Abstract
Glucagon-like peptide-1(7–36)amide (GLP-1) is a secreted peptide that acts as a key determinant of blood glucose homeostasis by virtue of its abilities to slow gastric emptying, to enhance pancreatic insulin secretion, and to suppress pancreatic glucagon secretion. GLP-1 is secreted from L cells of the gastrointestinal mucosa in response to a meal, and the blood glucose-lowering action of GLP-1 is terminated due to its enzymatic degradation by dipeptidyl-peptidase-IV (DPP-IV). Released GLP-1 activates enteric and autonomic reflexes while also circulating as an incretin hormone to control endocrine pancreas function. The GLP-1 receptor (GLP-1R) is a G protein-coupled receptor that is activated directly or indirectly by blood glucose-lowering agents currently in use for the treatment of type 2 diabetes mellitus (T2DM). These therapeutic agents include GLP-1R agonists (exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide, and langlenatide) and DPP-IV inhibitors (sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin). Investigational agents for use in the treatment of T2DM include GPR119 and GPR40 receptor agonists that stimulate the release of GLP-1 from L cells. Summarized here is the role of GLP-1 to control blood glucose homeo-stasis, with special emphasis on the advantages and limitations of GLP-1-based therapeutics.
1. Introduction
Systemic blood glucose homeostasis in humans is under the control of glucagon-like peptide-1(7–36)amide (GLP-1), a peptide secreted from intestinal enteroendocrine L cells in response to a meal.1 L cells are located within the gastrointestinal mucosa and they act as nutrient sensors to release GLP-1 in response to luminal sugars, amino acids, and fatty acids.2 Released GLP-1 acts locally within the intestinal wall to activate enteroenteric reflexes important to the control of gastric motility, thereby slowing gastric emptying.3 Simultaneously, released GLP-1 activates vagal sensory nerve terminals that innervate the intestinal wall, and in this manner, GLP-1 initiates vagal–vagal autonomic reflexes that control endocrine pancreas function.4 Circulating GLP-1 also acts as a hormone at the islets of Langerhans in the endocrine pancreas to stimulate the release of insulin, while suppressing the release of glucagon. During the postprandial phase of blood glucose control, these immediate and multiple actions of GLP-1 act in concert to lower levels of blood glucose.
Clinical studies demonstrate that the blood glucose-lowering action of GLP-1 is itself glucose-dependent.6–8 More specifically, GLP-1 reduces levels of blood glucose only when concentrations of blood glucose are elevated above fasting levels, as is the case after a meal. As the postprandial blood glucose levels fall in response to GLP-1, the blood glucose-lowering action of GLP-1 is self-terminating. This remarkable glucose-dependent property of GLP-1 action results in a situation in which intravenously administered GLP-1 fails to reduce levels of blood glucose below fasting levels.6–8 Since administered GLP-1 does not produce hypoglycemia, these clinical findings have led to the use of GLP-1 receptor (GLP-1R) agonists as a new class of blood glucose-lowering agents for use in the treatment of type 2 diabetes mellitus (T2DM).9,10
2. GLP-1 Biosynthesis, Secretion, and Degradation
Proglucagon gene expression in the intestinal L cells generates proglucagon (PG) that is processed by prohormone convertases (PC1/3) to liberate the GLP-1(1–37) peptide precursor.11,12 Endopeptidase-catalyzed cleavage of GLP-1(1–37) generates two peptides with insulin secretagogue properties. These are GLP-1(7–37) that is processed by amidating enzyme to generate GLP-1(7–36)amide.13–15 Although glucagon gene expression also generates PG in islet α-cells, it was thought that α-cells fail to synthesize GLP-1 due to the fact that these endocrine cells contain a prohormone convertase (PC2) that preferentially processes PG to glucagon.16 However, it is now apparent that endocrine cell “plasticity” exists within the islets such that α-cells synthesize GLP-1 under stressful or pathophysiological conditions including T2DM.17 Thus, it seems likely that GLP-1 can also act as an intraislet paracrine hormone but in a context-dependent manner.
GLP-1 is packaged in secretory granules and it is released from intestinal L cells by exocytosis in response to an elevation of cytosolic Ca2+ and cAMP.18 In this regard, it is important to note that L cells are electrically excitable and that glucose transporter-mediated uptake of glucose by L cells is Na+-dependent and electrogenic. Thus, L cells respond to orally administered glucose by generating action potentials that trigger depolarization-induced Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs).19 Ca2+ mobilized from intracellular Ca2+ stores is also a stimulus for GLP-1 secretion, and this Ca2+ mobilization is initiated by the binding of fatty acids to a receptor designated as GPR40 located on L cells.20 GLP-1 secretion is also stimulated by fatty acid amides (oleoylethanolamide) and monoacylglycerols (2-oleoyl glycerol) that activate GPR119, a receptor that is positively coupled to cAMP production in L cells.20
GLP-1 released from L cells acts locally within the hepato-portal circulation to activate the GLP-1R located on vagal sensory neurons. These neurons constitute the hepato-portal glucose sensor that communicates with brainstem neurons in order to regulate whole-body metabolism.4 Highest concentrations of released GLP-1 are found immediately within the hepato-portal circulation since GLP-1 is quickly metabolized to GLP-1 (9–36)amide by dipeptidyl-peptidase-IV (DPP-IV).21 DPP-IV exists in two forms—a 766-amino-acid transmembrane protein and a smaller soluble form found in the plasma.22 Both forms of DPP-IV have enzymatic activity, and the preferred substrates are peptides such as GLP-1 that contain penultimate N-terminus alanine or proline residues. The half-life for intravenously administered GLP-1 is less than 5 min owing to its rapid degradation by DPP-IV.23 However, picomolar concentrations of GLP-1 activate the GLP-1R,24 and concentrations of circulating GLP-1 are sufficiently high to allow it to activate the GLP-1R on islet β-cells.
Exenatide is a DPP-IV-resistant peptide that shares structural homology with GLP-1.25 It is the synthetic form of exendin-4, a peptide isolated from the Gila monster lizard Heloderma.26 Exenatide is a GLP-1R agonist in humans, and it has a half-life of ca. 20 min when it is administered by the intravenous route.9 Patients with T2DM receive exenatide by subcutaneous injection, and circulating exenatide produces a blood glucose-lowering effect since it directly activates the GLP-1R.9,10 Orally administrable DPP-IV inhibitors such as sitagliptin and vildagliptin are also useful for the treatment of T2DM.27,28 By slowing degradation of GLP-1, these DPP-IV inhibitors enhance the action of endogenous GLP-1 to activate vagal sensory neurons that compose the hepato-portal glucose sensor.29 This intestinal action of DPP-IV inhibitors is of significance since it is the predominant means by which low concentrations of DPP-IV inhibitors exert a blood glucose-lowering effect.29
3. Insulinotropic and Growth Factor Properties of GLP-1
Soon after the cloning of the anglerfish PG gene by the Habener lab-oratory in 1982, the sequence of a hamster PG cDNA was reported by Bell and coworkers.12 Bioinformatics analysis of the hamster PG cDNA revealed that it encoded GLP-1(1–37) derived from exon 4 of the PG gene.12 Subsequently, it was demonstrated by the laboratories o Creutzfeldt,30 Holst,31 Habener,13 Weir,32 and Bloom15 that pancreatic insulin secretion could be stimulated in a glucose-dependent manner by derivates of GLP-1(1–37) that included GLP-1(1–36), GLP-1(7–36)amide, and GLP-1(7–37). Cloning of the 463-amino-acid rat pancreatic islet GLP-1R by Thorens in 1992 revealed that nanomolar high-affinity agonist binding to the recombinant GLP-1R was measurable using a truncated metabolite of GLP-1(1–37) that corresponded to GLP-1(7–36)amide.33
It is now recognized that GLP-1(7–36)amide is the predominant bioactive GLP-1 present in human serum. It has an MW of 3298 Da and is composed of 30-amino-acid residues with the sequence HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH2.34 This truncated and fully bioactive GLP-1 binds to the human GLP-1R35,36 expressed on islet β-cells in order to potentiate glucose-stimulated insulin secretion in vivo (GSIS).6–8 Importantly, the insulin secretagogue action of GLP-1 is accompanied by its ability to stimulate insulin gene transcription, insulin mRNA translation, and proinsulin biosynthesis in β-cells.37–40 Potentially just as important, GLP-1 also acts as a β-cell growth factor so that in mice, it stimulates β-cell proliferation while also exerting a prosurvival (antiapoptosis) action to protect against β-cell death.41–45 Thus, there is great interest to determine whether such preclinical findings concerning GLP-1 are applicable to T2DM patients treated with GLP-1R agonists.
In addition to improving β-cell insulin secretion and insulin biosynthesis, a modern treatment for T2DM might also be capable of increasing β-cell “mass,” either by stimulating β-cell replication or by slowing β-cell death. Furthermore, for certain forms of T2DM, it might be useful to identify GLP-1R agonists that selectively “bias” GLP-1R signal transduction in order to achieve a desired therapeutic outcome. In this regard, attention has recently focused on whether it might be possible to synthesize GLP-1R agonists that allosterically induce a GLP-1R conformation that enhances receptor coupling to select downstream effectors such as GTP-binding proteins, mitogen-activated protein kinases, c-src kinase, and β-arrestin.46 In this context, it is valuable to summarize what is currently known concerning signal transduction pathways activated by the GLP-1R.
The GLP-1R is a G protein-coupled receptor (GPCR) that is a member of the secretin receptor-like family of seven transmembrane-spanning domain proteins. These group B receptors include GPCRs that selectively bind secretin, glucagon, glucose-dependent insulinotropic peptide (GIP), vasoactive intestinal peptide (VIP), and pituitary adenylyl cyclase-activating peptide (PACAP).47 Heterotrimeric GS GTP-binding proteins are activated in response to agonist binding to the GLP-1R, and they couple GLP-1R agonist occupancy to the stimulation of transmembrane adenylyl cyclases (TMACs). In β-cells, TMACs catalyze conversion of ATP to cytosolic cAMP, a second messenger that activates either protein kinase A (PKA) or the cAMP-regulated guanine nucleotide exchange factor designated as Epac2.48 PKA is a serine/threonine protein kinase that phosphorylates key substrate proteins of the β-cell stimulus-secretion coupling and gene regulatory networks. In contrast, Epac2 acts via Rap1 GTPase to activate a novel phospholipase C-epsilon (PLCε) that specifically hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2).49
In β-cells, there exists a PKA-mediated action of GLP-1R agonists to phosphorylate Snapin.50 Snapin is a protein that associates with SNAP-25, a component of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex that couples an increase of cytosolic Ca2+ concentration to insulin secretory granule exocytosis. By phosphorylating Snapin, GLP-1R agonists potentiate GSIS, thus lowing levels of blood glucose.50 An additional PKA-mediated action of GLP-1R agonists controls β-cell gene expression by phosphorylating CREB, a cAMP response element-binding protein.51 Activated CREB binds cAMP response elements (CREs) located in 5′ gene promoters, and it couples PKA activation to the stimulation of gene transcription. This CREB-mediated action of PKA is facilitated by CREB coactivators such as p300, CREB-binding protein (CBP), and CRTC (CREB-regulated transcriptional coregulator).52 Numerous CREB-regulated genes are under the control of GLP-1R agonists in β-cells, as demonstrated for CREB-dependent stimulation of insulin gene expression and insulin receptor substrate-2 (IRS-2) gene expression.51–53
Particularly interesting is the role of Epac2 in the control of pancreatic insulin secretion. Studies of mice with a knockout (KO) of Epac2 gene expression demonstrate that Epac2 mediates the action of GLP-1R agonist exendin-4 to potentiate the first phase kinetic component of GSIS.54 Since first phase GSIS is defective in patients with T2DM,55 and since exendin-4 restores first phase GSIS under conditions of T2DM,56 it appears that Epac2 activation might play an especially important role when considering how GLP-1-based therapeutic agents restore normoglycemia in patients with T2DM. Interestingly, Epac proteins may also play a role in the central nervous system (CNS) control of glucose homeostasis and energy expenditure since their activation appears to reduce leptin sensitivity in neural networks controlling feeding behavior.57
When considering how GLP-1R agonists act as β-cell trophic factors to increase β-cell mass, studies of β-cell lines or neonatal mouse β-cells indicate that it is PKA that mediates transcriptional induction of cyclin D1 expression by GLP-1 in order to stimulate β-cell proliferation.58 Interestingly, a proliferative action of GLP-1 also results from PKA-mediated phosphorylation of β-catenin, thereby indicating that the β-cell cAMP–PKA signaling branch exhibits signal transduction cross talk with a noncanonical Wnt signaling pathway that uses the transcription factor TCF7L2 to control gene expression.59 An additional surprising finding is that a truncated GLP-1 designated as GLP-1(28–36)amide stimulates cAMP production in β-cells, thereby activating the β-catenin/TCF7L2 signaling pathway.60 Furthermore, GLP-1(28–36)amide protects against β-cell glucotoxicity by improving mitochondrial function.61 GLP-1(28–36)amide is a cell-penetrating peptide that does not exert its effects by binding to the GLP-1R, but instead acts intracellularly.61 Thus, it is not clear how GLP-1(28–36)amide stimulates cAMP production.
