOI] [PubMed] [Google Scholar]
- 24.Murray DR, Mummidi S, Valente AJ, Yoshida T, Somanna NK, Delafontaine P, Dinarello CA, Chandrasekar B. β2 Adrenergic activation induces the expression of IL-18 binding protein, a potent inhibitor of isoproterenol induced cardiomyocyte hypertrophy in vitro and myocardial hypertrophy in vivo. J Mol Cell Cardiol. 2012;52:206–218. doi: 10.1016/j.yjmcc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol. 1997;273:R1885–R1890. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Zhu X, Yao WZ, Yang YL, A LT, Chen L. Yohimbine protects against endotoxin-induced acute lung injury by blockade of alpha 2a adrenergic receptor in rats. Chin Med J. 2011;124:1069–1074. [PubMed] [Google Scholar]
- 29.Fernandez S, Jose P, Avdiushko MG, Kaplan AM, Cohen DA. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J Immunol. 2004;172:2613–2620. doi: 10.4049/jimmunol.172.4.2613. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 32.Whyte CS, Bishop ET, Ruckerl D, Gaspar-Pereira S, Barker RN, Allen JE, Rees AJ, Wilson HM. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–854. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- 33.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 34.Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. Ship represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann NY Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 39.Mesotten D, Vanhorebeek I, van den Bergh G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat Clin Pract Endocrinol Metab. 2008;4:496–505. doi: 10.1038/ncpendmet0921. [DOI] [PubMed] [Google Scholar]