PKA-mediated induction of IRS-2 expression also promotes β-cell growth in response to GLP-1,53,62 and PKA mediates the action of GLP-1 to promote translocation of transcription factor PDX-1 to the nucleus, thereby enhancing the differentiated state of β-cells. In contrast, Epac2 participates in the protection of β-cells from cytotoxicity induced by reactive oxygen species (ROS).64,65 Redox control in β-cells is under the control of thioredoxin (TxN), and TxNIPs are thioredoxin-interacting proteins that downregulate the ROS buffering capacity of thioredoxin.66 Thus, it is significant that GLP-1 acts via Epac2 to suppress TxNIP expression and to enhance ROS buffering in β-cells.64 To what extent Epac2 also mediates actions of GLP-1R agonists to control β-cell mass and/or survival remains an active field of investigation.
4. Molecular Basis of GLP-1 Receptor Activation
Like other group B GPCRs, the GLP-1R possesses a long extracellularly oriented N-terminus of ca. 150 amino acids in which three pairs of disulfide bonds create a secondary structure important to ligand binding. This N-terminal extracellular domain is connected to a core domain of the receptor consisting of seven transmembrane α-helices interconnected by three extracellular loops (ECL 1–3) and three intracellular loops (ICL 1–3). Based on findings originally obtained in studies of the group GPCR for parathyroid hormone (PTH),67 a two-domain model for GLP-1R activation exists in which the α-helical C-terminus of GLP-1 (7–36)amide interacts with the receptor's N-terminal domain, while the N-terminus of GLP-1(7–36)amide interacts with ECL-1 and ECL-2 of the GLP-1R.68–72 The affinity and selectivity of ligand binding is determined by interactions of the GLP-1(7–36)amide C-terminus with the receptor's N-terminal domain, whereas coupling of the GLP-1R to intracellular signaling pathways is strongly influenced by the receptor core domain and its intracellular loops. In this regard, ICL-3 is of major importance to GLP-1R-stimulated adenylyl cyclase activity.73
There exists an alternative model of GLP-1R activation in which it is proposed that binding of GLP-1(7–36)amide to the receptor results in a structural rearrangement of the receptor's N-terminal domain so that a pentapeptide NRTFD signature sequence within the N-terminus of the receptor acts as an endogenous agonist at ECL-2 or ECL-3 of the receptor.74 In this model, the signature sequence acts as a “tethered ligand” to promote GLP-1R activation.74 Adding to this complexity, the signaling properties of the GLP-1R are also dictated by its ability to form homodimers in which receptor dimerization occurs at the interface of transmembrane helix four of each receptor protomer.75
Since small molecule GLP-1R agonists are highly desired for the treatment of T2DM, considerable effort has been exerted in an attempt to identify the precise mechanisms of ligand binding to the GLP-1R. Nuclear magnetic resonance (NMR) analysis using isolated N-terminal extracellular domains of group B GPCRs reveals that these ligand-binding domains contain a core structure composed of two antiparallel β-sheets stabilized by three disulfide bonds. X-ray crystallographic analysis also reveals that these β-sheets are linked to an N-terminal α-helix in order to form a “fold” that is highly conserved among the group B GPCRs.69 Binding of GLP-1(7–36) amide within this fold leads to a structural rearrangement of GLP-1(7–36) amide so that it transitions from its disordered solution structure to an induced α-helical conformation with hydrophobic residues buried within the fold.69
Since binding of GLP-1(7–36)amide to the GLP-1R is accompanied by a structural rearrangement of the peptide in order for it to stimulate receptor signaling, it is understandable that the rationale design of small molecule GLP-1R agonists is complex and is not guided simply by the disordered solution structure of GLP-1(7–36)amide. However, the flexibility of GLP-1(7–36)amide to adopt multiple conformations might be of significance in view of current efforts to design small molecule allosteric modulators of the GLP-1R.76–78 These modulators bind regions of the receptor distinct from GLP-1(7–36)amide, and they might cause GLP-1(7–36)amide to adopt a conformation that “biases” its signal transduction properties so that it activates select downstream pathways. Given that potentially dangerous islet cell hyperplasia is reported to occur in some T2DM patients treated with GLP-1R agonists,79 it might be possible to design allosteric modulators of the GLP-1R that preferentially stimulate insulin secretion rather than islet growth.
5. Control of Pancreatic β-Cell Insulin Secretion by GLP-1
In studies of isolated islets, a stepwise increase of the glucose concentration from 2.8 to 16.7 mM leads to an initial first phase kinetic component of insulin secretion, followed by a delayed second phase, and the amplitudes of both phases of GSS are potentiated by GLP-1.80–83 These insulin secretagogue actions of GLP-1 are also measurable in vivo under conditions of a glucose clamp in which a GLP-1R agonist is infused intravenously while raising the blood glucose concentration in a stepwise manner.56 In patients with prediabetes, there is a characteristic loss of first phase GSIS that can be restored quickly under conditions of administered GLP-1. As T2DM progresses, there is an additional loss of second phase GSIS, and it too can be restored under conditions of acute GLP-1 administration. Such findings indicate that in T2DM, GLP-1 has the capacity to quickly restore GSIS independently of any long-term action to increase islet insulin content. Presumably, such acute actions of GLP-1 reflect, at least in part, its physiological role as an incretin hormone in which it activates the GLP-1R located on islet β-cells.
When considering the acute insulin secretagogue action of GLP-1 in vivo, it is also thought that oral administration of glucose leads to activation of vagal–vagal reflexes that allow GLP-1 to control insulin exocytosis indirectly. Thus, GLP-1 released from L cells activates vagal sensory neurons that project to the brainstem in order to initiate efferent vagal reflexes via the parasympathetic branch of the autonomic nervous system. Parasympathetic neurons release acetylcholine (ACh) in the islets in order to activate muscarinic cholinergic receptors that stimulate Ca2+ mobilization in β-cells, and these neurons also release PACAP to stimulate cAMP production in β-cells. The net effect is an indirect and neurally mediated action of GLP-1 to potentiate GSIS.84
As illustrated in Fig. 2.1, the direct action of GLP-1 to activate the GLP-1R on β-cells leads to dual activation of the PKA and Epac2 branches of the cAMP signaling mechanism. In this manner, GLP-1 facilitates glucose-dependent closure of ATP-sensitive K+ channels (KATP).85 The net effect is β-cell depolarization with consequent activation of VDDCs in order to allow Ca2+ influx that stimulates Ca2+-dependent insulin secretion. Simultaneously, GLP-1 enhances a mechanism of Ca2+-induced Ca2+ release (CICR) in which Ca2+ influx triggers the release of Ca2+ from intra-cellular Ca2+ stores.86–90 This mobilized Ca2+ then acts as an additional stimulus for Ca2+-dependent insulin secretion. In patients with T2DM, β-cell glucose metabolism is dysfunctional so that glucose is not fully capable of closing KATP channels in order to stimulate Ca2+ influx.84 Under these pathophysiological conditions, glucose alone fails to generate the critically important cytosolic Ca2+ signal that initiates insulin exocytosis. By facilitating glucose-dependent KATP channel closure and by enhancing CICR, GLP-1 restores the Ca2+ signal, thereby allowing GSIS to occur.
Figure 2.1.
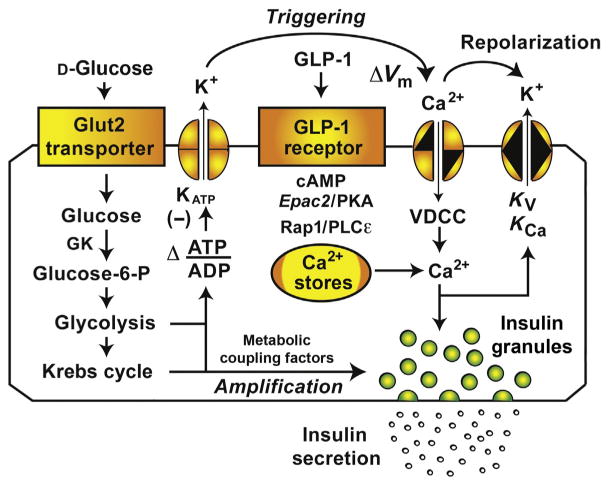
Role of GLP-1 in β-cell stimulus-secretion coupling. GLP-1 binds to its GPCR in order to stimulate cAMP production and to potentiate GSIS. One cAMP-dependent action of GLP-1 is mediated by PKA that phosphorylates secretory granule-associated proteins (e.g., Snapin) in order to facilitate Ca2+-dependent exocytosis of insulin. The PKA-independent action of GLP-1 is mediated by the cAMP-regulated guanine nucleotide exchange factor Epac2. Binding of cAMP to Epac2 results in sequential activation of Rap1 GTPase and PLCε, thereby promoting PIP2 hydrolysis and intracellular Ca2+ mobilization. GLP-1 also exerts PKA and Epac2-mediated actions to enhance glucose-dependent KATP channel closure, thereby promoting Ca2+ influx through VDCCs. The primary role of GLP-1 relevant to insulin secretion is to act as a β-cell glucose sensitizer in order to enhance insulin exocytosis mediated by the triggering and amplification pathways of GSIS. Abbreviations: GK, glucokinase; ΔVm, depolarization; Kv, voltage-dependent K+ channel, KCa, calcium-activated K+ channel.
When considering how GLP-1 potentiates GSIS from β-cells of healthy individuals, a different scenario exists. Under these nonpathological conditions, coupling of glucose metabolism to KATP channel closure is not disturbed, so that glucose is fully capable of generating the cytosolic Ca2+ signal that stimulates insulin exocytosis. Importantly, single cell studies demonstrate that this Ca2+ signal is a more efficient stimulus for insulin exocytosis under conditions in which β-cells are treated with GLP-1.91 Such a facilitation of exocytosis by GLP-1 is explained by its PKA- and Epac2-mediated actions that occur at “late” steps of β-cell stimulus-secretion coupling and that promote Ca2+-dependent fusion of secretory granules with the plasma membrane (Fig. 2.1).
Since a KATP channel-dependent action of GLP-1 is likely to explain insulin secretagogue properties of GLP-1 in patients with T2DM, it is useful to summarize what is known concerning this effect. Restoration of KATP channel closure by GLP-1 is measurable under conditions in which rat P-cells are initially exposed to a glucose-free solution that depletes intracellular ATP.85 Transient reintroduction of glucose weakly inhibits KATP channel activity, and this action of glucose is greatly potentiated by GLP-1. Such a restorative action of GLP-1 reflects its ability to alter the adenine nucleotide sensitivity of KATP channels so that these channels close more efficiently in response to the increase of cytosolic ATP/ADP concentration ratio that glucose metabolism produces. In fact, PKA reduces the stimulatory action of Mg-ADP at SUR1,92 whereas Epac2 enhances the inhibitory action of ATP at Kir6.2.93,94 These dual mechanisms of KATP channel modulation underlie the ability of GLP-1 to act as a β-cell glucose sensitizer so that it may facilitate glucose metabolism-dependent depolarization of β-cells.
Studies of mice lacking the sulfonylurea receptor-1 (SUR1) and pore-forming Kir6.2 subunits of KATP channels provide additional evidence for a KATP channel-dependent action of GLP-1 to stimulate insulin secretion. In these SUR1 and Kir6.2 KO mice, potentiation of GSIS by GLP-1 is absent95,96 or reduced.97 Furthermore, in mice harboring a tyrosine to stop codon (Y12STOP) mutation in the gene coding for Kir6.2, KATP channel expression and GLP-1-stimulated insulin secretion are absent.98 Important findings are also provided by a study of patients with neonatal diabetes mellitus (NDM) owing to gain-of-function mutations (C435R; R1380H) in the gene coding for SUR1.99 These mutations lead to overactive KATP channels and a consequent reduction of GSIS. Remarkably, administration of a GLP-1R agonist restores insulin secretion in these patients.
6. Altered GLP-1 Action in a Rodent Model of Insulin Resistance
It is interesting to note that expression of Epac2 within β-cells is of critical importance to β-cell compensation that occurs in mice fed with a high-fat diet (HFD).54 The HFD induces insulin resistance, and under these conditions, GSIS is enhanced in order to compensate for insulin resistance.100 When comparing wild-type (WT) and Epac2 KO mice fed with the HFD, it is possible to demonstrate that compensatory GSIS is lost in Epac2 KO mice.54 This unexpected role of Epac2 to enable GSIS is measurable in isolated islets and it does not require treatment of islets with GLP-1R agonists. Furthermore, this compensation under conditions of the HFD results from alterations of β-cell Ca2+ handling such that there is enhanced glucose-dependent Ca2+ influx and Ca2+ mobilization.54 Since Epac2 mediates stimulatory effects of GLP-1 on Ca2+ influx and mobilization,49,91,101–103 it appears that “plasticity” exists in the β-cell cAMP signaling network such that the HFD leads to an unexpected coupling of glucose metabolism to Epac2 activation and insulin secretion.54,104
Since GLP-1R expression is elevated in islets of mice fed with the HFD,105 it is evident that GLP-1 also participates in the functional adaptation of islets to diet-induced insulin resistance. In this regard, it is interesting to note that under conditions of the HFD, in vivo administration of a GLP-1R agonist leads to an additional compensatory increase of GSIS that is “durable” in that it is measurable in isolated islets in the complete absence of in vitro GLP-1R stimulation.106 This finding indicates that a GLP-1R agonist has the capacity to upregulate the expression and functionality of key components of the β-cell stimulus-secretion coupling mechanism, most likely including glucose-sensing, oxidative glucose metabolism, ion channel regulation, and Ca2+-dependent exocytosis.
When considering how the HFD also induces islet hyperplasia with a compensatory increase of β-cell mass, it could be that increased GLP-1R expression on β-cells plays a role. Thus, increased GLP-1R expression might favor increased β-cell sensitivity to circulating GLP-1, thereby allowing GLP-1 to efficiently signal through cAMP, PKA, and Epac2 to upregulate β-cell proliferation and survival. Although this is an attractive hypothesis, recent studies indicate that in mice fed with a normal diet, enhanced PKA activity per se does not increase β-cell mass.50,107 Furthermore, a KO of Epac2 expression does not lead to decrease of β-cell mass in mice fed with a normal diet.54 However, it could be that in mice fed with an HFD, a role for PKA and Epac2 in the induction of islet hyperplasia might be revealed.
Since cAMP-independent actions of GLP-1 exist, such actions might also play a role in promoting adaptive responses of β-cells under conditions of the HFD. Thus, it is of interest to summarize what is known concerning cAMP-independent actions of GLP-1 that allow it to act as a β-cell trophic factor. Studies performed primarily with β-cell lines or mouse β-cells demonstrate cAMP-independent actions of GLP-1R agonists to counteract endoplasmic reticulum stress108 and to signal via the GLP-1R through β-arrestin109,110 and epidermal growth factor (EGF) receptor trans-activation111 in order to downregulate the activities of proapoptotic protein BAD,112 the SirT1 deacetylase,113 and transcription factor FoxO1.114 GLP-1 also upregulates the activities of c-Src kinase,115 phosphatidylinositol 3-kinase (PI-3-kinase),116 protein kinase B (PKB),117 protein kinase c-zeta (PKC-ζ),118 and extracellular signal-regulated protein kinases (ERK1/2).119 Conceivably, these growth factor-like signaling pathways might be selectively activated by allosteric GLP-1R agonists that have “biased” signal transduction properties and that bind the GLP-1R in order to promote β-cell compensation under conditions of the HFD.
7. Glucoregulatory Properties of GLP-1 Mediated by the Nervous System
A high-profile area of research concerns GLP-1 action in the nervous system, and it is recently appreciated that such actions of GLP-1 are of importance to glucoregulation. Vagal–vagal reflexes are activated by injection of a GLP-1R agonist into the hepatic portal vein, and they are measurable as increased electrical activity in vagal sensory afferent neurons and also in vagal efferent neurons.120 By measuring insulin secretion induced by intraportal administration of glucose, it is also possible to demonstrate that GSiS is potentiated by coadministration of glucose with GLP-1.121 This action of GLP-1 to potentiate GSIS is reduced by a ganglionic blocker,121 as expected if vagal–vagal reflexes activate neurons within pancreatic ganglia in order to stimulate insulin secretion. Vagal sensory neurons express GLP-1 receptors,122–124 and direct application of GL-1 to vagal afferent neuron cell bodies within the nodose ganglion leads to action potential generation.125
Vagal sensory neurons activated by GLP-1 project to the brainstem and hypothalamus, and under conditions in which the GLP-1R agonist exendin-4 is administered intraperitoneally, a surgical subdiaphragmatic vagotomy blunts activation of neurons located within the hypothalamic and paraventricular nuclei.126 Such findings obtained with rats indicate that peripherally administered exendin-4 acts via the vagus nerve to stimulate neural activity within the brain and that this effect of exendin-4 complements its more direct action to cross the blood–brain barrier in order to activate CNS GLP-1 receptors.127–129 A vagus nerve-mediated action of GLP-1R agonists also occurs in humans since in vagotomized patients treated for pyloroplasty, there is a reduced ability of intravenously infused GLP-1 to suppress appetite, to slow gastric emptying, to stimulate insulin secretion, and to suppress glucagon secretion.130
GLP-1 receptors are widely expressed within the brain where they are activated by neuronally released GLP-1. Thus, GLP-1 is a neuropeptide, and neuroanatomical studies demonstrate that it is contained within neuronal cell bodies located in the medullary caudal nucleus tractus solitarius (NTS), the raphe obscurus, and the intermediate reticular nucleus.131,132 Axons of these neurons project to regions of the brain that are involved in the control of appetite, metabolism, water intake, stress, and cardiovascular functions.133–136 These regions include the dorsal vagal nucleus, dorsomedial and paraventricular hypothalamic nuclei, ventrolateral periaqueductal gray, and thalamic paraventricular nucleus.131,132 GLP-1-containing neurons project to the brainstem where they synapse on cholinergic vagal motor neurons, some of which project to the pancreas.137,138 Collectively, these findings indicate three mechanisms by which GLP-1 controls vagal efferent activity: (1) vagal–vagal reflexes in which GLP-1 initially activates the GLP-1R located on vagal sensory nerve terminals, (2) an action of circulating GLP-1 that requires its action at, or transit across, the blood–brain barrier in order to activate brainstem neural circuits, and (3) direct or indirect synaptic relays in which GLP-1 released within the brain activates vagal motor neurons.
When considering the physiological significance of such neural influences of GLP-1, it is important to note that neural control of insulin secretion is not an absolute requirement in order to measure an insulin secretagogue action of a GLP-1R agonist. This fact is demonstrated in studies of glucoregulation using Pdx1-hGLP1R:Glp1r−/− mice administered with the DPP-IV-resistant GLP-1R agonist exendin-4.139 These engineered mice do not express the mouse GLP-1R in any tissue, whereas they express recombinant human GLP-1 receptors only in the pancreas. In such mice, exendin-4 exerts its normal action to potentiate GSIS and to improve glucose tolerance in the absence of neural GLP-1R activation.139
Despite the fact that GLP-1R agonist action is preserved in Pdx1-hGLP1R:Glp1r−/− mice, there is reason to believe that the nervous system does in fact mediate important glucoregulatory actions of GLP-1.140–145 For example, under conditions in which pancreatic insulin secretion is induced by intragastric infusion of mice with glucose, an intracerebroventricular (i.c.v.) injection of GLP-1R antagonist exendin(9–39) results in less insulin being secreted.142,146 Furthermore, glucose uptake and glycogen synthesis within skeletal muscle are enhanced by intragastric infusion of glucose, and this effect of glucose is blocked by exendin(9–39) delivered by the i.c.v. route.142,146 Such findings indicate that during the initial prandial state of intestinal glucose absorption, GLP-1 “primes” whole-body metabolism by acting within the brain to facilitate pancreatic insulin secretion while also enhancing skeletal muscle glucose disposal.
Interestingly, the neurally mediated action of GLP-1 that is important to glucoregulation may be different under conditions that mimic the postprandial state when levels of blood glucose are rising. In studies of mice using infusion clamp techniques that elevate levels of blood glucose and insulin, it is reported that i.c.v. administration of GLP-1R agonist exendin-4 reduces blood flow and glucose uptake within skeletal muscle.142,146 Simultaneously, insulin secretion is stimulated in order to enhance insulin-dependent hepatic glucose uptake.142,146 Thus, in contrast to the initial prandial state of glucose absorption described earlier, GLP-1 acts in the postprandial state to shift glucose disposal from muscle to liver. Resultant hepatic glycogen synthesis allows for sufficient glycogen mobilization and hepatic glucose production during the subsequent fasting state. What remains to be demonstrated is that such neurally mediated effects of GLP-1 occur in healthy humans and/or patients with T2DM.
It is also recognized that GLP-1 receptors located on neurons within the arcuate nucleus (Arc) are activated in order for GLP-1 to stimulate insulin secretion while also suppressing hepatic glucose production.147–149 One mechanism that may explain how GLP-1 alters neural function in the Arc is provided by the finding that transmission in these neural circuits is modulated by neuropeptides, nutrients, and hormones that control KATP channels.149–151 In this regard, GLP-1 may inhibit KATP channel activity within the Arc in order to regulate blood glucose homeostasis. It will be interesting to assess whether this action of GLP-1 is selective for glucose-responsive neurons in the Arc and whether the inhibition of KATP channel activity results from Epac2 activation, as described for β-cells. Furthermore, since leptin activates KATP channels in the Arc,150,151 it could be that GLP-1 and leptin act as counterregulatory hormones to control Arc circuits important to glucoregulation.
Finally, it is interesting to note that GLP-1R agonists are under evaluation for use in the treatment of neurological disorders.152 In an in vitro model of Alzheimer's disease, GLP-1 protects hippocampal neurons from cytotoxicity induced by amyloid-beta peptide.153 Also surprising is the report that such neuroprotection is conferred by GLP-1(9–36)amide, which is the metabolite generated by DPP-IV-catalyzed degradation of GLP-1(7–36)amide.154 This finding suggests the existence of a nonconventional GLP-1R, although its identity remains unknown. Just as interesting, there is a potential usefulness of GLP-1R agonists to treat Parkinson's disease.155,156 Collectively, the available evidence suggests that these neuroprotective actions of GLP-1 might be secondary to its ability to alter glucose homeostasis in the brain. For example, under conditions of hyperglycemia, peripherally administered GLP-1 increases the phosphorylation velocity (Vmax) of neuronal hexokinase while also increasing blood–brain glucose transport capacity (Tmax).157,158
8. GLP-1 Receptor Agonists
Drug development strategies have led to the identification of GLP-1R agonists that are either peptide-based or small molecule-based. For peptide-based GLP-1R agonists, a further subdivision exists in order to classify “incretin mimetics” or “GLP-1 analogs,” as summarized in Table 2.1. Exenatide, also known as Byetta, is the prototypical incretin mimetic and it is the synthetic form of exendin-4 (Ex-4). In contrast, the prototypical GLP-1 analog is liraglutide, also known as Victoza. Liraglutide is structurally equivalent to GLP-1(7–37) except that lysine residue 26 is acylated by its conjugation to a hexadecanoyl (C16) side chain, whereas residue 34 contains arginine rather than the lysine residue found within native GLP-1. Exenatide and liraglutide are both high-affinity agonists at the GLP-1R, yet they are relatively resistant to hydrolysis by DPP-IV. For example, after intravenous administration, the half-life of circulating GLP-1 is only 1.5–5.0 min, whereas the half-lives of exenatide and liraglutide are 26 min and 8 h, respectively. Thus, exenatide and liraglutide exert prolonged blood glucose-lowering actions when they are administered by subcutaneous injection to patients with T2DM.
Table 2.1. Pharmacological properties of GLP-1R agonists currently in use or under study for the treatment of T2DM.
| GLP-1R agonist | Parental peptide | Modifications | Half-life | Route of administration | HbAlc reduction | Weight reduction | Refs. |
|---|---|---|---|---|---|---|---|
| Exenatide BID | Ex-4 | None | 2.4 h | SC 5 or l0 μg BID | 0.7–0.9% | 2.8–3.1 kg | 159 |
| Lixisenatide | Ex-4 | Proresidue deleted from C-terminus, six Lys residues added to C-terminus | 3h | SC 20 μg QD | 0.8–0.9% | 1.8–3.0 kg | 160 |
| Exenatide LAR | Ex-4 | Injectable microspheres of biopolymer with entrapped exenatide | 5–6 d | SC 2 mg QW | 1.3–1.9% | 3.6 kg | 161,162 |
| Liraglutide | GLP-1 | Palmitic acid conjugated to Lys-26, Lys-34/Arg substitution | 11–13 h | SC 1.2 mg QD | 1.1–1.8% | 2.0–3.0 kg | 163,164 |
| Semaglutide | GLP-1 | Palmitic acid conjugated to Lys-26, Gly-8/aminoisobutyric acid, and Lys-34/Arg substitutions | 6–7 d | SC 0.1–1.6 mg QW | 1.7% | 4.8 kg | 165 |
| Albiglutide | GLP-1 | Two molecules of GLP-1 fused as a tandem and conjugated to albumin; Ala-2/Gly substitution | 6–8 d | SC 50 mg QW | 0.8% | 0.6 kg | 166–168 |
| CJC-1134-PC | Ex-4 | Peptide coupled to albumin by a linker | 8d | SC 2 mg QW | 1.4% | 169 | |
| Dulaglutide | GLP-1 | Two molecules of GLP-1 covalently linked to a IgG4-Fc heavy chain; Ala-8/ Gly, Gly-26/Glu, Arg-36/Gly substitutions | 4d | SC 1.5 mg QW | 1.5% | NS | 170,171 |
| Langlenatide | Ex-4 | Peptide fused to Fc region | 6d | SC 1–4 mg QW SC 8–16 mg QMT |
NDA | NDA | 172 |
| VRS-859 | Ex-4 | Peptide fused to Xten protein | 3d | SC 200 mg QMT | NDA | NDA | 173 |
Ex-4, exendin-4; BED, twice daily dosing; QD, once daily dosing; QW, once weekly dosing; QMT, once monthly dosing; SC, subcutaneous administration; HbAlc, hemoglobin Alc.
Mechanistically, the hexadecanoyl side chain of liraglutide allows this peptide to bind to plasma albumin via hydrophobic interactions, thereby minimizing hydrolysis by DPP-IV. In the GLP-1 analog albiglutide, two molecules of GLP-1(7–36) are fused in tandem, and the tandem is covalently conjugated to recombinant human albumin in order to achieve DPP-IV resistance. Simultaneously, a glycine substitution is introduced at residue 8 in order to improve DPP-IV resistance. In dulaglutide, a different approach is taken in which GLP-1(7–36) is fused to human immunoglobulin heavy constant γ4 chain (IgGγ4-Fc) to create a monomer that then dimerizes with itself in order to generate the DPP-IV-resistant GLP-1R agonist.
Attempts to identify small molecule GLP-1R agonists are complicated by the complex ligand–receptor interactions that are characteristic of group B GPCRs.46,174 Despite this complication, new ago-allosteric modulators of the GLP-1R are described. These small molecules not only act as GLP-1R agonists (ago control) but also modify the ability of GLP-1 itself to activate the GLP-1R (allosteric control). Synthetic ago-allosteric modulators currently under preclinical investigation include substituted quinoxaline76,175–177 and cyclobutane derivatives.178–180 substituted quinoxaline designated as compound 2 acts as a partial agonist at the GLP-1R, but it is particularly revealing that the efficacy of compound 2 as a cAMP-elevating agent is enhanced rather than reduced by GLP-1R antagonist exendin(9–39). This finding is consistent with the concept that allostery results from binding of compound 2 to a site on the GLP-1R that is not recognized by peptide-based agonists and antagonists.181 Expanding on these findings, it is reported that novel substituted pyrimidines also act as GLP-1R agonists and that they do not compete with radiolabeled GLP-1 for binding to the GLP-1R.182
GLP-1R activation by the quinoxaline compound 3 is strongly influenced by mutations introduced into transmembrane α-helices 2 and 7, whereas such mutations do not alter the action of GLP-1.183 These findings indicate that small molecule agonists activate or modulate the GLP-1R in a manner that is distinct from that of GLP-1. In fact, a quinoxaline (compound 2) and a pyrimidine (compound B) act in an additive manner to activate the GLP-1R under conditions in which the receptor is truncated to remove the N-terminal extracellular domain at which the C-terminus of GLP-1 binds.184 Just as intriguing, GLP-1R-mediated signaling properties of ago-allosteric modulators are not identical, thereby suggesting that such agonists could be used in order to achieve signal transduction bias.77,184–187
9. DPP-IV Inhibitors
DPP-IV encoded by the DPP4 gene is a member of the prolyl oligopeptidase family of serine proteases, and it plays a role in the control of immune function and is a key determinant of incretin hormone action. DPP-IV exists as a soluble circulating form188 or as a type transmembrane serine exopeptidase.189 Both forms of the enzyme catalyze the cleavage of dipeptides from the N-terminus of peptide substrates that contain on average 30-amino-acid residues and that have a proline or alanine residue in the penultimate position.189 These substrates include chemokines (CCL5), neuropeptides (PYY and NPY), and hormones (GLP-1 and GIP).190 Terminology exists in which DPP-IV is also known as adenosine deaminase complexing protein 2 (ADCP 2) or as the T-cell activation antigen CD26. DPP-IV is highly expressed on endothelial cells, differentiated epithelial cells, and lymphocytes. In the immune system, DPP-IV exists as an integral membrane glycoprotein in which it acts as a cofactor to control intracellular signaling pathways that are of importance to T-cell proliferation and T-cell activation.191
Crystallographic analysis combined with molecular modeling reveals that the 766-amino-acid residue DPP-IV contains an N-terminal β-propeller domain and a C-terminal α/β hydrolase domain that together form a cavity in which the enzyme's active site is located.192,193 A distinguishing feature of DPP-IV is that the enzyme's α/β hydrolase domain contains a serine–aspartate–histidine catalytic triad, whereas the β-propeller domain contains two glutamate residues that are necessary for catalytic function and that align the substrate peptide so that only the penultimate proline or alanine residues may engage the active site. This structural feature of DPP-IV explains its substrate specificity in which it hydrolyzes peptides with N-terminal X-proline or X-alanine residues.194
Summarized in Table 2.2 are the pharmacological properties of small molecule DPP-IV inhibitors now in use for the treatment of T2DM. The xanthine class of DPP-IV inhibitors includes sitagliptin, linagliptin, and alogliptin, whereas vildagliptin and saxagliptin are members of the cyanopyrrolidine class of DPP-IV inhibitors. Inhibition of DPP-IV activity by sitagliptin is achieved by its noncovalent binding to the conserved glutamate residues 205 and 206 located within the enzyme's β-propeller, whereas saxagliptin binds not only to these glutamate residues but also to the serine residue located within the catalytic triad of the α/β hydroxylase domain.190 In general, cyanopyrrolidines such as saxagliptin are competitive inhibitors rather than noncompetitive inhibitors of DPP-IV enzymatic activity since they form reversible covalent bonds with serine residue 630 located within the enzyme's active site.190
Table 2.2. Pharmacological properties of DPP-IV inhibitors currently in use or under study for the treatment of T2DM.
| Inhibitor | Half-life | Route of administration | Plasma DPP-IV inhibition | Plasma GLP-1 increase | HbAlc reduction | Route of elimination | Refs. |
|---|---|---|---|---|---|---|---|
| Sitagliptin | 11–13h | PO 25–200 mg QD | 80% with 50 mg | 2 times | 0.6–0.8% | Mostly renal | 195–197 |
| Vildagliptin | 1.7–3 h | PO 25–200 mg QD or BID | 80–90% | 2–3 times | 0.5–1.5% | Mostly renal | 197–199 |
| Saxagliptin | 2.2-3.8 h 3.0–7.4 h | PO 2.5–50 mg QD | 70% | 1.5–2 times | 0.5–0.9% | Mostly renal | 23,197,200,201 |
| Linagliptin | 113–260 h | PO 0.5–10 mg QD | 46% with 0.5 mg 78% with 2.5 mg 90% with l0 mg |
2 times (0.5 mg) 3 times (2.5 mg) 4 times (10 mg) |
0.4–0.8% | Hepatic (biliary excretion) | 197,202,203 |
| Alogliptin | 12–21 h | PO 25–800 mg QD | 74–97% | 2–4 times | 0.5–0.9% | Mostly renal | 197,204,205 |
DPP-IV catalyzes the hydrolysis of GLP-1(7–36)amide to generate GLP-1(9–36)amide and the N-terminal histidine–alanine dipeptide. Therefore, DPP-IV inhibitors raise levels of endogenous GLP-1(7–36)amide, and it could be that at clinically relevant doses, this action of DPP-IV inhibitors produces a relatively selective increase of GLP-1(7–36)amide in the hepato-portal circulation or at the interface of L cells and vagal sensory nerve terminals. Since DPP-IV inhibitors suppress enzymatic production of GLP-1(9–36)amide, while also preventing release of the histidine–alanine dipeptide, it is of concern that these two metabolites might have important biological actions that would be missing in patients administered with DPP-IV inhibitors. In fact, GLP-1(9–36)amide exerts prosurvival actions in neurons and cardiomyocytes154,206 while also suppressing hepatic glucose production in obese patients.207,208. Furthermore, the histidine–alanine dipeptide is reported to influence glucose tolerance and insulin secretion in mice.209
10. GLP-1-Based Strategies for the Treatment of T2DM
A GLP-1-based strategy for the treatment of T2DM is indicated in view of the fact that GLP-1R agonists and DPP-IV inhibitors exert a beneficial constellation of physiological effects that include (1) glucose-dependent stimulation of insulin secretion, (2) suppression of glucagon secretion, (3) normalization of blood glucose without an attendant risk of hypoglycemia, (4) slowing of gastric emptying, (5) appetite suppression, and (6) weight loss. Potential additional benefits are actions to promote β-cell survival by slowing apoptosis or to promote β-cell regeneration by stimulating β-cell proliferation. Thus, it was originally anticipated that such a GLP-1-based therapy might lead to a long-term remission and possibly a cure for T2DM.210 Since the notion of a GLP-1-based therapy has led to the term “incretin therapy,” it is important to note that when considering the use of incretins for the treatment of T2DM, only GLP-1 is effective, whereas GIP is ineffective.8,211
Presently available GLP-1R agonists include exenatide (approved in the United States in 2005) and liraglutide (approved in the United States in 2010), both of which are administered by subcutaneous injection. Exenatide is approved for use twice a day, and liraglutide is approved for use once a day. An extended release (ER) formulation of exenatide is intended for use once a week. Additional long-acting formulations are under investigation including one depot preparation of exenatide that can be given once every 6 months. In contrast to exenatide and liraglutide, the DPP-IV inhibitors are orally administrable and are therefore a more convenient means by which to treat T2DM. Currently, in the United States, there are four approved drugs of this class. Sitagliptin was first approved in 2006, and since then, three additional DPP-IV inhibitors have been approved. They are saxagliptin, linagliptin, and alogliptin. In addition, vildagliptin and gemigliptin are available in other countries.
When considering the use of GLP-1R agonists or DPP-IV inhibitors for the treatment of T2DM, it is important to note that DPP-IV inhibitors raise levels of circulating GLP-1 by approximately twofold, whereas GLP-1R agonists exert a dose-dependent pharmacological effect that is considerably more potent since their circulating levels easily exceed endogenous GLP-1 levels by eightfold.212 These pharmacological differences may explain why GLP-1R agonists are more effective inhibitors of gastric emptying, while also suppressing appetite and promoting weight loss. In fact, in some patients, the high potency of GLP-1R agonists can lead to adverse side effects of nausea and vomiting.213
GLP-1R agonists and DPP-IV inhibitors are approved for use in patients with T2DM, typically as adjuncts to diet and exercise and as either a monotherapy or a combination therapy with other antidiabetic medications.214–217 Exenatide ER and liraglutide are not recommended as first-line therapies although they may be considered for monotherapy in patients who are unable to use other first-line therapies because of a lack of efficacy or due to contraindications such as allergic hypersensitivity, end-stage renal disease, and gastrointestinal diseases. DPP-IV inhibitors are also contraindicated in patients with hypersensitivity reactions such as urticaria, angioedema, or bronchial hypersensitivity. In addition to a history of serious hypersensitivity as a contraindication, exenatide ER and liraglutide are also contraindicated in patients with a personal or family history of medullary thyroid cancer or with a history of multiple endocrine neoplasia syndrome type 2 (MEN2). Prescribing information, warning labels, and precaution sections for exenatide and liraglutide or various DPP-IV inhibitors also list pancreatitis as a potential adverse side effect of their use.214–217
A review of the clinical DPP-IV literature concerning monotherapy for the treatment of T2DM indicates 25 randomized control trials (RCTs) in adult patients with trial durations of at least 12 weeks.218 Sitagliptin and vildagliptin therapy results in an HbA1c reduction of 0.7% and 0.6%, respectively. In another review that includes 17 RCTs of 8 weeks minimum duration, monotherapy with GLP-1R agonists results in reductions of HbA1c of ca. 1%.219 Although β-cell function improves with GLP-1R agonist treatment, it is interesting to note that a rapid deterioration of glucoregulation can occur after withdrawal of these medications. Thus, unlike the situation reported for mice administered with a GLP-1R agonist,106 a “durable” effect of GLP-1R agonists is not so obvious in humans. This finding seems to argue that in humans, the primary effect of GLP-1R agonists is to exert an acute stimulatory effect on β-cell insulin secretion, rather than acting long term to alter β-cell gene expression.
GLP-1R agonists and DPP-IV inhibitors are also under study for use in combination with non-GLP-1-based medications such as insulin, sulfonylureas, the biguanide metformin, and the thiazolidinedione pioglitazone.220–225 Especially noteworthy is the 2011 approval in the United States of exenatide as an add-on therapy to basal insulin analog glargine for patients with T2DM who are not achieving adequate glycemic control using glargine alone. Although GLP-1R agonists and DPP-IV inhibitors can be used in combination with sulfonylureas, there is an increased risk of hypoglycemia so that caution should be exercised and preemptive dose reduction should be implemented. In this regard, an attractive alternative therapy is based on the use of metformin in combination with a GLP-1R agonist or DPP-IV inhibitor. This combination therapy has a reduced risk hypoglycemia, yet it still promotes beneficial weight loss in patients with T2DM.
In the DURATION clinical trial series,161,226–229 T2DM patients are reported to lose an average 2–4 kg body weight when treated with exenatide ER (2 mg per weekly as a single injection). The weight loss achieved with exenatide ER is similar to that observed in patients administered with non-ER exenatide twice daily (5–10 mcg per single injection). Importantly, body weight reduction is significantly larger for patients administered with exenatide ER in comparison to administered sitagliptin (−2.3 vs. −0.8 kg, respectively).226 The LEAD (Liraglutide Effect and Action in Diabetes) trial also reveals significant body weight reduction with liraglutide monotherapy.230 This weight loss is primarily due to reduced fat mass, mainly visceral adipose tissue.231
As summarized in Table 2.3, a GLP-1-based therapy for the treatment of T2DM is particularly attractive since it not only normalizes glycemia while reducing body weight but also improves cardiovascular function.241,242 GLP-1R agonist treatment has positive effects on cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and obesity. A pooled data analysis from six clinical trials investigating the outcomes of 6-month exenatide treatment in 2171 T2DM patients reveals significantly greater reductions in systolic blood pressure compared with placebo.243 Mechanistically, such a reduction of blood pressure is consistent with the report that liraglutide exerts an action in the mouse atrial myocardium to stimulate the release of atrial natriuretic factor (ANF) that then acts to relax vascular smooth muscle while also promoting renal excretion of sodium ion244. GLP-1R agonist therapy also results in favorable changes in circulating lipids, which are another important cardiovascular risk factor. Meta-analysis demonstrates that liraglutide lowers blood levels of total cholesterol, low-density lipoproteins, free fatty acids, and triglycerides.245
Table 2.3. Cardiovascular actions of GLP-1-based therapeutics.
| GLP-1R agonist | Experimental/clinical setting | Effect of GLP-1R agonist | Refs. |
|---|---|---|---|
| Exenatide | TG9 mice (murine DCM model) | Improvement of glucose tolerance; increase 2-deoxyglucose uptake and GLUT4 expression in myocardium | 232 |
| GLP-1 | Dogs with pacing-induced DCM | Increase insulin sensitivity, basal and insulin-stimulated glucose extraction, and uptake in myocardium, and decrease plasma glucagon | 233 |
| GLP-1 GLP-1 (9–36) |
Dogs with pacing-induced DCM | Both peptides increase insulin sensitivity and basal and insulin-stimulated glucose uptake in myocardium, and decrease plasma glucagon | 234 |
| Exenatide | Diabetic (STZ-induced) rats | Increase myocardial glucose uptake | 235 |
| Vildagliptin | Model of murine heart failure | Increase plasma GLP-1, improvement of glucose tolerance | 236 |
| Liraglutide | Mice on HFD | Decrease insulin resistance | 237 |
| GLP-1 | Patients before, during, and after CABG | Decrease pre- and perioperative plasma glucose, decrease postoperative plasma glucagon, decrease postoperative insulin infusion required, decrease pharmacological or mechanical support to achieve hemodynamic stability in postoperative period | 238 |
| GLP-1 | T2DM patients after CABG | Decrease postoperative insulin infusion required Decrease dobutamine infusion required |
239 |
| GLP-1 | T1D patients | Decrease hyperglycemia- or hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction | 240 |
DCM, dilated cardiomyopathy; STZ, streptozotocin; HFD, high-fat diet; CABG, coronary artery bypass grafting.
11. Improved Glucoregulation after Bariatric Surgery
There is evidence that intestinally released GLP-1 might mediate the beneficial outcomes of bariatric surgery in which a Roux-en-Y gastric bypass (RYGB) leads to weight loss that is accompanied by elevated levels of plasma GLP-1 and improved glucoregulation in patients with T2DM.246–252 This is a clinically important issue to address since RYGB surgery results in weight loss in 30–40% of obese patients, whereas improved glucoregulation is observed in about 80% of T2DM patients.253–255 Unfortunately, our understanding how these beneficial outcomes of RYGB surgery are achieved is complicated by weaknesses in the experimental designs of published studies.256 In fact, the role of elevated plasma GLP-1 as a determining factor in the remission of T2DM is disputed,257 and it is instead reported that β-cells are rendered more sensitive to circulating GLP-1 after RYGB surgery.258 Evidently, RYGB induces compensatory changes in β-cells that lead to improved blood glucose control.258
Additional clinical observations reveal that levels of blood glucose are quickly normalized after RYGB surgery, even before significant weight loss is achieved.259 Though decreased caloric intake or reduced intestinal nutrient absorption is an obvious cause for the weight loss, there appear to be additional important factors that explain a remission of T2DM after RYGB surgery. This conclusion is supported by the following observations: (1) Remission occurs in the immediate postoperative period before any weight loss occurs, (2) remission is more pronounced after RYGB surgery as compared with outcomes achieved by dieting in order to achieve comparable weight loss, and (3) remission is more pronounced after RYGB surgery as compared with other forms of bariatric surgery (sleeve gastrectomy and gastric banding).
Differences in the outcomes achieved following RYGB or dieting are clearly evident since RYGB, but not dieting, leads to enhanced postprandial release of GLP-1, thereby restoring the incretin effect in patients with T2DM.248 Under postoperative conditions of RYGB in T2DM, there is also a restoration of the missing first phase kinetic component of GSIS, and there is an accompanying improvement of oral glucose tolerance.248 Unfortunately, the physiological basis for immediate or long-term endocrine and metabolic changes after RYGB is not fully elucidated. Changes in the rate of eating, gastric emptying, nutrient absorption and sensing, incretin hormone release, bile acid metabolism, and intestinal microbiota composition may all be important.260–263
Increased intestinal GLP-1 secretion after gastric bypass surgery appears to be sustained and can potentially have beneficial effects in terms of weight loss and long-term remission of T2DM. In addition to this surgery's acute stimulatory effect on insulin secretion, it could be that the sustained elevation of blood GLP-1 might regenerate β-cells. However, a potential drawback to surgery is that it is not yet clear whether a postoperative remission of T2DM is permanent or only temporary.256,264–267 Furthermore, this gastric bypass surgery can lead to hyperinsulinemic hypoglycemia, thereby necessitating pancreatectomy.268,269 Interestingly, the hyperinsulinemia in some patients who have had gastric bypass surgery does not appear to be secondary to an increase of β-cell mass, as might be expected if bypass surgery upregulates long-term actions of GLP-1 to stimulate β-cell proliferation. In one study of gastric bypass patients undergoing partial pancreatectomy to correct for hyperinsulinemia, histological analyses of pancreatic sections reveal no change in β-cell mass, proliferation, neogenesis, or apoptosis.269 Thus, the nature of the adaptive change that underlies remission of T2DM after gastric bypass surgery remains to be determined.
12. Safety Considerations for GLP-1-based Therapeutics
An ongoing controversy concerns whether the use of GLP-1-based therapeutics predisposes to unexpected side effects including inflammation of the pancreas (pancreatitis) or even pancreatic cancer in patients with T2DM.270–274 Furthermore, postmortem histological analyses of pancreatic tissue from patients treated with a GLP-1R agonist or DPP-IV inhibitors provide evidence for an increased incidence of pancreatic exocrine cell dysplasia accompanied by hyperplasia of glucagon-secreting α-cells of the endocrine pancreas.79 These findings have raised the specter that chronic GLP-1R activation in humans might lead to the appearance of exocrine cell adenocarcinomas or neuroendocrine tumors such as glucagonomas or insulinomas. Additional studies of rodents indicate that chronic stimulation of GLP-1 receptors on calcitonin-secreting C cells of the thyroid can lead to C-cell hyperplasia with eventual medullary thyroid cancer,275 although this outcome is not measurable in nonhuman primates276 and has yet to be demonstrated for humans. Countering these findings, it is argued that the benefits of GLP-1-based therapeutics outweigh their risks when considering their usefulness for the treatment of T2DM.277,278 Currently, these safety concerns remain debated, and it is pointed out that in the published literature, there is no direct demonstration of causality linking GLP-1-based therapeutics to human pancreatitis, pancreatic cancer, or thyroid cancer. Despite this fact, the Food and Drug Administration acted in 2007 to issue a safety alert concerning the potential for pancreatitis in patients treated with exenatide. Furthermore, a black box warning is now provided with prescription information for both exenatide and liraglutide. In 2013, both the American Diabetes Association and the Endocrine Society called for independent review of findings relating to these potential adverse side effects of GLP-1R agonists and DPP-IV inhibitors.
13. Conclusion
Nearly 30 years of basic science and clinical research has culminated with the recognition that GLP-1-based therapies for the treatment of T2DM are highly effective. Unanticipated are the surprising beneficial cardiovascular and neuroprotective actions of this class of blood glucose-lowering agents. Since GLP-1R agonists also produce substantial weight loss in obese patients, it is clear that pharmacological GLP-1R activation can be particularly useful for treating or reversing the increasingly common metabolic syndrome of hyperglycemia, impaired cardiovascular function, excess weight, and neuropathology. Although safety concerns are increasingly debated, the general consensus at the present time is that additional clinical research is necessary in order to establish whether the use of GLP-1R agonists or DPP-IV inhibitors predisposes to pancreatitis or cancer. Looking to the future, it is anticipated that a new approach to drug development will be popularized in order to identify GLP-1R agonists that have a reduced propensity to promote cell growth while retaining their capacity to stimulate pancreatic insulin secretion. Particularly useful will be new approaches that allow oral delivery of GLP-1R agonists, either as synthetic small molecule compounds or as novel peptide conjugates.279
Acknowledgments
G. G. H. and O. G. C. acknowledge the support of a Basic Science Award (7-12-BS-077) from the American Diabetes Association. All authors also acknowledge the institutional support of SUNY Upstate Medical University.
List of Abbreviations
- Ach
acetylcholine
- ANF
atrial natriuretic factor
- CICR
Ca2+-induced Ca2+ release
- CNS
central nervous system
- CBP
CREB-binding protein
- CRE
cAMP response element
- CREB
cAMP response element-binding protein
- CRTC
CREB-regulated transcriptional coregulator
- DPP-IV
dipeptidyl-peptidase-IV
- Epac
cAMP-regulated guanine nucleotide exchange factor
- ER
extended release
- GLP-1R
GLP-1 receptor
- GPCR
G protein-coupled receptor
- GSIS
glucose-stimulated insulin secretion
- HFD
high-fat diet
- IRS-2
insulin receptor substrate 2
- KATP
ATP-sensitive K+ channel
- KO
knockout
- MEN2
multiple endocrine neoplasia syndrome type 2
- NDM
neonatal diabetes mellitus
- NPY
neuropeptide tyrosine
- PACAP
pituitary adenylyl cyclase-activating peptide
- PG
proglucagon
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- PTH
parathyroid hormone
- PYY
peptide tyrosine tyrosine
- RCT
randomized control trial
- RYGB
Roux-en-Y gastric bypass
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SUR1
sulfonylurea receptor type 1
- T2DM
type 2 diabetes mellitus
- TMAC
transmembrane adenylyl cyclase
- TxNIP
thioredoxin-interacting protein
- VDCC
voltage-dependent Ca2+ channel
- WT
wild type
Footnotes
All authors declare no conflict of interest concerning any of the concepts addressed in this review of the literature.
References
- 1.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ. The physiology of glucagon-like peptide-1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Burcelin R. The gut-brain axis: a major glucoregulatory player. Diabetes Metab. 2010;36(Suppl 3):S54–S58. doi: 10.1016/S1262-3636(10)70468-7. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 7.Gutniak M, Ø´rskov C, Holst JJ, Ahrén B, Efendić S. Antidiabetic effect of glucagon-like peptide-1 (7-36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 8.Nauk MA, Heimesaat MM, Ø´rskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide-1[7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holz GG, Chepurny OG. Glucagon-like peptide-1synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 11.Lund PK, Goodman RH, Dee PC, Habener JF. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc Natl Acad Sci U S A. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- 13.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide-1(7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreymann B, Yiangou Y, Kanse S, Williams G, Ghatei MA, Bloom SR. Isolation and characterization of GLP-1 7-36 amide from rat intestine. Elevated levels in diabetic rats. FEBS Lett. 1988;242:167–170. doi: 10.1016/0014-5793(88)81008-1. [DOI] [PubMed] [Google Scholar]
- 15.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 16.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- 17.Habener JF, Stanojevic V. Alpha cells come of age. Trends Endocrinol Metab. 2013;24:153–163. doi: 10.1016/j.tem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide-1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 22.Augustyns K, Bal G, Thonus G, et al. The unique properties of dipeptidyl-peptidase IV (DPP IV / CD26) and the therapeutic potential of DPP IV inhibitors. Curr Med Chem. 1999;6:311–327. [PubMed] [Google Scholar]
- 23.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51:501–514. doi: 10.1007/BF03261927. [DOI] [PubMed] [Google Scholar]
- 24.Shigeto M, Katsura M, Matsuda M, Ohkuma S, Kaku K. Low, but physiological, concentration of GLP-1 stimulates insulin secretion independent of the cAMP-dependent protein kinase pathway. J Pharmacol Sci. 2008;108:274–279. doi: 10.1254/jphs.08090fp. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen LL, Young AA, Parkes DG. Pharmacology of Exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Eng J, Kleinman WA, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 27.Drucker D, Easley C, Kirkpatrick P. Sitagliptin. Nat Rev Drug Discov. 2007;6:109–110. doi: 10.1038/nrd2245. [DOI] [PubMed] [Google Scholar]
- 28.Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70:2089–2112. doi: 10.2165/11206370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 2013;25:570–579. doi: 10.1016/j.cellsig.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ, Ø´rskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211:169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 32.Weir GC, Mojsov S, Heinrich G, Habener JF. Glucagon-like peptide-1(7-37) actions on endocrine pancreas. Diabetes. 1989;38:338–342. doi: 10.2337/diab.38.3.338. [DOI] [PubMed] [Google Scholar]
- 33.Thorens B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ø´rskov C, Jeppesen J, Matsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorens B, Porret A, Bühler L, Deng SP, Morrel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 36.Dillon JS, Tanizawa Y, Wheeler MB, et al. Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology. 1993;133:1907–1910. doi: 10.1210/endo.133.4.8404634. [DOI] [PubMed] [Google Scholar]
- 37.Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-1(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 38.Skoglund G, Hussain MA, Holz GG. Glucagon-like peptide-1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes. 2000;49:1156–1164. doi: 10.2337/diabetes.49.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chepurny OG, Hussain MA, Holz GG. Exendin-4 as a stimulator of rat insulin I gene promoter activity via bZIP/CRE interactions sensitive to serine/threonine protein kinase inhibitor Ro 31-8220. Endocrinology. 2002;143:2303–2313. doi: 10.1210/endo.143.6.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay CW, Sinclair EM, Bermano G, Durward E, Tadayyon M, Docherty K. Glucagon-like peptide-1 stimulates human insulin promoter activity in part through cAMP-responsive elements that lie upstream and downstream of the transcription start site. J Endocrinol. 2005;186:353–365. doi: 10.1677/joe.1.06205. [DOI] [PubMed] [Google Scholar]
- 41.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 42.Buteau J. GLP-1 receptor signaling: effects on pancreatic β-cell proliferation and survival. Diabetes Metab. 2008;34(Suppl 2):S73–S77. doi: 10.1016/S1262-3636(08)73398-6. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh CH, Widenmaier S, Kim SJ. Pleiotropic actions of the incretin hormones. Vitam Horm. 2010;84:21–79. doi: 10.1016/B978-0-12-381517-0.00002-3. [DOI] [PubMed] [Google Scholar]
- 44.Portha B, Tourrel-Cuzin C, Movassat J. Activation of the GLP-1 receptor signaling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. 2011;2011:376509. doi: 10.1155/2011/376509. http://dx.doi.org/10.1155/2011/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yabe D, Seino S. Two incretin hormones GLP-1 and GIP: comparison of their actions on insulin secretion and β cell preservation. Prog Biophys Mol Biol. 2011;107:248–256. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Willard FS, Bueno AB, Sloop KW. Small molecule drug discovery at the glucagon-like peptide-1 receptor. Exp Diabetes Res. 2012;2012:709893. doi: 10.1155/2012/709893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001 Nov 23;2:reviews3013.1–reviews3013.10. doi: 10.1186/gb-2001-2-12-reviews3013. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol. 2010;654:281–304. doi: 10.1007/978-90-481-3271-3_13. [DOI] [PubMed] [Google Scholar]
- 49.Leech CA, Chepurny OG, Holz GG. Epac2-dependent Rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm. 2010;84:279–302. doi: 10.1016/B978-0-12-381517-0.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song WJ, Seshadri M, Ashraf U, et al. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011;13:308–319. doi: 10.1016/j.cmet.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalle S, Quoyer J, Varin E, Costes S. Roles and regulation of the transcription factor CREB in pancreatic β -cells. Curr Mol Pharmacol. 2011;4:187–195. doi: 10.2174/1874467211104030187. [DOI] [PubMed] [Google Scholar]
- 52.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jhala US, Canettieri G, Screaton RA, et al. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song WJ, Mondal P, Li Y, Lee SE, Hussain MA. Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A. Diabetes. 2013;62:2796–2807. doi: 10.2337/db12-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng K, Andrikopoulos S, Gunton JE. First phase insulin secretion and type 2 diabetes. Curr Mol Med. 2013;13:126–139. [PubMed] [Google Scholar]
- 56.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 2011;13:331–339. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedrichsen BN, Neubauer N, Lee YC, et al. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188:481–492. doi: 10.1677/joe.1.06160. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shao W, Wang Z, Ip W, et al. GLP-1(28–36) improves β-cell mass and glucose disposal in streptozotocin induced diabetes mice and activates PKA-β-catenin signaling in beta-cells in vitro. Am J Physiol Endocrinol Metab. 2013;304:E1263–E1272. doi: 10.1152/ajpendo.00600.2012. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1 (28-36)amide protects pancreatic β-cells from glucolipotoxicity. J Endocrinol. 2012;213:143–154. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guettier JM, Gautam D, Scarselli M, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 2001;142:1820–1827. doi: 10.1210/endo.142.5.8128. [DOI] [PubMed] [Google Scholar]
- 64.Shao W, Yu Z, Fantus IG, Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal. 2010;22:1240–1246. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Mukai E, Fujimoto S, Sato H, et al. Exendin-4 suppresses SRC activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60:218–226. doi: 10.2337/db10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2006;346:1067–1074. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Bergwitz C, Gardella TJ, Flannery MR, et al. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand-receptor interaction. J Biol Chem. 1996;271:26469–26472. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- 68.Miller LJ, Dong M, Harikumar KG, Gao F. Structural basis of natural ligand binding and activation of the Class II G-protein-coupled secretin receptor. Biochem Soc Trans. 2007;35:709–712. doi: 10.1042/BST0350709. [DOI] [PubMed] [Google Scholar]
- 69.Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem Sci. 2009;34:303–310. doi: 10.1016/j.tibs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2011;166:27–41. doi: 10.1111/j.1476-5381.2011.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Q, Pinon DI, Miller LJ, Dong M. Spatial approximations between residues 6 and 12 in the amino-terminal region of glucagon-like peptide-1 and its receptor. A region critical for biological activity. J Biol Chem. 2010;285:24508–24518. doi: 10.1074/jbc.M110.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koole C, Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM. Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) has a critical role in GLP-1 peptide binding and receptor activation. J Biol Chem. 2012;287:3642–3658. doi: 10.1074/jbc.M111.309328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathi SKI, Chan Y, Li X, Wheeler MB. Scanning of the glucagon-like peptide-1 receptor localizes G protein-activating determinants primarily to the N terminus of the third intracellular loop. Mol Endocrinol. 1997;11:424–432. doi: 10.1210/mend.11.4.9913. [DOI] [PubMed] [Google Scholar]
- 74.Dong M, Gao F, Pinon DI, Miller LJ. Insights into the structural basis of endogenous agonist activation of family B G protein-coupled receptors. Mol Endocrinol. 2008;22:1489–1499. doi: 10.1210/me.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harikumar KG, Wootten D, Pinon DI, et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc Natl Acad Sci U S A. 2012;109:18607–18612. doi: 10.1073/pnas.1205227109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knudsen LB, Kiel D, Teng M, et al. Small-molecule agonists for the glucagon-like pep-tide 1 receptor. Proc Natl Acad Sci U S A. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koole C, Wootten D, Simms J, et al. Allosteric ligands of the glucagon-like peptide-1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol Pharmacol. 2010;78:456–465. doi: 10.1124/mol.110.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willard FS, Wootten D, Showalter AD, et al. Small molecule allosteric modulation of the glucagon-like peptide-1 receptor enhances the insulinotropic effect of oxyntomodulin. Mol Pharmacol. 2012;82:1066–1073. doi: 10.1124/mol.112.080432. [DOI] [PubMed] [Google Scholar]
- 79.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide-1. Pflugers Arch. 1998;435:583–594. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- 81.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res. 2004;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol. 2004;95:252–262. doi: 10.1111/j.1742-7843.2004.t01-1-pto950502.x. [DOI] [PubMed] [Google Scholar]
- 83.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 84.Holz GG, Chepurny OG, Leech CA, Song WJ, Hussain MA. The Islets of Langerhans. 2nd. Springer; 2013. Molecular basis of cAMP signaling in pancreatic β-cells. in press. [Google Scholar]
- 85.Holz GG, 4th, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic β-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J Physiol. 2003;546:175–189. doi: 10.1113/jphysiol.2002.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang G, Joseph JW, Chepurny OG, et al. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang G, Chepurny OG, Rindler MJ, et al. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leech CA, Dzhura I, Chepurny OG, et al. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol. 2011;107:236–247. doi: 10.1016/j.pbiomolbio.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- 93.Kang G, Chepurny OG, Malester B, et al. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2008;586:1307–1319. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakazaki M, Crane A, Hu M, et al. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51:3440–3449. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- 96.Shiota C, Larsson O, Shelton KD, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 97.Miki T, Minami K, Shinozaki H, et al. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes. 2005;54:1056–1063. doi: 10.2337/diabetes.54.4.1056. [DOI] [PubMed] [Google Scholar]
- 98.Hugill A, Shimomura K, Ashcroft FM, Cox RD. A mutation in KCNJ11 causing human hyperinsulinism (Y12X) results in a glucose-intolerant phenotype in the mouse. Diabetologia. 2010;53:2352–2356. doi: 10.1007/s00125-010-1866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bourron O, Chebbi F, Halbron M, et al. Incretin effect of glucagon-like peptide-1 receptor agonist is preserved in presence of ABCC8/SUR1 mutation in β-cell. Diabetes Care. 2012;35:e76. doi: 10.2337/dc12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 101.Bode HP, Moormann B, Dabew R, Göke B. Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology. 1999;140:3919–3927. doi: 10.1210/endo.140.9.6947. [DOI] [PubMed] [Google Scholar]
- 102.Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holz GG, Chepurny OG, Leech CA. Epac2A makes a new impact in β-cell biology. Diabetes. 2013;62:2665–2666. doi: 10.2337/db13-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahlqvist L, Brown K, Ahrén B. Upregulated insulin secretion in insulin-resistant mice: evidence of increased islet GLP1 receptor levels and GPR119-activated GLP1 secretion. Endocr Connect. 2013;2:69–78. doi: 10.1530/EC-12-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winzell MS, Ahrén B. Durable islet effects on insulin secretion and protein kinase A expression following exendin-4 treatment of high-fat diet-fed mice. J Mol Endocrinol. 2008;40:93–100. doi: 10.1677/JME-07-0121. [DOI] [PubMed] [Google Scholar]
- 107.Kaihara KA, Dickson LM, Jacobson DA, et al. β-cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes. 2013;62:1527–1536. doi: 10.2337/db12-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. β-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc Natl Acad Sci U S A. 2008;105:6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalle S, Ravier MA, Bertrand G. Emerging roles for β-arrestin in the control of the pancreatic β-cell function and mass: new therapeutic strategies and consequences for drug screening. Cell Signal. 2011;23:522–528. doi: 10.1016/j.cellsig.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 111.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide-1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 112.Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J Biol Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bastien-Dionne PO, Valenti L, Kon N, Gu W, Buteau J. Glucagon-like peptide-1 inhibits the sirtuin deacetylase SirT1 to stimulate pancreatic β-cell mass expansion. Diabetes. 2011;60:3217–3222. doi: 10.2337/db11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes. 2006;55:1190–1196. doi: 10.2337/db05-0825. [DOI] [PubMed] [Google Scholar]
- 115.Talbot J, Joly E, Prentki M, Buteau J. β-Arrestin 1-mediated recruitment of c-Src underlies the proliferative action of glucagon-like peptide-1 in pancreatic β INS832/13 cells. Mol Cell Endocrinol. 2012;364:65–70. doi: 10.1016/j.mce.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 116.Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004;47:478–487. doi: 10.1007/s00125-004-1327-5. [DOI] [PubMed] [Google Scholar]
- 118.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Cζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 119.Arnette D, Gibson TB, Lawrence MC, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic β cells. J Biol Chem. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- 120.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 121.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2000;279:1449–1454. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 122.Nakagawa A, Satake H, Nakabayashi H, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 124.Bucinskaite V, Tolessa T, Pedersen J, et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:978, e78. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 125.Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 126.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1011–R1024. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 127.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Ralat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 128.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–3000. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 129.Merchenthaler I, Lane M, Shurhrue P. Distribution of pre-pro-glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 130.Plamboeck A, Veedfald S, Deacon CF, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1117–G1127. doi: 10.1152/ajpgi.00035.2013. [DOI] [PubMed] [Google Scholar]
- 131.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2011;229:130–143. doi: 10.1016/j.neuroscience.2012.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burcelin R, Serino M, Cabou C. A role of the gut-to-brain GLP-1-dependent axis in the control of metabolism. Curr Opin Pharmacol. 2009;9:744–752. doi: 10.1016/j.coph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Trapp S, Hisadome K. Glucagon-like peptide 1 and the brain: central actions-central sources? Auton Neurosci. 2011;161:14–19. doi: 10.1016/j.autneu.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 135.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.04.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lockie SH. Glucagon-like peptide-1 receptor in the brain: role in neuroendocrine control of energy metabolism and treatment target for obesity. J Neuroendocrinol. 2013;25:597–604. doi: 10.1111/jne.12039. [DOI] [PubMed] [Google Scholar]
- 137.Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1474–G1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]
- 138.Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007;28:2184–2191. doi: 10.1016/j.peptides.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 139.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012;122:388–402. doi: 10.1172/JCI42497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cabou C, Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud. 2011;8:418–431. doi: 10.1900/RDS.2011.8.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.D'Alessio DA, Sandoval DA, Seeley RJ. New ways in which GLP-1 can regulate glucose homeostasis. J Clin Invest. 2005;115:3406–3408. doi: 10.1172/JCI27207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav. 2012;106:413–416. doi: 10.1016/j.physbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ionut V, Liberty IF, Hucking K, et al. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab. 2006;291:E779–E785. doi: 10.1152/ajpendo.00106.2005. [DOI] [PubMed] [Google Scholar]
- 145.Thorens B. Central control of glucose homeostasis: the brain-endocrine pancreas axis. Diabetes Metab. 2010;36(Suppl 3):S45–S49. doi: 10.1016/S1262-3636(10)70466-3. [DOI] [PubMed] [Google Scholar]
- 146.Knauf C, Cani PD, Perrin C, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sandoval DA. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol Behav. 2008;94:670–674. doi: 10.1016/j.physbeh.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ. Leptin inhibits hypo-thalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 151.Mirshamsi S, Laidlaw HA, Ning K, et al. Leptin and insulin stimulation of signaling pathways in arcuate nucleus neurons: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26:871–882. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ma T, Du X, Pick JE, Sui G, Brownlee M, Klann E. Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice. J Neurosci. 2012;32:13701–13708. doi: 10.1523/JNEUROSCI.2107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gejl M, Egefjord L, Lerche S, et al. Glucagon-like peptide-1 decreases intracerebral glucose content by activating hexokinase and changing glucose clearance during hyper-glycemia. J Cereb Blood Flow Metab. 2012;32:2146–2152. doi: 10.1038/jcbfm.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Geil M, Lerche S, Egefjord L, et al. Glucagon-like peptide-1 raises blood-brain glucose transfer capacity and hexokinase activity in human brain. Front Neuroenergetics. 2013;5:2. doi: 10.3389/fnene.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 160.Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–2951. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Drucker DJ, Buse JD, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 162.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 163.Niswender K, Pi-Sunyer X, Buse J, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15:42–54. doi: 10.1111/j.1463-1326.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 164.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 165.Nauck M, Petrie JR, Sesti G, et al. The once-weekly human GLP-1 analogue semaglutide provides significant reductions in HbA1C and body weight in patients with type 2 diabetes (T2D). Diabetologia; EASD 48th Annual Meeting; Berlin, Germany. 2012. p. S7. [Google Scholar]
- 166.St Onge EL, Miller SA. Albiglutide: a new GLP-1 analog for the treatment of the type 2 diabetes. Exp Opin Biol Ther. 2010;10:801–806. doi: 10.1517/14712598.2010.481281. [DOI] [PubMed] [Google Scholar]
- 167.Available from: http://us.gsk.com/html/media-news/pressreleases/2012/2012-pressrelease-1178461.htm
- 168.Available from: http://us.gsk.com/html/media-news/pressreleases/2012/2012-pressrelease-1125277.htm
- 169.Wang M, Matheson S, Picard J, Pezzullo J. Abstracts of 69th Scientific Sessions of the American Diabetes Association; New Orleans. 5–9 June 2009; Abstract 553-P. [Google Scholar]
- 170.Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK. Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther. 2010;12:790–797. [PubMed] [Google Scholar]
- 171.Grunberger G, Chang A, Garsia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double blind, placebo-controlled study. Diabet Med. 2012;29:1260–1267. doi: 10.1111/j.1464-5491.2012.03745.x. [DOI] [PubMed] [Google Scholar]
- 172.Available from: http://www.hanmi.co.kr/korea/research/120607_ADA_poster_HM11260C%20%28LAPS-Exendin-4%29.pdf
- 173.Available from: http://www.diartispharma.com/content/newsandevents/releases/100212.htm
- 174.Hoare SR. Allosteric modulators of class B G-protein-coupled receptors. Curr Neuropharmacol. 2007;5:168–179. doi: 10.2174/157015907781695928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bahekar RH, Jain MR, Gupta AA, et al. Synthesis and antidiabetic activity of 3,6,7-trisubstituted-2-(1H-imidazol-2-ylsulfanyl)quinoxalines and quinoxalin-2-yl isothioureas. Arch Pharm. 2007;340:359–366. doi: 10.1002/ardp.200700024. [DOI] [PubMed] [Google Scholar]
- 176.Teng M, Johnson MD, Thomas C, et al. Small molecule ago-allosteric modulators of the human glucagon-like peptide-1 (hGLP-1) receptor. Bioorg Med Chem Lett. 2007;17:5472–5478. doi: 10.1016/j.bmcl.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 177.Irwin N, Flatt PR, Patterson S, Green BD. Insulin-releasing and metabolic effects of small molecule GLP-1 receptor agonist 6,7-dichloro-2-methylsulfonyl-3-N-tert-butylaminoquinoxaline. Eur J Pharmacol. 2010;628:268–273. doi: 10.1016/j.ejphar.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 178.Su H, He M, Li H, et al. Boc5, a non-peptidic glucagon-like peptide-1 receptor agonist, invokes sustained glycemic control and weight loss in diabetic mice. PLoS ONE. 2008;3:e2892. doi: 10.1371/journal.pone.0002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen D, Liao J, Zhou C, et al. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci U S A. 2007;104:943–948. doi: 10.1073/pnas.0610173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Q, Li N, Yuan Y, et al. Cyclobutane derivatives as novel nonpeptidic small molecule agonists of glucagon-like peptide-1 receptor. J Med Chem. 2012;55:250–267. doi: 10.1021/jm201150j. [DOI] [PubMed] [Google Scholar]
- 181.Coopman K, Huang Y, Johnston N, Bradley SJ, Wilkinson GF, Willars GB. Comparative effects of the endogenous agonist glucagon-like peptide-1 (GLP-1)-(7-36) amide and the small-molecule ago-allosteric agent “compound 2” at the GLP-1 receptor. J Pharmacol Exp Ther. 2010;334:795–808. doi: 10.1124/jpet.110.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sloop KW, Willard FS, Brenner MB, et al. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes. 2010;59:3099–3107. doi: 10.2337/db10-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rye Underwood C, Möller Knudsen S, Schjellerup Wulff B, et al. Transmembrane α-helix 2 and 7 are important for small molecule-mediated activation of the GLP-1 receptor. Pharmacology. 2011;88:340–348. doi: 10.1159/000334338. [DOI] [PubMed] [Google Scholar]
- 184.Cheong YH, Kim MK, Son MH, Kaang BK. Two small molecule agonists of glucagon-like peptide-1 receptor modulate the receptor activation response differently. Biochem Biophys Res Commun. 2012;417:558–563. doi: 10.1016/j.bbrc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 185.Li N, Lu J, Willars GB. Allosteric modulation of the activity of the glucagon-like peptide-1 (GLP-1) metabolite GLP-1 9-36 amide at GLP-1 receptor. PLoS One. 2012;7:e47936. doi: 10.1371/journal.pone.0047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wootten D, Savage EE, Willard FS, et al. Differential activation and modulation of the glucagon-like peptide-1 receptor by small molecule ligands. Mol Pharmacol. 2013;83:822–834. doi: 10.1124/mol.112.084525. [DOI] [PubMed] [Google Scholar]
- 187.Koole C, Wootten D, Simms J, et al. Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Mol Pharmacol. 2011;80:486–497. doi: 10.1124/mol.111.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ozeki N, Terasawa T, Naruse R, et al. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159:25–31. doi: 10.1016/j.trsl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 189.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 190.Kirby M, Yu DM, O'Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond) 2009;118:31–41. doi: 10.1042/CS20090047. [DOI] [PubMed] [Google Scholar]
- 191.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 192.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- 193.Engel M, Hoffmann T, Wagner L, et al. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci U S A. 2003;100:5063–5068. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abbott CA, McCaughan GW, Gorrell MD. Two highly conserved glutamic acid residues in the predicted beta propeller domain of dipeptidyl peptidase IV are required for its enzyme activity. FEBS Lett. 1999;458:278–284. doi: 10.1016/s0014-5793(99)01166-7. [DOI] [PubMed] [Google Scholar]
- 195.Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 196.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 197.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247–1258. doi: 10.1016/j.clinthera.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 198.He YL, Yamaguchi M, Ito H, Terao S, Sekiguchi K. Pharmacokinetics and pharmacodynamics of vildagliptin in Japanese patients with type 2 diabetes. Int J Clin Pharmacol Ther. 2010;48:582–595. doi: 10.5414/cpp48582. [DOI] [PubMed] [Google Scholar]
- 199.He YL, Valencia J, Zhang Y, et al. Hormonal and metabolic effects of morning or evening dosing of the dipeptidyl peptidase IV inhibitor vildagliptin in patients with type 2 diabetes. Br J Clin Pharmacol. 2010;70:34–42. doi: 10.1111/j.1365-2125.2010.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Boulton DW, Geraldes M. Safety, tolerability, pharmacokinetics and pharmacodynamics of once daily oral doses of saxagliptin for 2 weeks in type 2 diabetics and healthy subjects. Diabetes; American Diabetes Association, 67th Scientific Sessions; Chicago, IL. 2007. p. AI61. Poster 606-P. [Google Scholar]
- 201.Kania DS, Gonzalvo JD, Weber ZA. Saxagliptin: a clinical review in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33:1005–1022. doi: 10.1016/j.clinthera.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 202.Horie Y, Kanada S, Watada H, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor linagliptin: a 4-week multicenter, randomized, double-blind, placebo-controlled phase IIa study in Japanese type 2 diabetes patients. Clin Ther. 2011;33:973–989. doi: 10.1016/j.clinthera.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 203.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13:258–267. doi: 10.1111/j.1463-1326.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 204.Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30:499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 205.Christopher R, Covington P, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin Ther. 2008;30:513–527. doi: 10.1016/j.clinthera.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 206.Ban K, Kim KH, Cho CK, et al. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 207.Elahi D, Egan JM, Shannon RP, et al. GLP-1 (9-36) amide, cleavage product of GLP-1 (7-36) amide, is a glucoregulatory peptide. Obesity. 2008;16:1501–1509. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- 208.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: the extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Waget A, Cabou C, Masseboeuf M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–3029. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- 210.Holst JJ, Deacon C, Toft-Nielsen MB, Bjerre-Knudsen L. On the treatment of diabetes mellitus with glucagon-like peptide-1. Ann N Y Acad Sci. 1998;865:336–343. doi: 10.1111/j.1749-6632.1998.tb11193.x. [DOI] [PubMed] [Google Scholar]
- 211.Vilsbøll T, Knop FK, Krarup T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 212.Gentilella R, Bianchi C, Rossi A, Rotella CM. Exenatide: a review from pharmacology to clinical practice. Diabetes Obes Metab. 2009;11:544–556. doi: 10.1111/j.1463-1326.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- 213.Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD. Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev. 2004;20:411–417. doi: 10.1002/dmrr.499. [DOI] [PubMed] [Google Scholar]
- 214.Bond A. Exenatide (Byetta) as a novel treatment option for type 2 diabetes mellitus. Proc (Bayl Univ Med Cent) 2006;19:281–284. doi: 10.1080/08998280.2006.11928181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gough SC. Liraglutide: from clinical trials to clinical practice. Diabetes Obes Metab. 2012;14(Suppl 2):33–40. doi: 10.1111/j.1463-1326.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- 216.White JR. Dipeptidyl peptidase-IV inhibitors: pharmacological profile and clinical use. Clin Diabetes. 2008;26:53–57. [Google Scholar]
- 217.Pathak R, Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharm Ther. 2010;35:509–513. [PMC free article] [PubMed] [Google Scholar]
- 218.Ritcher B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2:CD006739. doi: 10.1002/14651858.CD006739.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like pep-tide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;10:CD006423. doi: 10.1002/14651858.CD006423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18:17–25. doi: 10.4158/EP11097.OR. [DOI] [PubMed] [Google Scholar]
- 221.Garber AJ. Liraglutide in oral antidiabetic drug combination therapy. Diabetes Obes Metab. 2012;14(Suppl 2):13–19. doi: 10.1111/j.1463-1326.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- 222.St Onge EL, Miller S, Clements E. Sitagliptin/Metformin (janumet) as combination therapy in the treatment of type-2 diabetes mellitus. Proc Natl Acad Sci U S A. 2012;37:699–708. [PMC free article] [PubMed] [Google Scholar]
- 223.Harashima SI, Ogura M, Tanaka D, et al. Sitagliptin add-on to low dosage sulphonylureas: efficacy and safety of combination therapy on glycaemic control and insulin secretion capacity in type 2 diabetes. Int J Clin Pract. 2012;66:465–476. doi: 10.1111/j.1742-1241.2012.02903.x. [DOI] [PubMed] [Google Scholar]
- 224.Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:442–451. doi: 10.1111/j.1463-1326.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 225.Jonas D, Van Scoyoc E, Gerrald K, et al. Drug class review: newer diabetes medications, TZDs, and combinations: final original report. Portland, OR: Oregon Health & Science University; 2011. Internet. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54209/ [PubMed] [Google Scholar]
- 226.Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomized trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 227.Diamant M, Van Gaal L, Stranks S, et al. Once weekly Exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomized trial. Lancet. 2010;375:2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 228.Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255–1261. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–1310. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 230.Nauk M, Frid A, Hermansen K, et al. Efficacy and safety comparison of Liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jendle J, Nauk M, Matthews DR, et al. Weight loss with Liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 232.Vyas AK, Yang KC, Woo D, et al. Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS ONE. 2011;6:e17178. doi: 10.1371/journal.pone.0017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 234.Nikolaidis LA, Elahi D, Shen Y, Shennon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 235.Wang D, Luo P, Wang Y, et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. 2013;62:1697–1708. doi: 10.2337/db12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Takahashi A, Asakura M, Ito S, et al. Dipeptidyl-peptidase IV inhibition improves pathophysiology of heart failure and increases survival rate in pressure-overloaded mice. Am J Physiol Heart Circ Physiol. 2013;304:H1361–H1369. doi: 10.1152/ajpheart.00454.2012. [DOI] [PubMed] [Google Scholar]
- 237.Noyan-Ashraf MH, Shikatani EA, Schuiki I, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 238.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 239.Müssig K, Onkü A, Lindauer P, et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol. 2008;101:646–647. doi: 10.1016/j.amjcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 240.Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide-1 reduces endothelial dysfunction, inflammation and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mundil D, Cameron-Vendrig A, Husain M. GLP-1 receptor agonists: a clinical perspective on cardiovascular effects. Diab Vasc Dis Res. 2012;9:95–108. doi: 10.1177/1479164112441526. [DOI] [PubMed] [Google Scholar]
- 242.Okerson T, Chilton RJ. The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther. 2012;30:e146–e155. doi: 10.1111/j.1755-5922.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334–339. doi: 10.1038/ajh.2009.245. [DOI] [PubMed] [Google Scholar]
- 244.Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 245.Plutzky J, Garber A, Toft AD, Poulter LR. Meta-analysis demonstrates that liraglutide, a once-daily human GLP-1 analogue, significantly reduces lipids and other markers of cardiovascular risks in type 2 diabetes. Diabetologia. 2009;52(Suppl 1):S299–S300. [Google Scholar]
- 246.Morinigo R, Moizé V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 247.Patriti A, Facchiano E, Gullà N, Aisa MC, Annetti C. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2007;245:157–158. doi: 10.1097/01.sla.0000250941.44516.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity. 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 250.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 255.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 256.Rhee NA, Vilsbøll T, Knop FK. Current evidence for a role of GLP-1in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab. 2012;14:291–298. doi: 10.1111/j.1463-1326.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 257.Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg. 2013;257:894–899. doi: 10.1097/SLA.0b013e31826b8603. [DOI] [PubMed] [Google Scholar]
- 258.Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon- like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks. Diabetes Care. 2011;34(Suppl 2):S361–S266. doi: 10.2337/dc11-s254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Laferrere B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine. 2011;40:162–167. doi: 10.1007/s12020-011-9514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are evidence? Obes Surg. 2009;19:217–229. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mason EE. The mechanism of surgical treatment of type 2 diabetes. Obes Surg. 2005;15:459–461. doi: 10.1381/0960892053723330. [DOI] [PubMed] [Google Scholar]
- 263.Lee WJ, Hur KY, Lakadawala M, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013;9:379–384. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 264.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 265.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zimmet P, Alberti KG. Surgery or medical therapy for obese patients with type 2 diabetes? N Engl J Med. 2012;366:1635–1636. doi: 10.1056/NEJMe1202443. [DOI] [PubMed] [Google Scholar]
- 267.Mason E. Gila Monster's guide to Surgery for obesity and diabetes. J Am Coll Surg. 2008;206:357–360. doi: 10.1016/j.jamcollsurg.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 268.Service GJ. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 269.Patti ME. Severe hypoglycemia post-gastric bypass requiring partial pancreatectomy : evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 270.Butler PC, Dry S, Elashoff R. GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care. 2010;33:453–455. doi: 10.2337/dc09-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spranger J, Gundert-Remy U, Stammschulte T. GLP-1-based therapies: the dilemma of uncertainty. Gastroenterology. 2011;141:20–23. doi: 10.1053/j.gastro.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 273.Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike pep-tide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534–539. doi: 10.1001/jamainternmed.2013.2720. [DOI] [PubMed] [Google Scholar]
- 274.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118–2125. doi: 10.2337/dc12-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chiu WY, Shih SR, Tseng CH. A review on the association between glucagon-like peptide-1 receptor agonists and thyroid cancer. Exp Diabetes Res. 2012;2012:924168. doi: 10.1155/2012/924168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 277.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 278.Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126–2132. doi: 10.2337/dc12-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Clardy-James S, Chepurny OG, Leech CA, Holz GG, Doyle RP. Synthesis, characterization and pharmacodynamics of vitamin B12-conjugated glucagon-like peptide-1. ChemMedChem. 2013;8:582–586. doi: 10.1002/cmdc.201200461. [DOI] [PMC free article] [PubMed] [Google Scholar]


